Immunotherapy and the Tumor Microenvironment in Brain Metastases from Non-Small Cell Lung Cancer: Challenges and Future Directions
Abstract
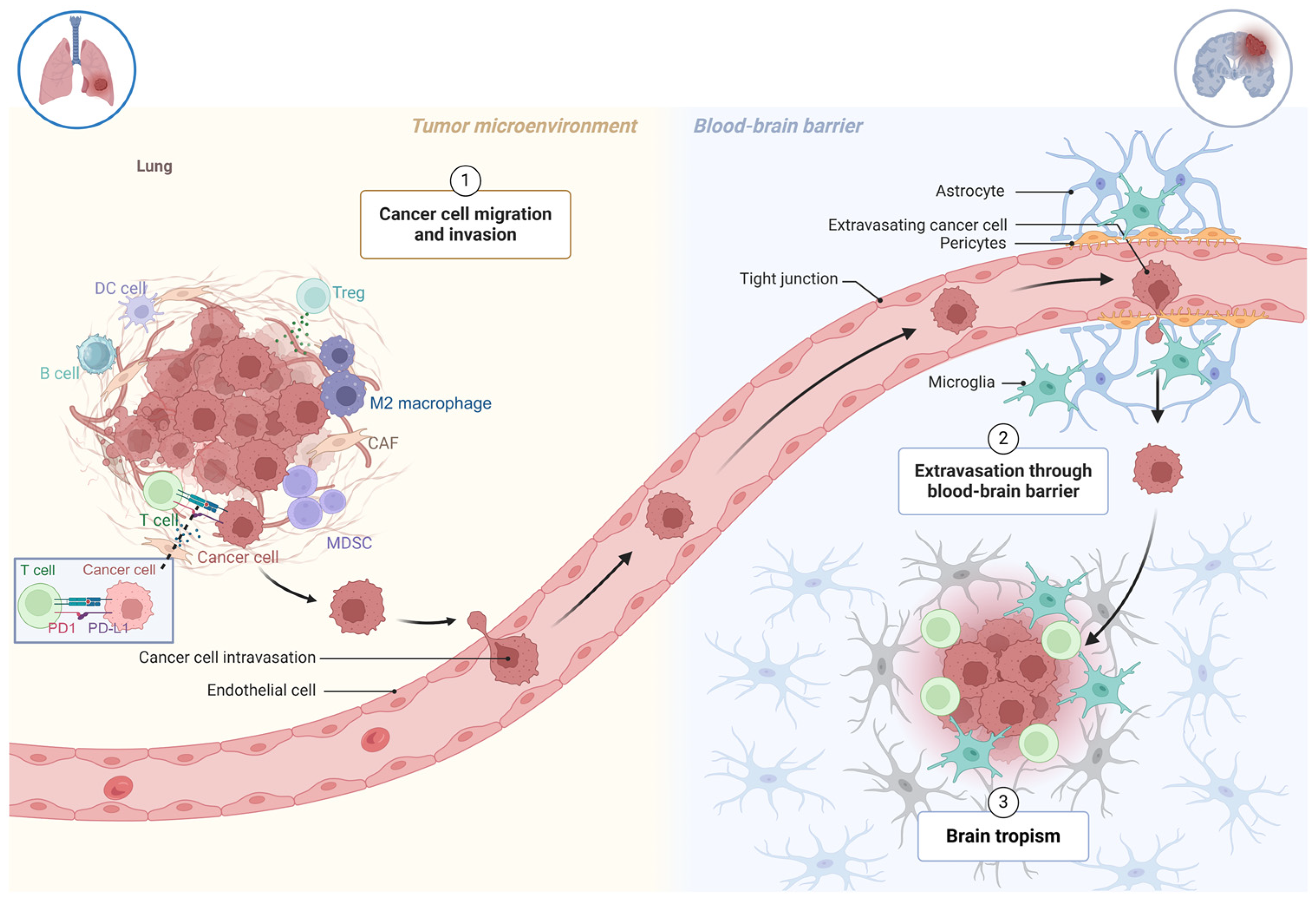
1. Introduction
2. The Formation of Brain Metastases in NSCLC
3. Blood–Brain Barrier
4. Astrocytes
5. Microglia
6. NSCLC Tumor Microenvironment
7. Immunotherapy Targeting the BM
7.1. Immunotherapy Alone
7.2. Immunotherapy Combined with Chemotherapy
7.3. Limitation of ICI and Future Strategies
8. Targeted Therapies in NSCLC with BM
8.1. EGFR
8.2. ALK
8.3. ROS1
8.4. Other Targeted Therapies
9. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nguyen, D.X.; Bos, P.D.; Massague, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–284. [Google Scholar] [CrossRef]
- Eichler, A.F.; Chung, E.; Kodack, D.P.; Loeffler, J.S.; Fukumura, D.; Jain, R.K. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 2011, 8, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.M.; Meza, J.L.; Lin, C. Association of Immunotherapy with Survival Among Patients with Brain Metastases Whose Cancer Was Managed with Definitive Surgery of the Primary Tumor. JAMA Netw. Open 2020, 3, e2015444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Perez-Soler, R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018, 19, e43–e55. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Riihimaki, M.; Hemminki, A.; Fallah, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic sites and survival in lung cancer. Lung Cancer 2014, 86, 78–84. [Google Scholar] [CrossRef]
- Strickland, M.R.; Alvarez-Breckenridge, C.; Gainor, J.F.; Brastianos, P.K. Tumor Immune Microenvironment of Brain Metastases: Toward Unlocking Antitumor Immunity. Cancer Discov. 2022, 12, 1199–1216. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain endothelial cell-cell junctions: How to “open” the blood brain barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef]
- Sun, Z.W.; Wang, X.; Zhao, Y.; Sun, Z.X.; Wu, Y.H.; Hu, H.; Zhang, L.; Wang, S.D.; Li, F.; Wei, A.J.; et al. Blood-brain barrier dysfunction mediated by the EZH2-Claudin-5 axis drives stress-induced TNF-alpha infiltration and depression-like behaviors. Brain Behav. Immun. 2024, 115, 143–156. [Google Scholar] [CrossRef]
- Nduom, E.K.; Yang, C.; Merrill, M.J.; Zhuang, Z.; Lonser, R.R. Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms. J. Neurosurg. 2013, 119, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, D.; Priego, N.; Fustero-Torre, C.; Valiente, M. Reactive Astrocytes in Brain Metastasis. Front. Oncol. 2017, 7, 298. [Google Scholar] [CrossRef]
- Valiente, M.; Obenauf, A.C.; Jin, X.; Chen, Q.; Zhang, X.H.; Lee, D.J.; Chaft, J.E.; Kris, M.G.; Huse, J.T.; Brogi, E.; et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014, 156, 1002–1016. [Google Scholar] [CrossRef]
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martinez, L.; Martinez-Saez, E.; Ramon, Y.C.S.; et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 2018, 24, 1024–1035. [Google Scholar] [CrossRef]
- Ghoochani, A.; Schwarz, M.A.; Yakubov, E.; Engelhorn, T.; Doerfler, A.; Buchfelder, M.; Bucala, R.; Savaskan, N.E.; Eyupoglu, I.Y. MIF-CD74 signaling impedes microglial M1 polarization and facilitates brain tumorigenesis. Oncogene 2016, 35, 6246–6261. [Google Scholar] [CrossRef]
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; Lopez-Soto, A.; Jacob, L.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016, 533, 493–498. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Mayer, M.G.; Fischer, T. Microglia at the blood brain barrier in health and disease. Front. Cell. Neurosci. 2024, 18, 1360195. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Izraely, S.; Ben-Menachem, S.; Sagi-Assif, O.; Telerman, A.; Zubrilov, I.; Ashkenazi, O.; Meshel, T.; Maman, S.; Orozco, J.I.J.; Salomon, M.P.; et al. The metastatic microenvironment: Melanoma-microglia cross-talk promotes the malignant phenotype of melanoma cells. Int. J. Cancer 2019, 144, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Lynch, M.A. The Multifaceted Profile of Activated Microglia. Mol. Neurobiol. 2009, 40, 139–156. [Google Scholar] [CrossRef]
- Thored, P.; Heldmann, U.; Gomes-Leal, W.; Gisler, R.; Darsalia, V.; Taneera, J.; Nygren, J.M.; Jacobsen, S.E.W.; Ekdahl, C.T.; Kokaia, Z.; et al. Long-Term Accumulation of Microglia with Proneurogenic Phenotype Concomitant with Persistent Neurogenesis in Adult Subventricular Zone After Stroke. Glia 2009, 57, 835–849. [Google Scholar] [CrossRef]
- Andreou, K.E.; Soto, M.S.; Allen, D.; Economopoulos, V.; de Bernardi, A.; Larkin, J.R.; Sibson, N.R. Anti-inflammatory Microglia/Macrophages As a Potential Therapeutic Target in Brain Metastasis. Front. Oncol. 2017, 7, 251. [Google Scholar] [CrossRef]
- Yuan, A.; Hsiao, Y.J.; Chen, H.Y.; Chen, H.W.; Ho, C.C.; Chen, Y.Y.; Liu, Y.C.; Hong, T.H.; Yu, S.L.; Chen, J.J.W.; et al. Opposite Effects of M1 and M2 Macrophage Subtypes on Lung Cancer Progression. Sci. Rep. 2015, 5, 14273. [Google Scholar] [CrossRef]
- Doron, H.; Pukrop, T.; Erez, N. A Blazing Landscape: Neuroinflammation Shapes Brain Metastasis. Cancer Res. 2019, 79, 423–436. [Google Scholar] [CrossRef]
- Jin, Y.; Kang, Y.; Wang, M.; Wu, B.; Su, B.; Yin, H.; Tang, Y.; Li, Q.; Wei, W.; Mei, Q.; et al. Targeting polarized phenotype of microglia via IL6/JAK2/STAT3 signaling to reduce NSCLC brain metastasis. Signal Transduct. Target. Ther. 2022, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, C.; Cianciulli, A.; Calvello, R.; Dragone, T.; Iacobazzi, F.; Panaro, M.A. The PI3K/Akt pathway is required for LPS activation of microglial cells. Immunopharmacol. Immunotoxicol. 2012, 34, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Porro, C.; Calvello, R.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- Goc, J.; Germain, C.; Vo-Bourgais, T.K.; Lupo, A.; Klein, C.; Knockaert, S.; de Chaisemartin, L.; Ouakrim, H.; Becht, E.; Alifano, M.; et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014, 74, 705–715. [Google Scholar] [CrossRef]
- Bravaccini, S.; Bronte, G.; Ulivi, P. TMB in NSCLC: A Broken Dream? Int. J. Mol. Sci. 2021, 22, 6536. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Wang, H.; Xu, Z.; Wang, Y.; Li, S.; Liu, J.; Chen, Y.; Luo, H.; Wu, L.; et al. Massive PD-L1 and CD8 double positive TILs characterize an immunosuppressive microenvironment with high mutational burden in lung cancer. J. Immunother. Cancer 2021, 9, e002356. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- Brody, R.; Zhang, Y.; Ballas, M.; Siddiqui, M.K.; Gupta, P.; Barker, C.; Midha, A.; Walker, J. PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017, 112, 200–215. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, T.; She, Y.; Wu, K.; Gu, S.; Li, L.; Dong, C.; Chen, C.; Zhou, Y. N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol. Cancer 2021, 20, 105. [Google Scholar] [CrossRef]
- Kim, S.; Jang, J.Y.; Koh, J.; Kwon, D.; Kim, Y.A.; Paeng, J.C.; Ock, C.Y.; Keam, B.; Kim, M.; Kim, T.M.; et al. Programmed cell death ligand-1-mediated enhancement of hexokinase 2 expression is inversely related to T-cell effector gene expression in non-small-cell lung cancer. J. Exp. Clin. Cancer Res. 2019, 38, 462. [Google Scholar] [CrossRef] [PubMed]
- Pulanco, M.C.; Madsen, A.T.; Tanwar, A.; Corrigan, D.T.; Zang, X. Recent advancements in the B7/CD28 immune checkpoint families: New biology and clinical therapeutic strategies. Cell Mol. Immunol. 2023, 20, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef]
- Conway, E.M.; Pikor, L.A.; Kung, S.H.; Hamilton, M.J.; Lam, S.; Lam, W.L.; Bennewith, K.L. Macrophages, Inflammation, and Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 116–130. [Google Scholar] [CrossRef]
- Quatromoni, J.G.; Eruslanov, E. Tumor-associated macrophages: Function, phenotype, and link to prognosis in human lung cancer. Am. J. Transl. Res. 2012, 4, 376–389. [Google Scholar] [PubMed]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Herbst, R.S.; de Castro, G.; Jr Hui, R.; Peled, N.; Kim, D.W.; Novello, S.; Satouchi, M.; Wu, Y.L.; Garon, E.B.; et al. Outcomes with Pembrolizumab Monotherapy in Patients with Programmed Death-Ligand 1-Positive NSCLC with Brain Metastases: Pooled Analysis of KEYNOTE-001, 010, 024, and 042. JTO Clin. Res. Rep. 2021, 2, 100205. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gumus, M.; Bondarenko, I.; Ozguroglu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Gumus, M.; Chen, C.I.; Ivanescu, C.; Kilickap, S.; Bondarenko, I.; Ozguroglu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Harnett, J.; et al. Patient-reported outcomes with cemiplimab monotherapy for first-line treatment of advanced non-small cell lung cancer with PD-L1 of >/=50%: The EMPOWER-Lung 1 study. Cancer 2023, 129, 118–129. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lee, J.S.; Ciuleanu, T.E.; Bernabe Caro, R.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Schalper, K.A.; Gettinger, S.N.; Mahajan, A.; Herbst, R.S.; Chiang, A.C.; Lilenbaum, R.; Wilson, F.H.; Omay, S.B.; Yu, J.B.; et al. Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 655–663. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Lukas, R.V.; Goldschmidt, J.; Conkling, P.; Park, K.; Cortinovis, D.; de Marinis, F.; Rittmeyer, A.; Patel, J.D.; von Pawel, J.; et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer 2019, 128, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.F.; Rodriguez-Abreu, D.; Langer, C.J.; Tafreshi, A.; Paz-Ares, L.; Kopp, H.G.; Rodriguez-Cid, J.; Kowalski, D.M.; Cheng, Y.; Kurata, T.; et al. Outcomes with Pembrolizumab Plus Platinum-Based Chemotherapy for Patients with NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J. Thorac. Oncol. 2021, 16, 1883–1892. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Carbone, D.P. Response to the Letter to the Editor Titled “First-Line Nivolumab Plus Ipilimumab with Chemotherapy for Metastatic NSCLC: The Updated Outcomes From CheckMate 9LA”. J. Thorac. Oncol. 2023, 18, e102–e103. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Rodriguez-Abreu, D.; Simo, M.; Massuti, B.; Juan, O.; Huidobro, G.; Lopez, R.; De Castro, J.; Estival, A.; Mosquera, J.; et al. Phase II Trial of Atezolizumab Combined with Carboplatin and Pemetrexed for Patients with Advanced Nonsquamous Non-Small-Cell Lung Cancer with Untreated Brain Metastases (Atezo-Brain, GECP17/05). J. Clin. Oncol. 2023, 41, 4478–4485. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, L.; Yang, Y.; Zhang, T.; Wu, Y.; Wang, L.; Bi, N. Efficacy and Safety of Combined Brain Radiotherapy and Immunotherapy in Non-Small-Cell Lung Cancer with Brain Metastases: A Systematic Review and Meta-Analysis. Clin. Lung Cancer 2022, 23, 95–107. [Google Scholar] [CrossRef]
- Kotecha, R.; Kim, J.M.; Miller, J.A.; Juloori, A.; Chao, S.T.; Murphy, E.S.; Peereboom, D.M.; Mohammadi, A.M.; Barnett, G.H.; Vogelbaum, M.A.; et al. The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro Oncol. 2019, 21, 1060–1068. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Sugawara, S.; Lee, J.S.; Kang, J.H.; Kim, H.R.; Inui, N.; Hida, T.; Lee, K.H.; Yoshida, T.; Tanaka, H.; Yang, C.T.; et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. 2021, 32, 1137–1147. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, J.; Wu, L.; Wang, L.; Liu, B.; Yao, J.; Zhong, H.; Li, J.; Cheng, Y.; Sun, Y.; et al. PL02.04 Phase 3 Study of Ivonescimab (AK112) vs. Pembrolizumab as First-line Treatment for PD-L1-positive Advanced NSCLC: Primary Analysis of HARMONi-2. J. Thorac. Oncol. 2024, 19, S1. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.; Fang, W.F.; Du, Y.; Zhao, Y.; Chen, J.; Luo, Y.; Yang, Y.; Xiong, A.; Zhao, H.; et al. 174P Intracranial (IC) activity of ivonescimab (ivo) alone or in combination with platinum doublet chemotherapy (PC) in patients (Pts) with advanced non-small cell lung cancer (aNSCLC) and brain metastases (BMs). ESMO Open 2024, 9, 102749. [Google Scholar] [CrossRef]
- Lu, C.; Tan, Y. Promising immunotherapy targets: TIM3, LAG3, and TIGIT joined the party. Mol. Ther. Oncol. 2024, 32, 200773. [Google Scholar] [CrossRef] [PubMed]
- Boldig, C.; Boldig, K.; Mokhtari, S.; Etame, A.B. A Review of the Molecular Determinants of Therapeutic Response in Non-Small Cell Lung Cancer Brain Metastases. Int. J. Mol. Sci. 2024, 25, 6961. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients with Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, JCO2018783118. [Google Scholar] [CrossRef]
- Planchard, D.; Janne, P.A.; Cheng, Y.; Yang, J.C.; Yanagitani, N.; Kim, S.W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef]
- Cho, B.C.; Lu, S.; Felip, E.; Spira, A.I.; Girard, N.; Lee, J.S.; Lee, S.H.; Ostapenko, Y.; Danchaivijitr, P.; Liu, B.; et al. Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2024, 391, 1486–1498. [Google Scholar] [CrossRef]
- Mok, T.; Camidge, D.R.; Gadgeel, S.M.; Rosell, R.; Dziadziuszko, R.; Kim, D.W.; Perol, M.; Ou, S.I.; Ahn, J.S.; Shaw, A.T.; et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.H.; Han, J.Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Garcia Campelo, M.R.; Kim, D.W.; et al. Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J. Thorac. Oncol. 2021, 16, 2091–2108. [Google Scholar] [CrossRef]
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs Crizotinib for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1617–1625. [Google Scholar] [CrossRef]
- Solomon, B.J.; Liu, G.; Felip, E.; Mok, T.S.K.; Soo, R.A.; Mazieres, J.; Shaw, A.T.; de Marinis, F.; Goto, Y.; Wu, Y.L.; et al. Lorlatinib Versus Crizotinib in Patients with Advanced ALK-Positive Non-Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study. J. Clin. Oncol. 2024, 42, 3400–3409. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Ou, S.H.; Bang, Y.J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Dziadziuszko, R.; Krebs, M.G.; De Braud, F.; Siena, S.; Drilon, A.; Doebele, R.C.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Camidge, D.R.; Lin, J.J.; Kim, S.W.; Solomon, B.J.; Dziadziuszko, R.; Besse, B.; Goto, K.; de Langen, A.J.; Wolf, J.; et al. Repotrectinib in ROS1 Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 118–131. [Google Scholar] [CrossRef]
- Drilon, A.; Subbiah, V.; Gautschi, O.; Tomasini, P.; de Braud, F.; Solomon, B.J.; Shao-Weng Tan, D.; Alonso, G.; Wolf, J.; Park, K.; et al. Selpercatinib in Patients with RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J. Clin. Oncol. 2023, 41, 385–394. [Google Scholar] [CrossRef]
- Griesinger, F.; Curigliano, G.; Thomas, M.; Subbiah, V.; Baik, C.S.; Tan, D.S.W.; Lee, D.H.; Misch, D.; Garralda, E.; Kim, D.W.; et al. Safety and efficacy of pralsetinib in RET fusion-positive non-small-cell lung cancer including as first-line therapy: Update from the ARROW trial. Ann. Oncol. 2022, 33, 1168–1178. [Google Scholar] [CrossRef]
- de Langen, A.J.; Johnson, M.L.; Mazieres, J.; Dingemans, A.C.; Mountzios, G.; Pless, M.; Wolf, J.; Schuler, M.; Lena, H.; Skoulidis, F.; et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: A randomised, open-label, phase 3 trial. Lancet 2023, 401, 733–746. [Google Scholar] [CrossRef]
- Barlesi, F.; Yao, W.; Duruisseaux, M.; Doucet, L.; Shi, J.; Juan Vidal, O.J.; Kim, Y.-C.; García Campelo, M.R.; Azkárate Martínez, A.; Lu, S.; et al. LBA57 Adagrasib (ADA) vs docetaxel (DOCE) in patients (pts) with KRASG12C-mutated advanced NSCLC and baseline brain metastases (BM): Results from KRYSTAL-12. Ann. Oncol. 2024, 35, S1247–S1248. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Yao, W.; Duruisseaux, M.; Doucet, L.; Martínez, A.A.; Gregorc, V.; Juan-Vidal, O.; Lu, S.; Bondt, C.D.; Marinis, F.D.; et al. KRYSTAL-12, Phase 3 study of adagrasib versus docetaxel in patients with previously treated advanced/metastatic non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. J. Clin. Oncol. 2024, 42, LBA8509. [Google Scholar] [CrossRef]
- Wolf, J.; Hochmair, M.; Han, J.Y.; Reguart, N.; Souquet, P.J.; Smit, E.F.; Orlov, S.V.; Vansteenkiste, J.; Nishio, M.; de Jonge, M.; et al. Capmatinib in MET exon 14-mutated non-small-cell lung cancer: Final results from the open-label, phase 2 GEOMETRY mono-1 trial. Lancet Oncol. 2024, 25, 1357–1370. [Google Scholar] [CrossRef]
- Thomas, M.; Garassino, M.; Felip, E.; Sakai, H.; Le, X.; Veillon, R.; Smit, E.; Mazieres, J.; Cortot, A.; Raskin, J.; et al. OA03.05 Tepotinib in Patients with MET Exon 14 (METex14) Skipping NSCLC: Primary Analysis of the Confirmatory VISION Cohort C. J. Thorac. Oncol. 2022, 17, S9–S10. [Google Scholar] [CrossRef]
- Drilon, A.; Tan, D.S.W.; Lassen, U.N.; Leyvraz, S.; Liu, Y.; Patel, J.D.; Rosen, L.; Solomon, B.; Norenberg, R.; Dima, L.; et al. Efficacy and Safety of Larotrectinib in Patients with Tropomyosin Receptor Kinase Fusion-Positive Lung Cancers. JCO Precis. Oncol. 2022, 6, e2100418. [Google Scholar] [CrossRef]
- Cho, B.C.; Chiu, C.H.; Massarelli, E.; Buchschacher, G.L.; Goto, K.; Overbeck, T.R.; Loong, H.H.F.; Chee, C.E.; Garrido, P.; Dong, X.; et al. Updated efficacy and safety of entrectinib in NTRK fusion-positive non-small cell lung cancer. Lung Cancer 2024, 188, 107442. [Google Scholar] [CrossRef]
| Trial | Type | Treatment | BM Eligibility | Patient Eligibility | Patient Number/BM Number | Systemic Outcome in Patients with BM | IC Outcome |
|---|---|---|---|---|---|---|---|
| Pooled analysis of KEYNOTE-001, -010, -024, -042 [47] | KEYNOTE-001 phase 1; KEYNOTE-010 phase 2/3; KEYNOTE -024 and -042 phase 3 | Pembro vs. Chemo | No active BM, no carcinomatous meningitis | Previously treated and treatment naïve, PD-L1 positive. No EGFR/ALK alteration, or failed EGFR or ALK TKI (KEYNOTE 001 and KEYNOTE 010) | 3170/293 | PD-L1 ≥ 50% and BM: ORR: 33.9% vs. 14.6% mPFS 4.1 m vs. 4.6 m (HR 0.70, 95% CI 0.47–1.03) mOS 19.7 m vs. 9.7 m (HR 0.67, 95% CI 0.44–1.02) PD-L1 ≥ 1% and BM: ORR: 26.1% vs. 18.1% mPFS 2.3 m vs. 5.2 m (HR 0.96, 95% CI 0.73–1.25) mOS 13.4 m vs. 10.3 m (HR 0.83, 95% CI 0.62–1.10) | / |
| IMPOWER-Lung 1 [48,49] | Phase 3, randomized, controlled study | CEMI vs. Chemo | Treated and clinically stable BM | Advanced NSCLC with PD-L1 ≥ 50%, no EGFR/ALK/ROS1 alterations | 710 enrolled, 563 PD-L1 ≥ 50%/68 | mPFS 10.4 m vs. 5.3 m (HR 0.45, 95% CI 022–0.92) mOS 18.7 m vs. 11.7 m (HR 0.17, 95% CI 0.04–0.76) | / |
| CheckMate-227 part 1 [50] | Phase 3, open label, randomized controlled study | Ipi + Nivo vs. Chemo | Treated and asymptomatic BM | Stage IV or recurrent NSCLC, treatment-naïve, no EGFR/ALK alterations | 1739/202 | ORR 32% vs. 26% mPFS 5.4 m vs. 5.8 m (HR 0.77, 95% CI 0.51–1.15) mOS 17.4 m vs. 13.7 m (HR 0.63, 95% CI 0.42–0.92) | IC PFS 8.6 m vs. 8.7 m (HR 0.82, 95% CI 0.52–1.30) 5-year IC PFS 16% vs. 6% New BM: 4% vs. 20% |
| Goldberg et al. [51] | Phase 2, open label, single arm | Pembro | Untreated or progressing after RT; no neurologic symptoms or steroid requirement | Stage IV NSCLC | 42/42 (cohort 1 PD-L1 ≥ 1%: 37; cohort 2 PD-L1 < 1% or unevaluable: 5) | Cohort 1: mPFS 2.3 m (95% CI 1.9-NE) mOS 9.9 m (95% CI 7.5–29.8) | Cohort 1: IC ORR 29.7% (95% CI 15.9–47.0) Cohort 2: IC ORR 0 |
| OAK study [52,53] | Phase 3, open label, randomized controlled study | Atezo vs. docetaxel | Treated, asymptomatic, supratentorial BM | Advanced NSCLC previously treated with platinum-based Chemo | 850/123 | mOS 20.1 m vs. 11.9 m (HR 0.54, 95% CI 0.31–0.94) | IC PFS NR vs. 9.5 m (HR 0.38, 95% CI 0.16–0.91, p = 0.024) IC OS 16.0 m vs. 11.9 m (HR 0.74, 95% CI 0.49–1.13, p = 0.16) |
| Pooled analysis of KEYNOTE-021, -189, -407 [54] | KEYNOTE-021 phase 2; KEYNOTE-189 and -407 phase 3 | Pembro + Chemo vs. Chemo | Treated or untreated (KEYNOTE-189 and KEYNOTE-407 only) stable BM | Stage IIIB or IV (KEYNOTE-021 cohort G), Stage IV (KEYNOTE-189 and -407) nonsquamous without EGFR/ALK alteration (KEYNOTE-021 cohort G and KEYNOTE-189) or squamous (KEYNOTE-407), chemotherapy naïve NSCLC | 1299/171 | ORR 39.0% vs. 19.7% mPFS 6.9 m vs. 4.1 m (HR 0.44, 95% CI 0.31–0.62) mOS 18.8 m vs. 7.6 m (HR 0.48, 95% CI 0.32–0.70) | / |
| CheckMate 9LA [55] | Phase 3, open label, randomized controlled study | Ipi + Nivo + Chemo vs. Chemo | Treated and asymptomatic BM | Stage IV or recurrent NSCLC without EGFR/ALK alterations | 719/101 | ORR 43% vs. 24% mPFS 9.7 m vs. 4.1 m (HR 0.44, 95% CI 0.28–0.69) mOS 19.3 m vs. 6.8 m (HR 0.45, 95% CI 0.29–0.70) | IC ORR 39% vs. 20% IC PFS 11.4 m vs. 4.6 m (HR 0.42, 95% CI 0.26–0.68) New BM in pt with baseline BM: 20% vs. 30% New BM in pt without baseline BM: 3.2% vs. 3.6% |
| Atezo-Brain [56] | Phase 2, single arm | Atezo + c arboplatin + pemetrexed | Untreated and asymptomatic BM | Advanced nonsquamous NSCLC with BM, no EGFR/ALK alterations | 40/40 | ORR 45% (95% credibility interval Crl 28.1–57.9) mPFS 8.9 m (95% CI 6.7–13.8) mOS 11.8 m (95% CI 7.6–16.9) | IC ORR 42.7% (95% Crl 28.1–57.9) IC PFS 6.9 m (95% CI 4.7–11.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Yang, J.; Wang, S.; Gill, H.; Cheng, H. Immunotherapy and the Tumor Microenvironment in Brain Metastases from Non-Small Cell Lung Cancer: Challenges and Future Directions. Curr. Oncol. 2025, 32, 171. https://doi.org/10.3390/curroncol32030171
Wang M, Yang J, Wang S, Gill H, Cheng H. Immunotherapy and the Tumor Microenvironment in Brain Metastases from Non-Small Cell Lung Cancer: Challenges and Future Directions. Current Oncology. 2025; 32(3):171. https://doi.org/10.3390/curroncol32030171
Chicago/Turabian StyleWang, Meng, Jihua Yang, Shuai Wang, Harjot Gill, and Haiying Cheng. 2025. "Immunotherapy and the Tumor Microenvironment in Brain Metastases from Non-Small Cell Lung Cancer: Challenges and Future Directions" Current Oncology 32, no. 3: 171. https://doi.org/10.3390/curroncol32030171
APA StyleWang, M., Yang, J., Wang, S., Gill, H., & Cheng, H. (2025). Immunotherapy and the Tumor Microenvironment in Brain Metastases from Non-Small Cell Lung Cancer: Challenges and Future Directions. Current Oncology, 32(3), 171. https://doi.org/10.3390/curroncol32030171





