Abstract
This study characterizes real-world treatment patterns and economic and healthcare resource utilization (HCRU) burden associated with first-line (1L) treatment of metastatic non-small cell lung cancer (NSCLC) without actionable alterations in the United States. This retrospective observational study used Optum Clinformatics® data. A total of 15,659 patients with metastatic NSCLC who started 1L treatment between January 2020 and March 2023 were included (52% male; mean age at the start of 1L treatment 71.7 years; 86% Medicare Advantage). The most frequent 1L regimens were immune checkpoint inhibitor (ICI) + platinum-based chemotherapy (PBCT) (47%), PBCT only (26%), and ICI only (20%). The median 1L treatment duration was 4.2 months (range 2.7–6.5) and was shorter with chemotherapy-only regimens. Outpatient visits accounted for the majority of HCRU (mean 6.6 visits per patient per month [PPPM]). Outpatient, inpatient, and emergency department visits were highest for chemotherapy-only regimens. Mean total (all-cause) healthcare costs were $32,215 PPPM and were highest for ICI + chemotherapy ($34,741–38,454 PPPM). Inpatient costs PPPM were highest for PBCT ($4725) and ICI + non-PBCT ($4648). First-line treatment of metastatic NSCLC without actionable alterations imposes a notable HCRU and cost burden, underscoring the need for better treatment options to improve outcomes and reduce economic impact.
1. Introduction
Lung cancer is a primary cause of cancer-related death in the United States (US) and globally [1,2]. The majority of lung cancer diagnoses are non-small-cell lung cancer (NSCLC) [3], with most diagnoses occurring at advanced or metastatic stages. Almost half of patients diagnosed with early-stage disease experience disease progression within 5 years [4]. Patients with advanced or metastatic NSCLC demonstrate poor survival despite advances in treatment, with 5-year survival rates of 10–15% [4,5]. Molecular subtypes, such as Kirstin rat sarcoma virus (KRAS) mutations, are known to significantly impact patient-reported outcomes and overall disease burden, emphasizing the need for tailored therapeutic approaches [6].
Patients with advanced/metastatic NSCLC have been historically treated with chemotherapy regimens [7]. However, the development of new systemic anticancer therapies (SACT) such as anti-angiogenic agents, immune checkpoint inhibitors (ICIs) and therapies that target specific alterations (e.g., endothelial growth factor receptor, anaplastic lymphoma kinase, Kristen rat sarcoma (KRAS)) [7,8] have led to a shift in the treatment landscape for NSCLC. Targeted therapies have demonstrated improved survival in patients with actionable alterations and are generally better tolerated than chemo(immuno)therapy [9]. For instance, sotorasib has been shown to improve clinical and patient-reported outcomes compared with docetaxel in patients with KRAS G12C-mutated NSCLC, as demonstrated in the CodeBreaK 200 trial [10,11]. However, 50–60% of patients with advanced NSCLC do not have actionable alterations [8,9], and treatment options for these patients have become increasingly complex. ICIs, which were approved in the US in 2016 for the first-line (1L) treatment of metastatic NSCLC, either as monotherapy or in combination with platinum-based chemotherapy (PBCT), have become established as 1L treatment [12,13] based on survival benefits demonstrated in clinical trials [14]. Despite advances in treatment options, decision-making in the real-world setting remains complex, particularly for patients without actionable alterations. Clinical trial populations often differ from real-world patients in terms of baseline characteristics, comorbidities, and treatment eligibility, which may impact outcomes and treatment choices [15]. It is important to understand the uptake of these treatments in real-world settings and to evaluate the overall economic and resource burden of 1L treatments on healthcare systems.
Although several studies have reported the burden of illness in US clinical practice for patients with metastatic NSCLC [16,17,18,19,20,21,22], most focus on second and later lines of therapy, evaluate targeted treatments or use data that largely precede the widespread adoption of ICIs. The current study aims to address this knowledge gap by evaluating real-world treatment patterns, healthcare resource utilization (HCRU), and healthcare costs, specifically among patients receiving SACTs for the 1L treatment of metastatic NSCLC without actionable alterations in the US. Understanding real-world outcomes can provide valuable insights for clinicians, policymakers, and payers in terms of managing healthcare expenditure and treatment options in the 1L treatment of NSCLC.
2. Materials and Methods
This was a retrospective observational cohort study utilizing data from the Optum Deidentified Clinformatics® database. The Optum database includes information on enrollment medical and pharmacy data for persons insured in the US via commercial insurance, as well as Medicare Advantage plans. Enrollment information includes age, sex, region, payer, plan type, and date of plan enrollment. Medical data include dates of service use, diagnosis, procedure information, and financial information. Pharmacy data include type of prescription filled, dates of filling, quantity, number of days’ supply, and financial information.
2.1. Study Design and Population
Eligible patients had a diagnosis of lung cancer (date of first diagnosis = Dx date) during the study period (1 January 2016 to 31 March 2023), a diagnosis of metastases on or after the Dx date (secondary malignancy date) and had received 1L SACT for lung cancer following the secondary malignancy date. The initiation of 1L SACT was determined as the index date. The study design is shown in Figure 1. The International Classification of Diseases, 10th Edition, Clinical Modification (ICD-10-CM) code C34 was used for identification of lung cancer diagnosis.
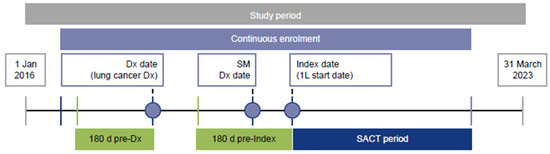
Figure 1.
Study design. The SACT period is the time from the index date until the earliest of 30 days after the last cycle of SACT during the LOT, the day before the next LOT or the end of follow-up. 1L, first-line; d, days; Dx, diagnosis; SACT, systemic anticancer treatment; SM, secondary malignancy.
Lines of treatment were determined using an adaptation of a previously published algorithm [23]: the date of first metastatic disease was determined to be the index date. The date of the first SACT received after the index date was the start date of 1L treatment. All SACTs received within the 30-day period beginning with the 1L treatment start date were classified as a part of the 1L regimen. The line of treatment (LOT) advanced when a new drug that was not part of the current regimen was introduced or if there was a treatment gap of at least 60 days. However, temporary pauses or reintroductions of drugs within the same regimen did not change the LOT. Substitutions between similar drugs (e.g., cisplatin and carboplatin, paclitaxel and albumin-bound paclitaxel) or the addition of National Comprehensive Cancer Network (NCCN)-recommended maintenance therapies for metastatic NSCLC did not advance the LOT. LOT is finished at the end of continuous enrollment, the end of the study period, or death.
Patients were also required to initiate 1L SACT on or after 1 January 2020 (to ensure capture of recent data), to be at least 18 years of age as of the index date and to have been continuously enrolled in medical and pharmacy benefits from a minimum of 180 days before the Dx date to a minimum of 30 days after the index date. Exclusion criteria included receiving treatments for actionable mutations (not including ICIs or vascular endothelial growth factor therapies). In order to ensure that patients with NSCLC were captured, those receiving treatments typically administered for small-cell lung cancer (SCLC) before the index date were excluded. Patients receiving SACT for lung cancer and those who had a diagnosis of metastases during the 180-day pre-diagnosis period were also excluded.
2.2. Treatment Classification
Treatment regimens for 1L were classified into the following categories: ICI therapy (mono/dual), ICI + PBCT, ICI + non-PBCT, PBCT mono/combination therapies (excluding ICIs), non-PBCT monotherapies, and non-PBCT combination therapies (excluding ICIs), plus any other regimens that were identified during the analysis.
2.3. Baseline Characteristics
Demographics assessed on the index date included age, region, sex and payer. Clinical characteristics included types of secondary metastases, comorbidities that are common among lung cancer patients (such as anemia, chronic obstructive pulmonary disease [COPD], chronic kidney disease, chronic liver disease, coronary heart disease, dementia, diabetes, hypertension and cerebrovascular disease), smoking history and National Cancer Institute (NCI) Adapted Charlson Comorbidity Index (NCI-CCI) [24]. Clinical characteristics were assessed during the 180 days up to but excluding the index date.
2.4. Study Outcomes
The duration of 1L treatment was defined as the time from the index date to the earliest maximum runout date for all the therapies in 1L, the day before the start date of the next line of treatment, study end date or end of follow-up. For patients who went on to receive second-line (2L) treatment, time to next treatment (TTNT) was estimated as the number of days from the start of 1L treatment to the day before the start of 2L treatment.
All-cause and NSCLC-related HCRU and healthcare costs were assessed during the SACT treatment period, which spanned from the index date to the earliest of 30 days after the maximum runout date for all treatments in 1L regimen, the day before the start of 2L, or the end of the follow-up period. All-cause and NSCLC-related HCRU and healthcare costs were evaluated at the per-patient per-month (PPPM) level and stratified into outpatient (including chemotherapy and ICI costs as these are generally administered in an outpatient setting), inpatient, emergency department (ED) and pharmacy (prescriptions). A claim was considered to be NSCLC-related if it had a diagnosis of lung cancer in any position or if it was for a lung-cancer-related medication.
2.5. Analysis
No statistical testing was carried out, and the analyses were presented descriptively. Means (standard deviation [SD] or medians (interquartile range [IQR]) are used for reporting continuous variables, and numbers and percentages are used for the reporting of categorical variables. To account for inflation, healthcare costs were adjusted to 2022 US dollars using the medical component of the Consumer Price Index [25]. To account for censoring, treatment duration was determined using Kaplan–Meier survival analysis.
STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting cohort studies were considered.
3. Results
3.1. Study Population
A total of 15,659 patients met the eligibility criteria (Table 1). Their mean age at the start of 1L treatment was 71.7 (SD 8.6) years; 52% were male, and the majority (86%) of patients were covered by Medicare Advantage (Table 2). The median duration from lung cancer diagnosis to initiation of 1L therapy was 1.5 (IQR 0.9–2.8) months. The mean NCI-CCI score was 2.3 (SD 1.9). The most common comorbidities reported were hypertension (73%), COPD (44%) and coronary heart disease (29%).

Table 1.
Selection of study population.

Table 2.
Baseline characteristics (n = 15,659).
3.2. Treatment Patterns
The most frequent 1L treatment regimens were ICI + PBCT (n = 7359; 47.0%), PBCT mono/combo (n = 4139; 26.4%) and ICI mono/dual (n = 3089; 19.7%) (Figure 2). Additional details on treatment patterns are provided in Supplementary Table S1.
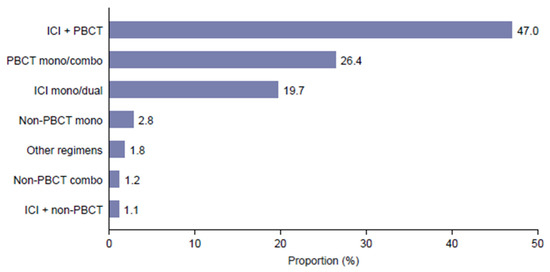
Figure 2.
Distribution of first-line treatments. ICI is an immune checkpoint inhibitor; PBCT is a platinum-based chemotherapy.
The mean duration of follow-up was 11.2 (SD 9.2) months. The median duration of 1L treatment (based on Kaplan–Meier analysis) was 4.2 months; the mean TTNT among patients who received 2L treatment was 8.0 months (SD 5.7). The treatment duration and TTNT were the shortest for regiments containing chemotherapy alone (Figure 3).
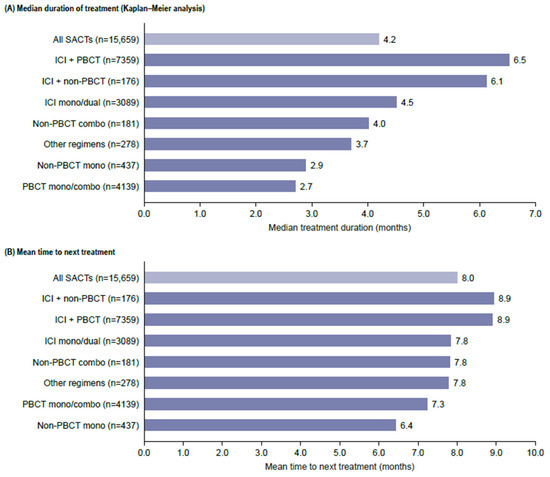
Figure 3.
Duration of treatment and time to next treatment. ICI is an immune checkpoint inhibitor; PBCT is a platinum-based chemotherapy; SACTs, systemic anti-cancer treatment.
3.3. HCRU
Chemotherapy-only regimens were associated with the greatest HCRU for outpatient, inpatient and ED visits across all treatment categories (Figure 4). Outpatient visits accounted for the majority of all-cause HCRU. The mean number of outpatient visits was 6.6 PPPM and was highest for PBCT mono/combo (10.5 PPPM) and non-PBCT monotherapy (6.6 PPPM) (Figure 4A). Almost three-quarters of outpatient visits (74%) were NSCLC-related (Supplementary Table S1). The mean number of all-cause inpatient admissions was 0.12 PPPM and was highest for non-PBCT combinations (0.19 PPPM), PBCT mono/combination therapies and non-PBCT therapies (both 0.16 PPPM). NSCLC-related inpatient admissions were also highest for PBCT-containing regimens (Figure 4B). The mean number of ED visits was 0.11 PPPM and was highest with PBCT mono/combination therapies (0.16 PPPM) (Figure 4C).
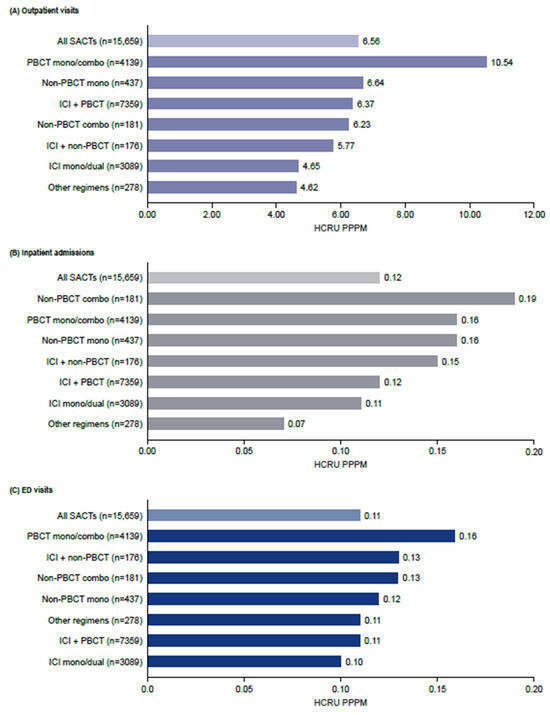
Figure 4.
Healthcare resource utilization during the first-line treatment of NSCLC. Combo, combination; HCRU, healthcare resource utilization; ICI, immune checkpoint inhibitor; PBCT, platinum-based chemotherapy; PPPM, per patient per month; SACT, systemic anticancer therapy.
3.4. Healthcare Costs
Figure 5 illustrates the relative contributions of the key cost categories (inpatient, outpatient, ER, prescriptions) to the total cost for each treatment type. Mean total (all-cause) healthcare costs during 1L treatment were $32,215 PPPM (SD 44,597) and were highest for ICI in combination with chemotherapy regimens: $38,454 (SD 55,678) for ICI + non-PBCT, $34,721 (SD 44,191) for ICI + PBCT (all PPPM) (Figure 5).
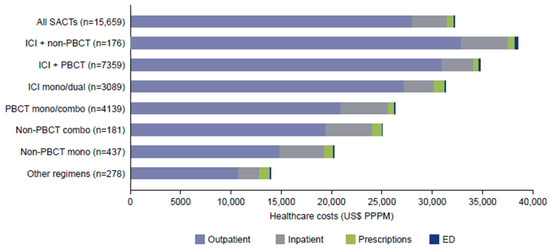
Figure 5.
Mean total (all-cause) healthcare costs during first-line treatment. Combo, combination; ED, emergency department; ICI, immune checkpoint inhibitor; PBCT, platinum-based chemotherapy; PPPM, per patient per month; SACT, systemic anticancer therapy.
All-cause healthcare costs were largely incurred in outpatient settings ($28,045 [SD 37,735] PPPM), followed by inpatient costs ($3412 [SD 21,791] PPPM). Outpatient costs (PPPM) were highest for ICI in combination with chemotherapy: $32,913 (SD 50,790) for ICI + non-PBCT, followed by $30,888 (SD 37,409) for ICI + PBCT. Inpatient costs were highest for PBCT mono/combination therapy ($4725 [SD 22,874] PPPM), followed by ICI + non-PBCT ($4648 [SD 24,323] PPPM).
Mean NSCLC-related healthcare costs during 1L treatment were $26,231 PPPM (SD 46,183) and were highest for ICI + PBCT ($31,690 [SD 44,358]). As with all-cause costs, most NSCLC-related costs were incurred in the outpatient setting ($23,388 [SD 40,111]), followed by inpatient costs ($2709 [SD 19,203]) (Supplementary Table S2).
The median and interquartile range for all-cause total costs by each treatment category are provided in Supplementary Table S3. Mean and median total healthcare costs were found to be similar.
4. Discussion
This study evaluated treatment patterns, HCRU and healthcare costs in the 1L treatment of metastatic NSCLC without actionable alterations in US real-world practice. While our study indicates widespread adoption of ICI-containing regimens in the 1L setting, it also identified a considerable burden in terms of HCRU and economic impact.
The most frequently used SACT in the 1L setting was ICI + PBCT (47%), followed by PBCT mono/combo (26%) and mono/dual ICI (19%). This is consistent with clinical practice guidelines, which recommend ICI + PBCT for 1L treatment [12,13]. Recent studies indicate a shift in treatment patterns compared with a 2018 study in which 93% received chemotherapy [17]. DaCosta Byfield and colleagues reported growing use of pembrolizumab (alone or in combination with chemotherapy) from fewer than 2% of claims in 2016 to almost half in 2018 (based on retrospective clinical data collected from a prior authorization tool linked with payer claims data from 2108 patients) [15]. Similarly, Kish and colleagues reported a decrease in the use of chemotherapy from 72% to 48% between 2017 and 2019, whereas the use of ICI + chemotherapy increased from 2% to 30% [26]. We found that 68% of regimens included an ICI (with or without chemotherapy), indicating that uptake has increased since earlier studies. It is possible that treatment patterns have changed within this data collection period, highlighting the importance of understanding how real-world treatment patterns evolve as new therapies are adopted into clinical use.
In the current study, treatment duration was found to be the lowest with chemotherapy-only regimens (2.7–4 months). This is consistent with the 2019–2021 US claims retrospective cohort study, which reported a shorter median time to treatment discontinuation with chemotherapy than with other regimens (2.4 months [IQR 2.1–3.7] vs. overall median of 3.4 [IQR 2.2–6.1]) [27], and a 2015–2018 study using claims data which reported shorter treatment duration with chemotherapy regimens compared with combined ICI + chemotherapy [15].
In the current study, all-cause outpatient, inpatient, and ED visits were all frequent with chemotherapy-only regimens, which is consistent with the greater toxicity of these regimens. Published studies have rarely compared HCRU associated with different treatment regimens in NSCLC. Pham and colleagues reported higher HCRU with ICI + chemotherapy combinations than with ICI alone [28], similar to the current study. A real-world study in Spain reported that HCRU for both disease management and management of adverse events was higher with chemotherapy than with ICIs; hospitalizations, ED visits and pharmacy visits were all more frequent with chemotherapy [29], as seen in the current study.
Outpatient visits were the main driver of HCRU in the 1L treatment of NSCLC in the current analysis, consistent with other studies [19,20,27]. However, the number of outpatient visits varies widely between studies. In the current study, the mean number of outpatient visits for all SACTs was 6.6 PPPM and ranged from 1.6 to 10.5 across the different treatment regimens. Spira and colleagues report a higher rate of mean outpatient visits (10.3 PPPM) in their study, which included fewer patients and appears to include targeted 1L treatments [27]. Notably, their study covered a different timeline from our study, which may also account for these differences. However, Spiral and colleagues reported similar numbers of inpatient stays (0.10 ± 0.3 PPPM) to the current study (0.12 PPPM) [27].
In terms of healthcare costs, the economic burden of non-targeted 1L treatment for metastatic NSCLC in the current study was $32,215 PPPM and was highest for patients receiving ICI in combination with chemotherapy ($34,721–38,454 PPPM). Consistent with the HCRU findings, the majority of healthcare costs were attributed to outpatient visits, potentially related to ICI-containing therapies. These costs are higher than those reported by Zhang and colleagues based on a US claims-based analysis of $22,633 PPPM, although this is an older study (claims from 2010 to 2019) and a largely commercially insured population [19]. Simmons and colleagues reported mean costs for 1L treatment of $36,717 ($35,658–37,777) PPPM for Medicare Advantage patients [20], which are similar to the costs reported in our study.
In the current study, total costs for 1L treatment were higher with ICI + chemotherapy regimens. Four other studies have reported a similar pattern. Kish and colleagues reported that total costs for 1L treatment were highest with ICI + chemotherapy ($32,436 PPPM) [26]. This study included a significant proportion of Medicaid patients (40%) who were not evaluated in our study, and the data were for a shorter and earlier period (January 2017 to May 2019). Spira and colleagues reported higher costs with pembrolizumab/immunotherapy plus chemotherapy compared with pembrolizumab/immunotherapy alone (US$44,678–45,682 vs. 31,774–33,371 PPPM) based on US claims data from January 2019 to June 2021 [27]. In another small claims-based study (2016–2021) of the 1L treatment of patients with advanced NSCLC and programmed death ligand 1 (PDL1) expression below 50% who made at least one claim for ICI-based chemotherapy, total adjusted all-cause medical costs were higher with ICI in combination than with chemotherapy alone (based on 88 patients in each group) [28]. The 2015–2018 study using claims data also identified higher total costs with pembrolizumab plus chemotherapy than with chemotherapy alone [15].
These studies together indicate that the 1L treatment of advanced/metastatic NSCLC without actionable alterations is associated with a substantial HCRU burden and healthcare costs. The existing literature suggests that newer therapies such as immunotherapies improve survival compared with chemotherapy alone [30,31] but are associated with higher upfront costs, as shown in the current analysis. While this study does not evaluate cost-effectiveness, our findings on the economic burden associated with 1L treatments provide valuable context for future studies evaluating the overall value of newer therapies. With the advent of novel therapies, real-world studies have become important to evaluate the value of treatment in terms of long-term and patient-reported outcomes and to guide evidence-based decision-making.
This study is subject to the limitations inherent in studies using administrative claims databases. There is no specific International Classification of Disease 10th edition (ICD10) code to define NSCLC, so patients with a lung cancer diagnosis code who were not receiving regimens for SCLC were determined to have NSCLC—an approach that has been used previously and validated against electronic medical record data [32]. History of smoking is based on claims related to nicotine dependence and is, therefore, likely to be underreported. An adaptation of a published algorithm was used to derive lines of treatment [23], which may differ from actual clinical practice. The Optum database used in this study mainly includes patients who are commercially insured or covered by Medicare Advantage plans; elderly patients with Medicare fee-for-service or Medicaid plans may be underrepresented, potentially limiting generalizability. Healthcare costs in this study are based on standardized costs reported in the database, which may not reflect actual reimbursements. Furthermore, this study descriptively assessed outcomes among patients with metastatic NSCLC and by 1L treatments received. Future studies could control the impact of patient characteristics to examine the association between 1L treatments and HCRU and costs.
5. Conclusions
The adoption of ICIs in the 1L treatment of metastatic NSCLC without actionable alterations has been increasing, and it is in line with treatment guidelines. However, a substantial economic burden persists, particularly with widely used ICI + chemotherapy regimens. Chemotherapy-only regimens accounted for a quarter of 1L treatments but were associated with the shortest treatment duration and high HCRU burden. The 1L treatment of metastatic NSCLC continues to impose a notable HCRU burden on patients, caregivers, and healthcare systems, as well as an economic burden for payers, highlighting the need for better treatment options in this setting.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/curroncol32030151/s1, Supplementary Table S1. All-cause and NSCLC-related healthcare resource utilization during first-line treatment; Supplementary Table S2. All-cause and NSCLC-related healthcare costs during first-line treatment. Supplementary Table S3. Median all-cause healthcare costs ($, per patient per month) during first-line treatment.
Author Contributions
Conceptualization, D.C., I.S., D.M.W. and B.S.; methodology, D.C., I.S., D.M.W. and B.S.; investigation, D.C. and I.S.; Resources, D.C. and I.S.; writing—original draft preparation, D.C. and I.S.; writing—review and editing, D.C., I.S., D.M.W. and B.S.; supervision, D.C. and I.S.; project administration, D.C., I.S. and D.M.W. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study and its publication was provided by Amgen, Inc., Thousand Oaks, CA, USA.
Institutional Review Board Statement
Institutional review board or ethics committee approval was not required as this was a retrospective study involving secondary use of pre-existing deidentified claims data. Permission to access and utilize data was obtained through an agreement with Optum.
Informed Consent Statement
This is a retrospective observational study based on secondary use of existing deidentified data rather than one directly involving human subjects. Because identifying information cannot be linked back to the patients from whom the data were originally collected, the subsequent use of the data does not constitute human-subject research requiring informed consent. Hence, informed consent is not required.
Data Availability Statement
The datasets used in this study were obtained from a third-party source and are subject to data-sharing restrictions. Consequently, the datasets generated and analyzed during this study are confidential and cannot be shared by the authors or Amgen.
Acknowledgments
The authors thank Alexander Lonshteyn and Thomas E Delea of Avalere Health, Boston, MA, USA, for conducting the analysis and Perscribo Medical Communications Ltd., Basingstoke, UK, for medical writing support.
Conflicts of Interest
D.C., I.S. and B.S. are employees and stockholder of Amgen. D.M.W. has received consulting fees from Bristol Myers Squibb (BMS), Amgen, Janssen, Merck, Fresenius Kabi, and Novartis; honoraria from BMS, AstraZeneca, Amgen, Janssen, EMD Serono, Fresenius Kabi, and Sanofi; support to attend meetings and/or travel from BMS and Amgen; and has participated on a data safety monitoring board or advisory board for BMS, AstraZeneca, AbbVie, Amgen, Janssen, Eisai, EMD Serono, Merck, Pfizer, Fresenius Kabi, Sanofi, Astellas, Gilead, Takeda, Daiichi, and Bayer. Employees of the Funders (Amgen Inc.) were involved in all stages of the study and the decision to publish the results, as set out in the Author Contributions statement.
Abbreviations
The following abbreviations are used in this manuscript:
| 1L | first-line |
| 2L | second line |
| COPD | chronic obstructive pulmonary disease |
| ED | emergency department |
| HCRU | healthcare resource utilization |
| ICD-10-CM | The International Classification of Diseases, 10th Edition, Clinical Modification |
| ICI | immune checkpoint inhibitor |
| KRAS | Kirstin rat sarcoma virus |
| NCI-CCI | National Cancer Institute-adapted Charlson Comorbidity Index |
| NCCN | National Comprehensive Cancer Network |
| NSCLC | non-small cell lung cancer |
| PBCT | platinum-based chemotherapy |
| PPPM | per patient per month |
| SACT | systemic anticancer therapy |
| SD | standard deviation |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TTNT | time to the next treatment |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Lung Cancer. Available online: https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (accessed on 6 June 2024).
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Chouaid, C.; Giannopoulou, A.; Starry, A.; Stollenwerk, B.; Bozorgmehr, F. The impact of KRAS mutational status on patient-reported outcomes in advanced non-small-cell lung cancer: A cross sectional study in France and Germany. J. Med. Econ. 2025, 28, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.M.; Zheng, M.-M.; Pan, Y.; Liu, S.-Y.; Li, Y.; Wu, Y.-L. Emerging evidence and treatment paradigm of non-small cell lung cancer. J. Hematol. Oncol. 2023, 16, 40. [Google Scholar] [CrossRef]
- Friedlaender, A.; Perol, M.; Banna, G.L.; Parikh, K.; Addeo, A. Oncogenic alterations in advanced NSCLC: A molecular super-highway. Biomark Res. 2024, 12, 24. [Google Scholar] [CrossRef]
- Sathiyapalan, A.; Ellis, P.M. Molecular Testing in Non-Small-Cell Lung Cancer: A Call to Action. JCO Oncol. Pract. 2024, 20, 7–9. [Google Scholar] [CrossRef]
- de Langen, A.J.; Johnson, M.L.; Mazieres, J.; Dingemans, A.C.; Mountzios, G.; Pless, M.; Wolf, J.; Schuler, M.; Lena, H.; Skoulidis, F.; et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS (G12C) mutation: A randomised, open-label, phase 3 trial. Lancet 2023, 401, 733–746. [Google Scholar] [CrossRef]
- Waterhouse, D.M.; Rothschild, S.; Dooms, C.; Mennecier, B.; Bozorgmehr, F.; Majem, M.; van den Heuvel, M.H.; Linardou, H.; Chul Cho, B.; Roberts-Thomson, R.; et al. Patient-reported outcomes in CodeBreaK 200: Sotorasib versus docetaxel for previously treated advanced NSCLC with KRAS G12C mutation. Lung Cancer 2024, 196, 107921. [Google Scholar] [CrossRef]
- Kim, S.Y.; Halmos, B. Choosing the best first-line therapy: NSCLC with no actionable oncogenic driver. Lung Cancer Manag. 2020, 9, Lmt36. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology Non-Small Cell Lung Cancer Version 11.2024—15 October 2024; NCCN: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- DaCosta Byfield, S.; Chastek, B.; Korrer, S.; Horstman, T.; Malin, J.; Newcomer, L. Real-world outcomes and value of first-line therapy for metastatic non-small cell lung cancer. Cancer Investig. 2020, 38, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, A.; Li, H.; Bittoni, M.A.; Camacho, R.; Cao, X.; Zhong, Y.; Lubiniecki, G.M.; Carbone, D.P. Real-World Treatment Patterns, Overall Survival, and Occurrence and Costs of Adverse Events Associated with Second-Line Therapies for Medicare Patients with Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, e783–e799. [Google Scholar] [CrossRef]
- Nadler, E.; Espirito, J.L.; Pavilack, M.; Boyd, M.; Vergara-Silva, A.; Fernandes, A. Treatment Patterns and Clinical Outcomes Among Metastatic Non-Small-Cell Lung Cancer Patients Treated in the Community Practice Setting. Clin. Lung Cancer 2018, 19, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Allo, M.A.; Gu, L.; Vashistha, V.; Press, A.; Kelley, M. Health outcomes and healthcare resource utilization among Veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS ONE 2023, 18, e0282020. [Google Scholar] [CrossRef]
- Zhang, X.; Beachler, D.C.; Masters, E.; Liu, F.; Yang, M.; Dinh, J.; Jamal-Allial, A.; Kolitsopoulos, F.; Lamy, F.X. Health care resource utilization and costs associated with advanced or metastatic nonsmall cell lung cancer in the United States. J. Manag. Care Spec. Pharm. 2022, 28, 255–265. [Google Scholar] [CrossRef]
- Simmons, D.; Welch, E.; Pyrih, N.; Jiang, Z.; Xiao, Y.; Jassim, R. EE270 The Economic Burden of Metastatic Non-Small Cell Lung Cancer in US Patients without an EGFR or ALK Mutation. Value Health 2023, 26, S108. [Google Scholar] [CrossRef]
- Vanderpoel, J.; Emond, B.; Ghelerter, I.; Milbers, K.; Lafeuille, M.H.; Lefebvre, P.; Ellis, L.A. Healthcare Resource Utilization and Costs in Patients with EGFR-Mutated Advanced Non-Small Cell Lung Cancer Receiving First-Line Treatment in the United States: An Insurance Claims-Based Descriptive Analysis. PharmacoEcon. Open 2023, 7, 617–626. [Google Scholar] [CrossRef]
- Chopra, D.; Waterhouse, D.; Sultan, I.; Lonshteyn, A.; Weycker, D.; Delea, T. Real-world treatment patterns, health care costs and health care utilization in US patients with non–small cell lung cancer receiving sotorasib. J. Manag. Care Spec. Pharm. 2023, 29, S16. [Google Scholar]
- Meng, W.; Ou, W.; Chandwani, S.; Chen, X.; Black, W.; Cai, Z. Temporal phenotyping by mining healthcare data to derive lines of therapy for cancer. J. Biomed. Inform. 2019, 100, 103335. [Google Scholar] [CrossRef]
- Klabunde, C.N.; Legler, J.M.; Warren, J.L.; Baldwin, L.M.; Schrag, D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann. Epidemiol. 2007, 17, 584–590. [Google Scholar] [CrossRef]
- US Bureau of Labor Statistics. CPI for All Urban Consumers (CPI-U), Medical Care in U.S. City Average, All Urban Consumers, Not Seasonally Adjusted; Series ID: CUUR0000SAM; US Bureau of Labor Statistics: Washington, DC, USA, 2023.
- Kish, J.; Liassou, D.; Hartman, J.; Lubinga, S.J.; Chopra, D.; Feinberg, B. Better together? costs of first-line chemoimmunotherapy for advanced non-small cell lung cancer. Am. J. Manag. Care 2023, 29, e129–e135. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.; Knoll, S.; Smith, T.; Scotchmer, A.; Bauer, M. Costs of first-line systemic therapy (1LT) for locally advanced or metastatic non-small cell lung cancer (a/mNSCLC)—A secondary analysis of claims data from the United States (US) (abstract). Value Health 2023, 26, S67. [Google Scholar] [CrossRef]
- Pham, T.T.; Gordon, A.S.; Chen, X.; Debono, D.; Fisch, M.J. Immunotherapy in combination with chemotherapy vs. immunotherapy alone for advanced non-small cell lung cancer and programmed death ligand 1 score < 50. Cancer Treat. Res. Commun. 2023, 37, 100769. [Google Scholar] [CrossRef]
- Gines Rubio, J.; Delgado, O.; Callejo, A.; Dominguez, M.; Torres, C. Healthcare Resource Utilization and Associated Costs among Patients with Advanced Non-Small-Cell Lung Cancer Receiving Chemotherapy or Immunotherapy in Spain: A Single-Center, Real-World, Exploratory Study. Cancers 2024, 16, 2068. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Turner, R.M.; Chen, Y.W.; Fernandes, A.W. Validation of a Case-Finding Algorithm for Identifying Patients with Non-small Cell Lung Cancer (NSCLC) in Administrative Claims Databases. Front. Pharmacol. 2017, 8, 883. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).