Safety and Efficacy in the Transcortical and Transsylvian Approach in Insular High-Grade Gliomas: A Comparative Series of 58 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Tumor MR Analysis
2.3. Surgical Technique
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Surgical Resection
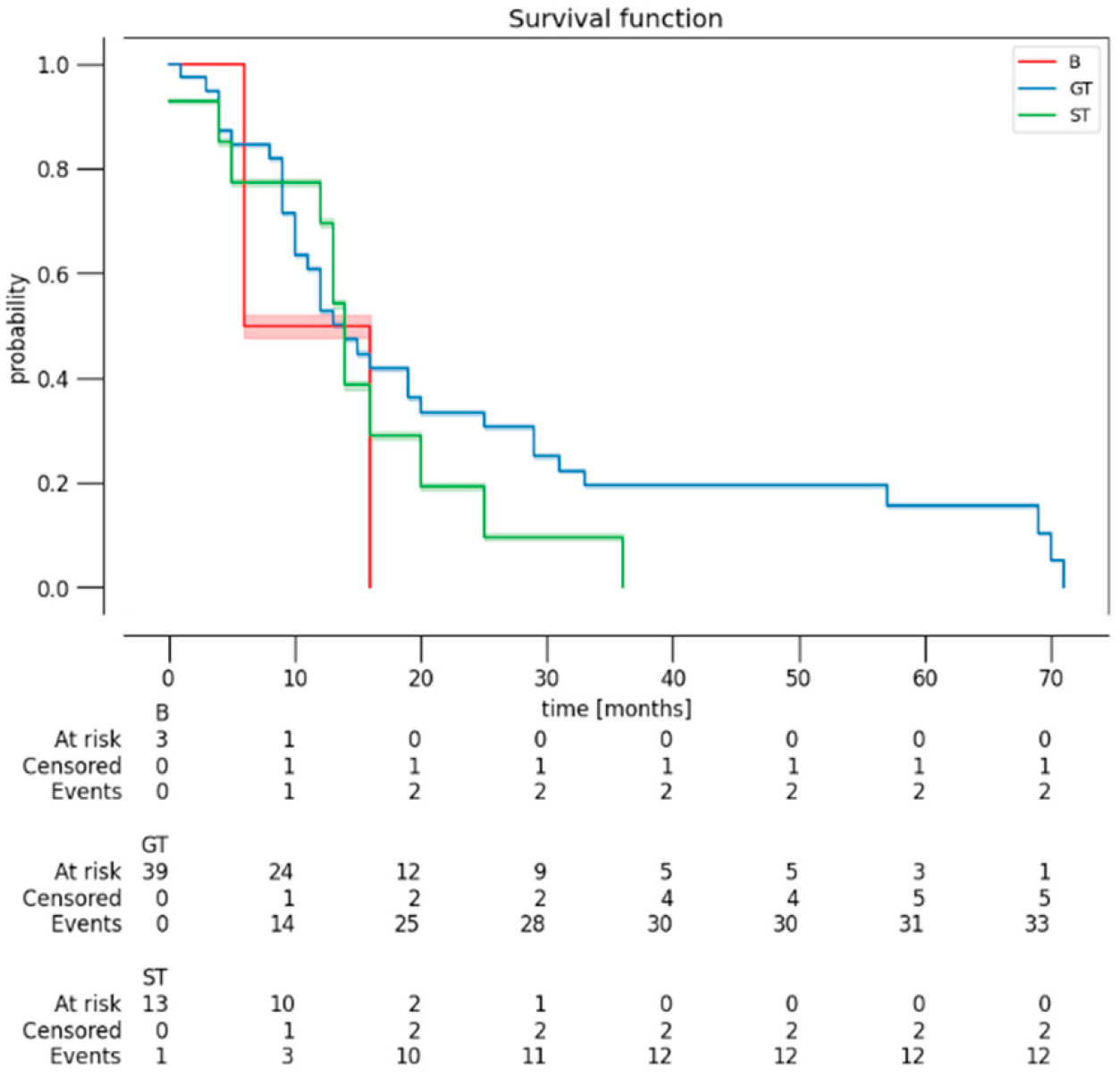
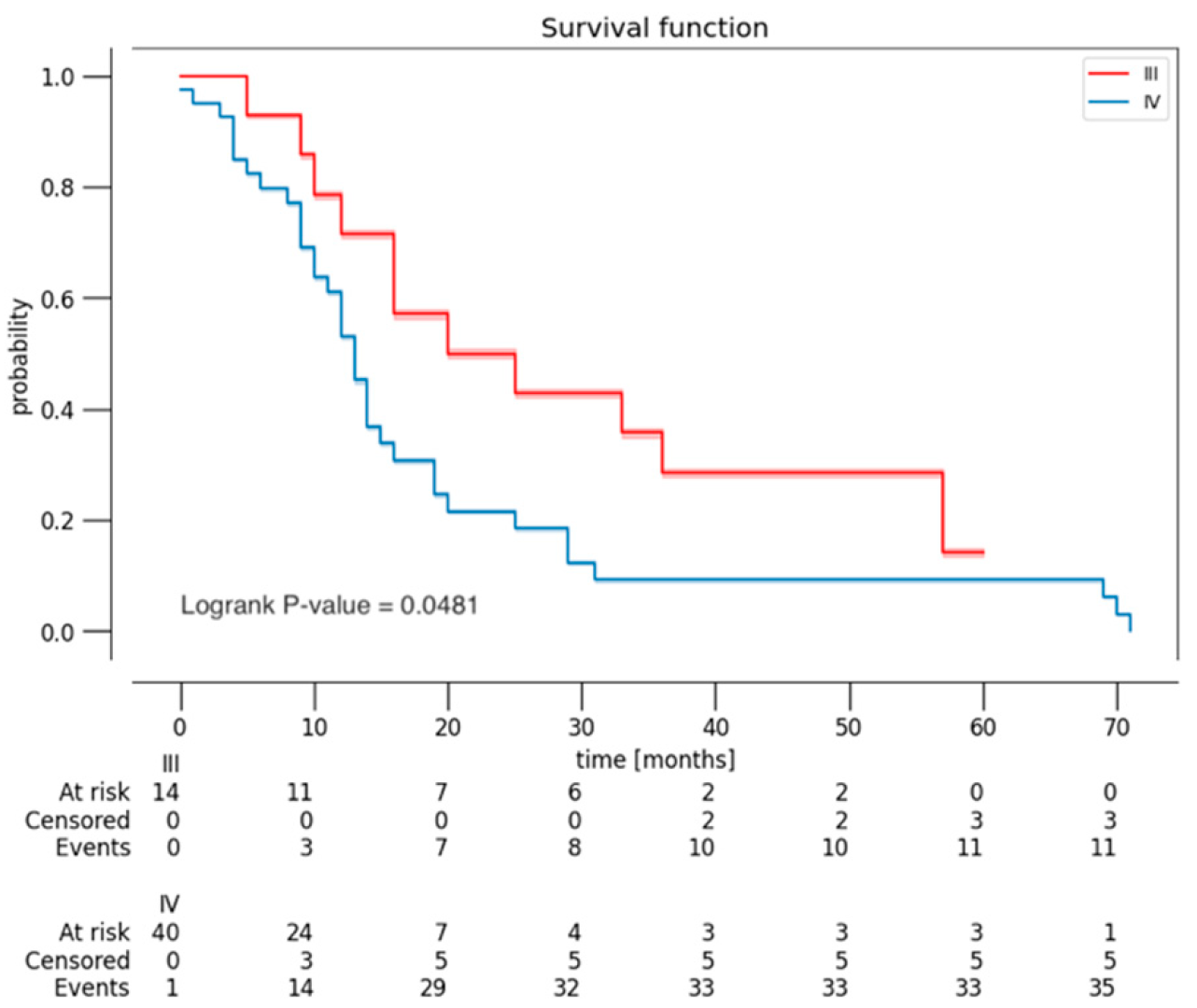
3.3. Follow-Up and Outcome
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KPS = Karnofsky performance status |
| PFS = progression-free survival |
| OS = overall survival |
| EOR = extent of resection |
| GTR = gross total resection |
| YC = Yaşargil classification |
| BSC = Berger–Sanai classification |
References
- Bellomo, J.; Spinello, A.; Morello, A.; Schubert, T.; Fierstra, J.; Piazza, A.; Serra, C. Human brain vasculature. In Encyclopedia of the Human Brain; Elsevier: Amsterdam, The Netherlands, 2025; pp. 62–87. [Google Scholar]
- Türe, U.; Yaşargil, D.C.; Al-Mefty, O.; Yaşargil, M.G. Topographic anatomy of the insular region. J. Neurosurg. 1999, 90, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Safaee, M.M.; Englot, D.J.; Han, S.J.; Lawton, M.T.; Berger, M.S. The transsylvian approach for resection of insular gliomas: Technical nuances of splitting the Sylvian fissure. J. Neurooncol. 2016, 130, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Przybylowski, C.J.; Hervey-Jumper, S.L.; Sanai, N. Surgical strategy for insular glioma. J. Neurooncol. 2021, 151, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Hervey-Jumper, S.L.; Li, J.; Osorio, J.A.; Lau, D.; Molinaro, A.M.; Benet, A.; Berger, M.S. Surgical assessment of the insula. Part 2: Validation of the Berger-Sanai zone classification system for predicting extent of glioma resection. J. Neurosurg. 2016, 124, 482–488. [Google Scholar] [CrossRef]
- Yaşargil, M.G.; von Ammon, K.; Cavazos, E.; Doczi, T.; Reeves, J.D.; Roth, P. Tumours of the limbic and paralimbic systems. Acta Neurochir. 1992, 118, 40–52. [Google Scholar] [CrossRef]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro Oncol. 2023, 25, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.; Mato, D.; De Lucas, E.M.; García-Porrero, J.A.; Gabarrós, A.; Fernández-Coello, A.; Vázquez-Barquero, A. Subcortical anatomy as an anatomical and functional landmark in insulo-opercular gliomas: Implications for surgical approach to the insular region. J. Neurosurg. 2015, 123, 1081–1092. [Google Scholar] [CrossRef]
- Duffau, H.; Capelle, L. Preferential brain locations of low-grade gliomas. Cancer 2004, 100, 2622–2626. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.Y.; Berger, M.S. Insular glioma resection: Assessment of patient morbidity, survival, and tumor progression—Clinical article. J. Neurosurg. 2010, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Morello, A.; Bianconi, A.; Rizzo, F.; Bellomo, J.; Meyer, A.C.; Garbossa, D.; Regli, L.; Cofano, F. Laser Interstitial Thermotherapy (LITT) in Recurrent Glioblastoma: What Window of Opportunity for This Treatment? Technol. Cancer Res. Treat. 2024, 23, 15330338241249026. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.; Das, K.; Gosal, J.; Attri, G.; Singh, A.; Bhaisora, K.; Mehrotra, A.; Sardhara, J.; Verma, P.; Srivastava, A.; et al. Surgery in high-grade insular tumors: Oncological and seizure outcomes from 41 consecutive patients. Asian J. Neurosurg. 2020, 15, 537–544. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Insular glioma surgery: An evolution of thought and practice. J. Neurosurg. 2019, 130, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Penfield, W.; Faulk, M.E. The insula: Further observations on its function. Brain 1955, 78, 445–470. [Google Scholar] [CrossRef]
- Zarino, B.; Sirtori, M.A.; Meschini, T.; Bertani, G.A.; Caroli, M.; Bana, C.; Borellini, L.; Locatelli, M.; Carrabba, G. Insular lobe surgery and cognitive impairment in gliomas operated with intraoperative neurophysiological monitoring. Acta Neurochir. 2021, 163, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, G.; Al-Saffar, Y.; Brigui, M.; Martin, H.; Bystam, J.; Benmakhlouf, H.; Shamikh, A.; Dodoo, E. Gamma knife radiosurgery in the management of endolymphatic sac tumors. Surg. Neurol. Int. 2018, 9, 18. [Google Scholar] [CrossRef]
- Hameed, N.U.F.; Qiu, T.; Zhuang, D.; Lu, J.; Yu, Z.; Wu, S.; Wu, B.; Zhu, F.; Song, Y.; Chen, H.; et al. Transcortical insular glioma resection: Clinical outcome and predictors. J. Neurosurg. 2019, 131, 706–716. [Google Scholar] [CrossRef]
- Isolan, G.R.; Buffon, V.; Maldonado, I.; Monteiro, J.M.; Yağmurlu, K.; Ribas, C.A.P.M.; Roesler, R.; Malafaia, O. Avoiding vascular complications in insular glioma surgery—A microsurgical anatomy study and critical reflections regarding intraoperative findings. Front. Surg. 2022, 9, 906466. [Google Scholar] [CrossRef] [PubMed]
- Yaşargil, M.G.; Krisht, A.F.; Türe, U.; Al-Mefty, O.; Yaşargil, D.C.H. Microsurgery of Insular Gliomas: Part IV—Surgical Treatment and Outcome. Contemp. Neurosurg. 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Kumar, N.S. Prognostic Factors and Resectability Predictors in Insular Gliomas: A Systematic Review. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2024, 85, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Mandonnet, E.; Capelle, L.; Duffau, H. Extension of paralimbic low grade gliomas: Toward an anatomical classification based on white matter invasion patterns. J. Neurooncol. 2006, 78, 179–185. [Google Scholar] [CrossRef]
- Das, K.K.; Gosal, J.S.; Khatri, D.; Singh, A.; Datta, A.; Kumar, A.; Bhaisora, K.; Verma, P.K.; Srivastava, A.K.; Jaiswal, A.K.; et al. Balancing the Extent of Resection and Ischemic Complications in Insular Glioma Surgery: Technical Nuances and Proposal of a Novel Composite Postoperative Outcome Index. Neurol. India 2022, 70, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Renfrow, J.J.; Julian, B.Q.; Brown, D.A.; Tatter, S.B.; Laxton, A.W.; Lesser, G.J.; Strowd, R.E.; Parney, I.F. A Review on the Surgical Management of Insular Gliomas. Can. J. Neurol. Sci. 2023, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Arumulla, S.; Manjunath, N.; Meena, R.; Doddamani, R.; Singh, P.; Chandra, S. Trans-Sylvian Resection of Giant Left Insular Glioma: Operative Technique and Nuances. Neurol. India 2021, 69, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Dezamis, E.; Trancart, B.; Pallud, J. How I do it: Trans-cortical approach for insular diffuse glioma. Acta Neurochir. 2020, 162, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Specchia, F.M.C.; Monticelli, M.; Zeppa, P.; Bianconi, A.; Zenga, F.; Altieri, R.; Pugliese, B.; Di Perna, G.; Cofano, F.; Tartara, F.; et al. Let Me See: Correlation between 5-ALA Fluorescence and Molecular Pathways in Glioblastoma: A Single Center Experience. Brain Sci. 2021, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, A.; La Cava, P.; Bonada, M.; Zeppa, P.; Melcarne, A.; Cofano, F.; Fiaschi, P.; Garbossa, D.; Bianconi, A. Combined Fluorescence-Guided Surgery with 5-Aminolevulinic Acid and Fluorescein in Glioblastoma: Technical Description and Report of 100 Cases. Cancers 2024, 16, 2771. [Google Scholar] [CrossRef]
- Dziedzic, T.A.; Bala, A.; Marchel, A. Anatomical aspects of the insula, opercula and peri-insular white matter for a transcortical approach to insular glioma resection. Neurosurg. Rev. 2022, 45, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Awake Surgery for Left Posterior Insular Low-Grade Glioma Through the Parietorolandic Operculum: The Need to Preserve the Functional Connectivity. A Case Series. Front. Surg. 2022, 8, 824003. [Google Scholar] [CrossRef]
- Benet, A.; Hervey-Jumper, S.L.; González Sánchez, J.J.; Lawton, M.T.; Berger, M.S. Surgical assessment of the insula. Part 1: Surgical anatomy and morphometric analysis of the transsylvian and transcortical approaches to the insula. J. Neurosurg. 2016, 124, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.T.; Cagnazzo, F.; Anania, Y.; Duffau, H.; Benedetto, N.; Morganti, R.; Perrini, P. Post-operative morbidity ensuing surgery for insular gliomas: A systematic review and meta-analysis. Neurosurg. Rev. 2020, 43, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sano, H.; Imizu, S.; Yoneda, M.; Viral, M.; Nagata, J.; Kanno, T. Surgical Strategies for Treatment of Giant or Large Intracranial Aneurysms: Our Experience with 139 Cases. min-Minim. Invasive Neurosurg. 2003, 46, 339–343. [Google Scholar]
- Wang, Y.; Wang, Y.; Fan, X.; Li, S.; Liu, X.; Wang, J.; Jiang, T. Putamen involvement and survival outcomes in patients with insular low-grade gliomas. J. Neurosurg. 2017, 126, 1788–1794. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, C.Y. Current Surgical Management of Insular Gliomas. Neurosurg. Clin. North Am. 2012, 23, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Das, K.K.; Khatri, D.; Singh, S.; Gosal, J.S.; Jaiswal, S.; Mishra, P.; Mehrotra, A.; Bhaisora, K.; Sardhara, J.; et al. Insular glioblastoma: Surgical challenges, survival outcomes and prognostic factors. Br. J. Neurosurg. 2023, 37, 26–34. [Google Scholar] [CrossRef]
- Cofano, F.; Bianconi, A.; De Marco, R.; Consoli, E.; Zeppa, P.; Bruno, F.; Pellerino, A.; Panico, F.; Salvati, L.F.; Rizzo, F.; et al. The Impact of Lateral Ventricular Opening in the Resection of Newly Diagnosed High-Grade Gliomas: A Single Center Experience. Cancers 2024, 16, 1574. [Google Scholar] [CrossRef] [PubMed]
- Ülgen, E.; Aras, F.K.; Coşgun, E.; Erşen-Danyeli, A.; Dinçer, A.; Usseli, M.I.; Özduman, K.; Pamir, M.N. Correlation of anatomical involvement patterns of insular gliomas with subnetworks of the limbic system. J. Neurosurg. 2021, 136, 323–334. [Google Scholar] [CrossRef]
- Pallud, J.; Zanello, M.; Moiraghi, A.; Peeters, S.; Trancart, B.; Edjlali, M.; Oppenheim, C.; Varlet, P.; Chrétien, F.; Dhermain, F.; et al. Surgery of Insular Diffuse Gliomas—Part 1: Transcortical Awake Resection Is Safe and Independently Improves Overall Survival. Neurosurgery 2021, 89, 565–578. [Google Scholar] [CrossRef]
- Rossi, M.; Gay, L.; Conti Nibali, M.; Sciortino, T.; Ambrogi, F.; Leonetti, A.; Puglisi, G.; Howells, H.; Zito, P.; Villa, F.; et al. Challenging Giant Insular Gliomas With Brain Mapping: Evaluation of Neurosurgical, Neurological, Neuropsychological, and Quality of Life Results in a Large Mono-Institutional Series. Front. Oncol. 2021, 11, 629166. [Google Scholar] [CrossRef] [PubMed]
- Pathiyil, R.K.; Moiyadi, A.V.; Shetty, P.; Singh, V.; Velayutham, P. Transopercular Approach to Resection of Dominant Hemisphere Diffuse Insular Glioma Using Multimodal Intraoperative Strategy with Awake Mapping. Neurol. India 2022, 70, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. A personal consecutive series of surgically treated 51 cases of insular WHO Grade II glioma: Advances and limitations—Clinical article. J. Neurosurg. 2009, 110, 696–708. [Google Scholar] [CrossRef] [PubMed]

| Parameter | No. (%) |
|---|---|
| Age at diagnosis (yrs) Median Range | 57.4 24–80 |
| Sex M F | 34 (58.6) 24 (41.4) |
| KPS score (median) At diagnosis Postop At 1-month follow-up | 90 [range 40–100] 80 [range 40–100] 90 [range 50–100] |
| CCI (median) | 2.9 |
| Side of tumor Left Right | 25 (43.1) 33 (56.9) |
| WHO tumor grade 3 4 | 13 (22.4) 45 (77.6) |
| IDH mutation | 13 (22.4) |
| MGMT methylation | 23 (36.7) |
| Extent of resection Total resection Subtotal resection Partial resection | 40/58 (69) 15/58 (26) 3/58 (5) |
| Preop enhancement tumor volume (cm3) Mean Range | 27 1.7–94.4 |
| Preop FLAIR volume (cm3) Mean Range | 64 1–144.5 |
| Vascular encasement (MCA) | 17 (29.3) |
| Surgical approach Transcortical Transsylvian | 47 (81) 11 (19) |
| Surgical time (minutes) Transcortical Transsylvian | 209.5 223.2 |
| Home discharge | 39 (67.2) |
| Patient postop chemotherapy Yes No | 44 (75.9) 14 (24.1) |
| Patient postop radiation Yes No | 45 (77.6) 13 (22.4) |
| OS (months) Median range | 18.5 3–71 |
| Outcome—Total No. of Patients (%) | |||
|---|---|---|---|
| Condition | Postop | At 1-Month Follow-Up | At 3-Month Follow-Up |
| Motor deficit | 24 (41.4) | 20 (34.5) | 9 (15.5) * |
| Speech deficit | 13 (22.4) | 11 (19) | 9 (15.5) |
| Sensory deficit | 17 (29.3) | 14 (24.1) | / |
| (A) | ||
| Variable | r | p -Value |
| CCI | −0.361 | 0.003 |
| MCA encasement | −0.314 | 0.043 |
| KPS at 1-month FU | 0.409 | 0.001 |
| Autonomous deambulation at 1 month | 0.339 | 0.027 |
| Radiotherapy | 0.321 | 0.009 |
| Histological | −0.261 | 0.027 |
| GTR | 0.231 | 0.045 |
| KPS preoperative | 0.160 | 0.122 |
| Tumor volume | −0.224 | 0.146 |
| Chemotherapy | 0.007 | 0.479 |
| (B) | ||
| Variable | r | p -Value |
| BSC | −0.518 | 0.002 |
| YC | −0.474 | 0.005 |
| MCA | 0.686 | 0.001 |
| Biopsy | 0.284 | 0.015 |
| GTR | −0.245 | 0.031 |
| KPS postoperative | −0.077 | 0.281 |
| KPS at 1-month FU | 0.151 | 0.129 |
| Autonomous deambulation at 1 month | 0.006 | 0.486 |
| Aphasia at 1 month | −0.130 | 0.231 |
| PFS | −0.102 | 0.230 |
| OS | −0.147 | 0.139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morello, A.; Rizzo, F.; Gatto, A.; Panico, F.; Bianconi, A.; Chiari, G.; Armocida, D.; Greco Crasto, S.; Melcarne, A.; Zenga, F.; et al. Safety and Efficacy in the Transcortical and Transsylvian Approach in Insular High-Grade Gliomas: A Comparative Series of 58 Patients. Curr. Oncol. 2025, 32, 98. https://doi.org/10.3390/curroncol32020098
Morello A, Rizzo F, Gatto A, Panico F, Bianconi A, Chiari G, Armocida D, Greco Crasto S, Melcarne A, Zenga F, et al. Safety and Efficacy in the Transcortical and Transsylvian Approach in Insular High-Grade Gliomas: A Comparative Series of 58 Patients. Current Oncology. 2025; 32(2):98. https://doi.org/10.3390/curroncol32020098
Chicago/Turabian StyleMorello, Alberto, Francesca Rizzo, Andrea Gatto, Flavio Panico, Andrea Bianconi, Giulia Chiari, Daniele Armocida, Stefania Greco Crasto, Antonio Melcarne, Francesco Zenga, and et al. 2025. "Safety and Efficacy in the Transcortical and Transsylvian Approach in Insular High-Grade Gliomas: A Comparative Series of 58 Patients" Current Oncology 32, no. 2: 98. https://doi.org/10.3390/curroncol32020098
APA StyleMorello, A., Rizzo, F., Gatto, A., Panico, F., Bianconi, A., Chiari, G., Armocida, D., Greco Crasto, S., Melcarne, A., Zenga, F., Rudà, R., Morana, G., Garbossa, D., & Cofano, F. (2025). Safety and Efficacy in the Transcortical and Transsylvian Approach in Insular High-Grade Gliomas: A Comparative Series of 58 Patients. Current Oncology, 32(2), 98. https://doi.org/10.3390/curroncol32020098






