First-Line Pyrotinib Combination Therapy for HER2-Mutated Advanced NSCLC: A Retrospective Cohort Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Method
2.3. Response Assessment
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Molecular Characteristics
3.3. Efficacy Analysis
3.4. Subgroup Analysis
3.5. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mina, S.A.; Shanshal, M.; Leventakos, K.; Parikh, K. Emerging Targeted Therapies in Non-Small-Cell Lung Cancer (NSCLC). Cancers 2025, 17, 353. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D'Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Guo, H.; Ma, J.; Lai, J.; Liu, L.; Yin, K.; Zhao, J.; Wang, J.; Jiang, F.; Xu, W.; et al. Clinical characteristics and prognostic factors of patients with non-small cell lung cancer having HER2 alterations. J. Cancer Res. Clin. Oncol. 2023, 149, 2029–2039. [Google Scholar] [CrossRef]
- Uy, N.F.; Merkhofer, C.M.; Baik, C.S. HER2 in Non-Small Cell Lung Cancer: A Review of Emerging Therapies. Cancers 2022, 14, 4155. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, B.; Choi, Y.L.; Kang, D.W.; Han, J. Clinicopathologic and Molecular Characteristics of HER2 (ERBB2)-Altered Non-Small Cell Lung Cancer: Implications for Precision Medicine. Mod. Pathol. 2024, 37, 100490. [Google Scholar] [CrossRef]
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients with Oncogenic Driver Molecular Alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef]
- Kris, M.G.; Camidge, D.R.; Giaccone, G.; Hida, T.; Li, B.T.; O’Connell, J.; Taylor, I.; Zhang, H.; Arcila, M.E.; Goldberg, Z.; et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann. Oncol. 2015, 26, 1421–1427. [Google Scholar] [CrossRef]
- Gatzemeier, U.; Groth, G.; Butts, C.; Van Zandwijk, N.; Shepherd, F.; Ardizzoni, A.; Barton, C.; Ghahramani, P.; Hirsh, V. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann. Oncol. 2004, 15, 19–27. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Zhou, C.; Wang, H.; Shu, Y.; Zhang, J.; Hua, H.; Huang, D.C.; Zhou, C. Afatinib in patients with advanced non-small cell lung cancer harboring HER2 mutations, previously treated with chemotherapy: A phase II trial. Lung Cancer 2020, 147, 209–213. [Google Scholar] [CrossRef]
- Dziadziuszko, R.; Smit, E.F.; Dafni, U.; Wolf, J.; Wasąg, B.; Biernat, W.; Finn, S.P.; Kammler, R.; Tsourti, Z.; Rabaglio, M.; et al. Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP). J. Thorac. Oncol. 2019, 14, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, X.; Wang, Q.; Gao, G.; Zhang, Y.; Chen, J.; Shu, Y.; Hu, Y.; Fan, Y.; Fang, J.; et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2020, 38, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Cornelissen, R.; Garassino, M.; Clarke, J.M.; Tchekmedyian, N.; Goldman, J.W.; Leu, S.Y.; Bhat, G.; Lebel, F.; Heymach, J.V.; et al. Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial. J. Clin. Oncol. 2022, 40, 710–718. [Google Scholar] [CrossRef]
- Liu, S.M.; Tu, H.Y.; Wei, X.W.; Yan, H.H.; Dong, X.R.; Cui, J.W.; Zhou, Z.; Xu, C.R.; Zheng, M.Y.; Li, Y.S.; et al. First-line pyrotinib in advanced HER2-mutant non-small-cell lung cancer: A patient-centric phase 2 trial. Nat. Med. 2023, 29, 2079–2086. [Google Scholar] [CrossRef]
- Yang, G.; Yang, Y.; Liu, R.; Li, W.; Xu, H.; Hao, X.; Li, J.; Xing, P.; Zhang, S.; Ai, X.; et al. First-line immunotherapy or angiogenesis inhibitor plus chemotherapy for HER2-altered NSCLC: A retrospective real-world POLISH study. Ther. Adv. Med. Oncol. 2022, 14, 17588359221082339. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Zehir, A.; Yaeger, R.; Wang, L.; Middha, S.; Zheng, T.; Hyman, D.M.; Solit, D.; Arcila, M.E.; Borsu, L.; et al. Identification of Targetable Kinase Alterations in Patients with Colorectal Carcinoma That are Preferentially Associated with Wild-Type RAS/RAF. Mol. Cancer Res. 2016, 14, 296–301. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Wilding, B.; Woelflingseder, L.; Baum, A.; Chylinski, K.; Vainorius, G.; Gibson, N.; Waizenegger, I.C.; Gerlach, D.; Augsten, M.; Spreitzer, F.; et al. Zongertinib (BI 1810631), an Irreversible HER2 TKI, Spares EGFR Signaling and Improves Therapeutic Response in Preclinical Models and Patients with HER2-Driven Cancers. Cancer Discov. 2025, 15, 119–138. [Google Scholar] [CrossRef]
- Wu, Y.; Opdam, F.; Barve, M.; Tu, H.; Berz, D.; Rohrbacher, M.; Sadrolhefazi, B.; Serra, J.; Yoh, K.; Yamamoto, N.J.E.O. 34P Updated data from Beamion LUNG-1, a phase (ph) Ia/b trial of the HER2-specific tyrosine kinase inhibitor (TKI), zongertinib (BI 1810631), in patients (pts) with HER2 mutation-positive (m+) NSCLC. ESMO Open 2024, 9, 3. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Zhao, C.; Li, X.; Liu, Q.; Mao, S.; Liu, Y.; Yu, X.; Wang, W.; Tian, Q.; et al. Exon 20 YVMA insertion is associated with high incidence of brain metastasis and inferior outcome of chemotherapy in advanced non-small cell lung cancer patients with HER2 kinase domain mutations. Transl. Lung Cancer Res. 2021, 10, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhao, S.; Liang, Y.; Yang, Y.; Yang, L.; Dong, X.; Zhang, L.; Tang, Y.; Wang, S.; Yang, Y.; et al. Mutation Variants and Co-Mutations as Genomic Modifiers of Response to Afatinib in HER2-Mutant Lung Adenocarcinoma. Oncologist 2020, 25, e545–e554. [Google Scholar] [CrossRef] [PubMed]

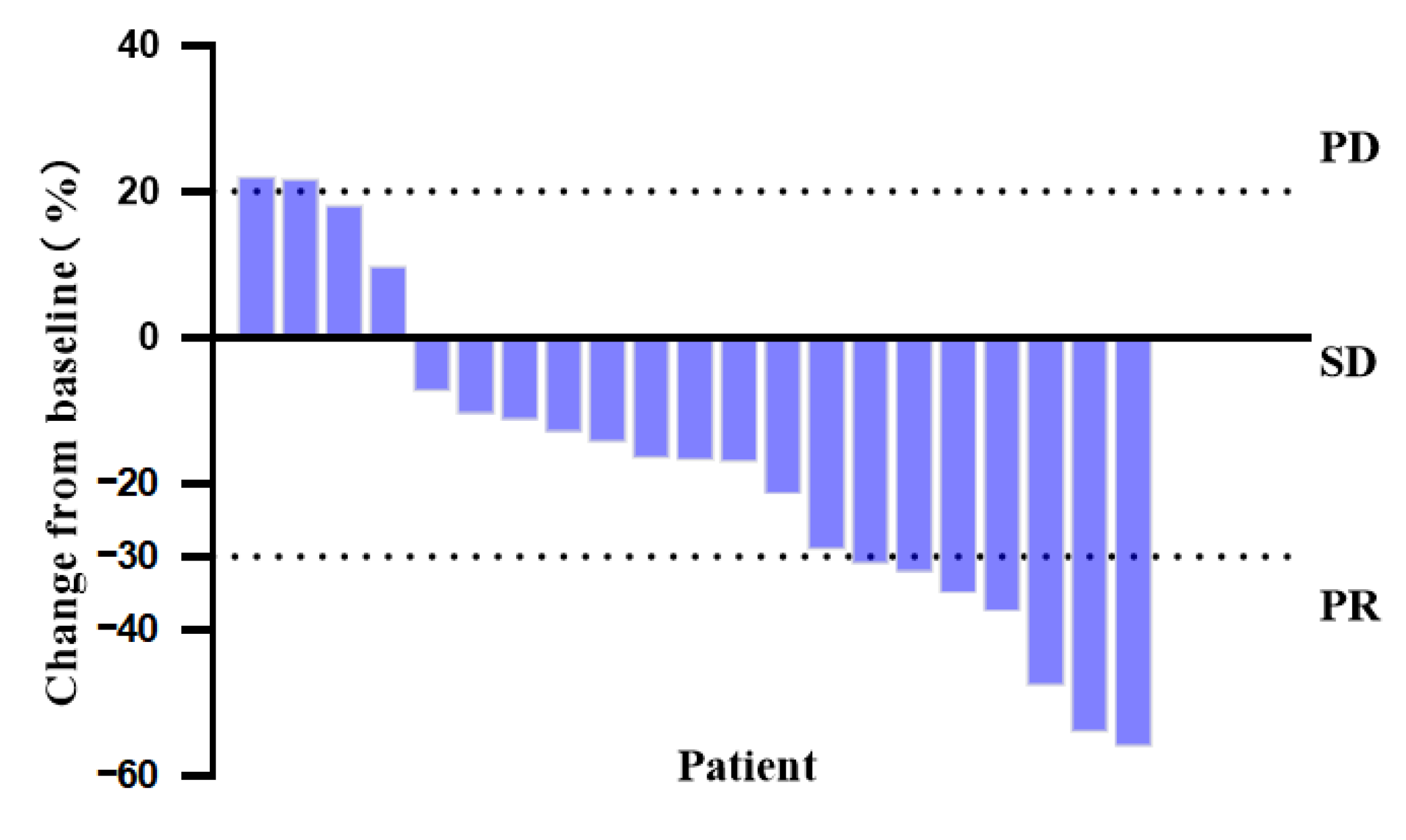
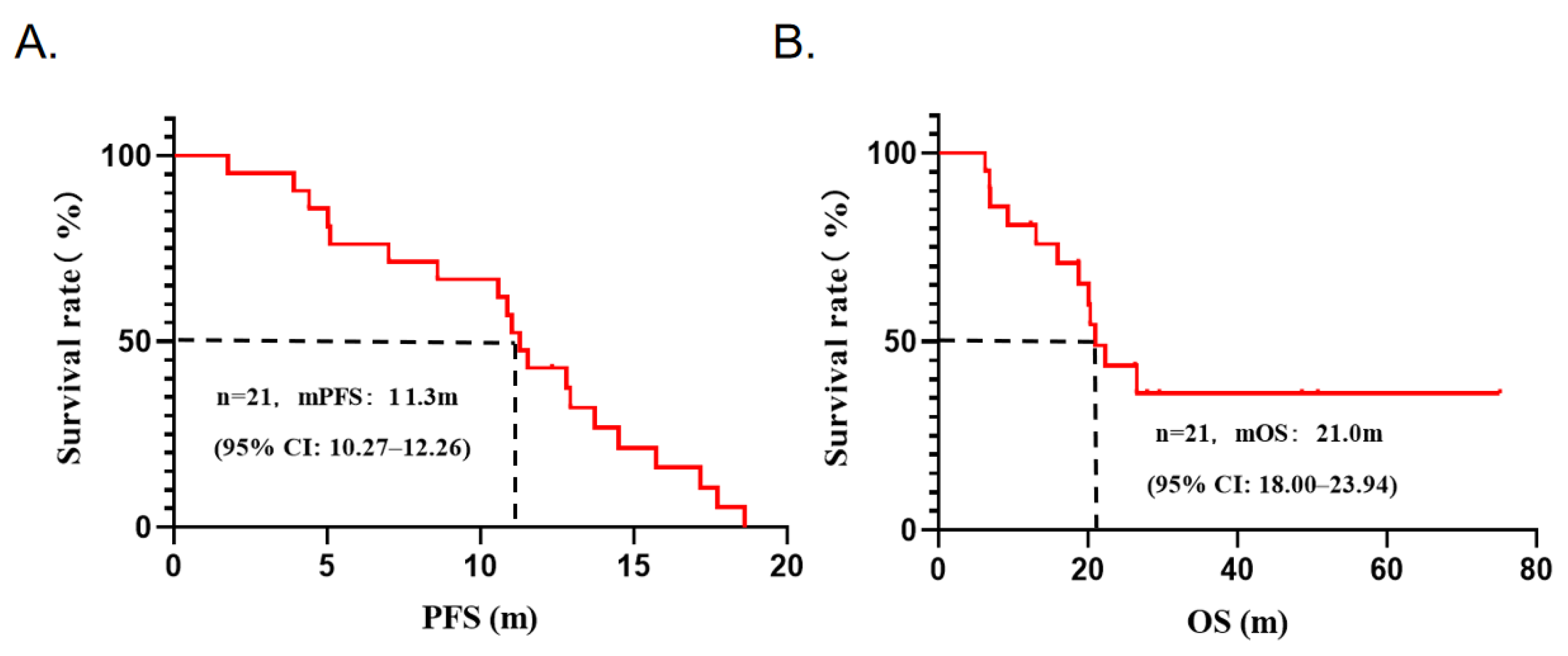
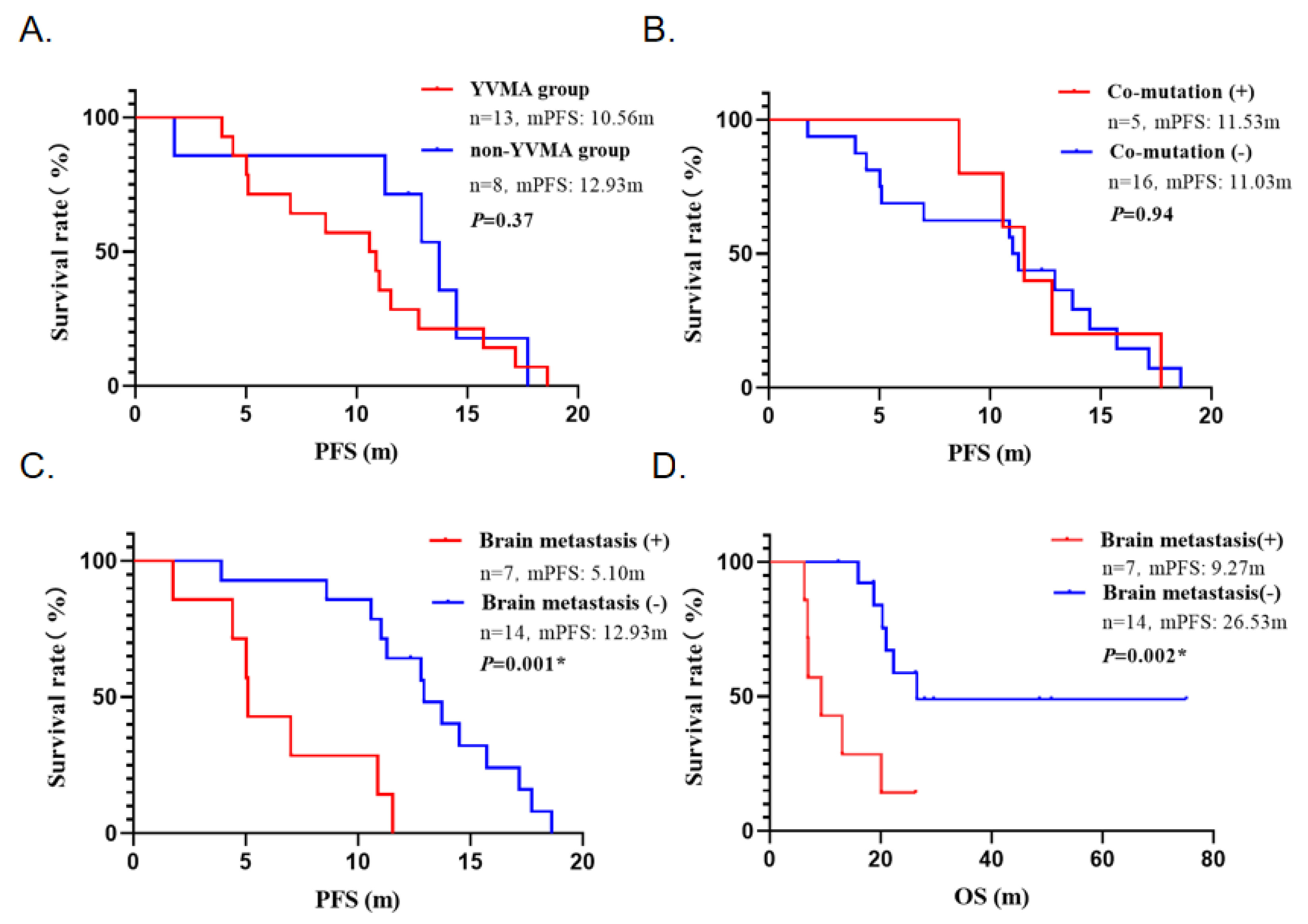
| Characteristics | All Patients | Pyrotinib Plus Chemotherapy |
|---|---|---|
| Total | 144 | 21 (43.8%) |
| Gender | ||
| Female | 87 (60.4%) | 15 (71.4%) |
| Male | 57 (39.6%) | 6 (28.6%) |
| Age | 62.0 ± 10.0 | 66.3 ± 9.4 |
| Smoking history | ||
| No | 106 (73.6%) | 12 (57.1%) |
| Yes | 38 (26.4%) | 9 (42.9%) |
| Physiology | ||
| Adenocarcinoma | 140 (97.2%) | 21 (100.0%) |
| Squamous cell carcinoma | 3 (2.1%) | 0 (0.0%) |
| Adenosquamous carcinoma | 1 (0.7%) | 0 (0.0%) |
| TNM Stage | ||
| I–II | 34 (23.6%) | 0 (0.0%) |
| III–IV | 110 (76.4%) | 21 (100.0%) |
| ECOG score | ||
| 0–1 | 95 (81.9%) | 17 (81.0%) |
| ≥2 | 21 (18.1%) | 4 (19.0%) |
| Brain metastasis | ||
| No | 120 (83.3%) | 14 (66.7%) |
| Yes | 24 (16.7%) | 7 (33.3%) |
| HER2 exon20 Insertion Subtypes | Number | Proportion |
|---|---|---|
| p.A775_G776insYVMA | 69 | 47.90% |
| p. G776delinsVC | 10 | 6.90% |
| p.P780_Y781insGSP | 7 | 4.90% |
| p. G776delinsLC | 3 | 2.10% |
| p. G776delinsVV | 2 | 1.40% |
| p.G778_S779insCPG | 2 | 1.40% |
| p.G776_V777delinsCVC | 1 | 0.70% |
| p.G776_V777delinsAVCG | 1 | 0.70% |
| p.V777_G778insCG | 1 | 0.70% |
| p.M774delinsVWL | 1 | 0.70% |
| Total | 97 | 67.4% |
| Case | Gender/ Age | Brain Metastases | HER2 Mutation Subtype | Co-Current Genes | First-Line Treatment | Efficacy | PFS/Months | OS/Months |
|---|---|---|---|---|---|---|---|---|
| 1 | M/76 | Yes | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Pemetrexed/Carboplatin | SD | 5.03 | 6.83 |
| 2 | M/82 | No | Exon8 p.S310F | / | Pyrotinib/Pemetrexed/Carboplatin | PR | 14.50 | 50.73+ |
| 3 | M/74 | No | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Pemetrexed/Carboplatin | SD | 17.17 | 27.87+ |
| 4 | F/83 | No | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Pemetrexed/Carboplatin | SD | 3.90 | 15.93 |
| 5 | F/73 | No | Exon20 p.A775_G776insYVMA | TP53 | Pyrotinib/Pemetrexed/Carboplatin | SD | 10.57 | 20.30 |
| 6 | F/65 | Yes | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Nab-paclitaxel/Carboplatin | SD | 10.87 | 26.27+ |
| 7 | F/67 | Yes | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Pemetrexed/Carboplatin | PR | 5.10 | 6.23 |
| 8 | M/56 | No | Exon 20 p.S783A | / | Pyrotinib/Pemetrexed/Carboplatin | SD | 13.73 | 18.77 |
| 9 | M/60 | Yes | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Pemetrexed/Carboplatin | PR | 7.00 | 13.03 |
| 10 | F/67 | No | Exon20 p.G776delinsVC | / | Pyrotinib/Pemetrexed/Carboplatin | SD | 11.27 | 26.53 |
| 11 | F/67 | No | Exon20 p.A775_G776insYVMA | TP53 | Pyrotinib/Pemetrexed/Carboplatin | SD | 12.80 | 75.10+ |
| 12 | F/67 | No | Exon20 p.A775_G776insYVMA | TP53 | Pyrotinib/Pemetrexed/Carboplatin | SD | 8.60 | 22.30 |
| 13 | F/47 | No | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Pemetrexed/Carboplatin/Bevacizumab | SD | 18.60 | 18.67+ |
| 14 | F/64 | No | Exon20 p.G778_S779insCPG | MDM2 | Pyrotinib/Pemetrexed/Carboplatin/Bevacizumab | PR | 17.73 | 48.60+ |
| 15 | F/63 | Yes | Exon20 p.A775_G776insYVMA | TP53 | Pyrotinib/Pemetrexed/Carboplatin/Bevacizumab | PR | 11.53 | 20.13 |
| 16 | F/59 | Yes | Exon19 p.L755S | / | Pyrotinib/Pemetrexed/Carboplatin/Bevacizumab | PD | 1.77 | 9.27 |
| 17 | F/58 | No | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Paclitaxel liposome/ Carboplatin/Bevacizumab | SD | 11.03 | 20.97 |
| 18 | F/50 | No | Exon19 p.L755A | / | Pyrotinib/Pemetrexed/Carboplatin/Bevacizumab | PR | 12.33+ | 12.33+ |
| 19 | F/76 | Yes | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Pemetrexed/Carboplatin/Bevacizumab | SD | 4.40 | 6.93 |
| 20 | F/71 | No | Exon17 p.I655V | / | Pyrotinib/Pemetrexed/Carboplatin/Bevacizumab | SD | 12.93 | 29.53+ |
| 21 | M/67 | No | Exon20 p.A775_G776insYVMA | / | Pyrotinib/Pemetrexed/Carboplatin | PR | 15.73 | 26.27+ |
| Adverse Events | Any Grade | Grade 1–2 (%) | Grade 3 (%) |
|---|---|---|---|
| Any events | 19 (90.5%) | ||
| Diarrhea | 18 (85.7%) | 16 (76.2%) | 2 (9.5%) |
| Myelosuppression | 11 (52.4%) | 10 (47.6%) | 1 (4.8%) |
| Nausea | 10 (47.6%) | 10 (47.6%) | 0 (0.0%) |
| Fatigue | 9 (42.9%) | 9 (42.9%) | 0 (0.0%) |
| Decreased appetite | 7 (33.3%) | 7 (33.3%) | 0 (0.0%) |
| Elevated transaminase | 6 (28.6%) | 6 (28.6%) | 0 (0.0%) |
| Electrolyte imbalance | 3 (14.3%) | 3 (14.3%) | 0 (0.0%) |
| Hand-foot syndrome | 2 (9.5%) | 2 (9.5%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Zhang, M.; Wang, Q.; Liu, J.; Zeng, L.; Sun, A.; Lu, K. First-Line Pyrotinib Combination Therapy for HER2-Mutated Advanced NSCLC: A Retrospective Cohort Analysis. Curr. Oncol. 2025, 32, 148. https://doi.org/10.3390/curroncol32030148
Xiang Y, Zhang M, Wang Q, Liu J, Zeng L, Sun A, Lu K. First-Line Pyrotinib Combination Therapy for HER2-Mutated Advanced NSCLC: A Retrospective Cohort Analysis. Current Oncology. 2025; 32(3):148. https://doi.org/10.3390/curroncol32030148
Chicago/Turabian StyleXiang, Yan, Meiling Zhang, Qian Wang, Jingwen Liu, Lulin Zeng, Ao Sun, and Kaihua Lu. 2025. "First-Line Pyrotinib Combination Therapy for HER2-Mutated Advanced NSCLC: A Retrospective Cohort Analysis" Current Oncology 32, no. 3: 148. https://doi.org/10.3390/curroncol32030148
APA StyleXiang, Y., Zhang, M., Wang, Q., Liu, J., Zeng, L., Sun, A., & Lu, K. (2025). First-Line Pyrotinib Combination Therapy for HER2-Mutated Advanced NSCLC: A Retrospective Cohort Analysis. Current Oncology, 32(3), 148. https://doi.org/10.3390/curroncol32030148






