Measurement of Intratumor Heterogeneity and Its Changing Pattern to Predict Response and Recurrence Risk After Neoadjuvant Chemotherapy in Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
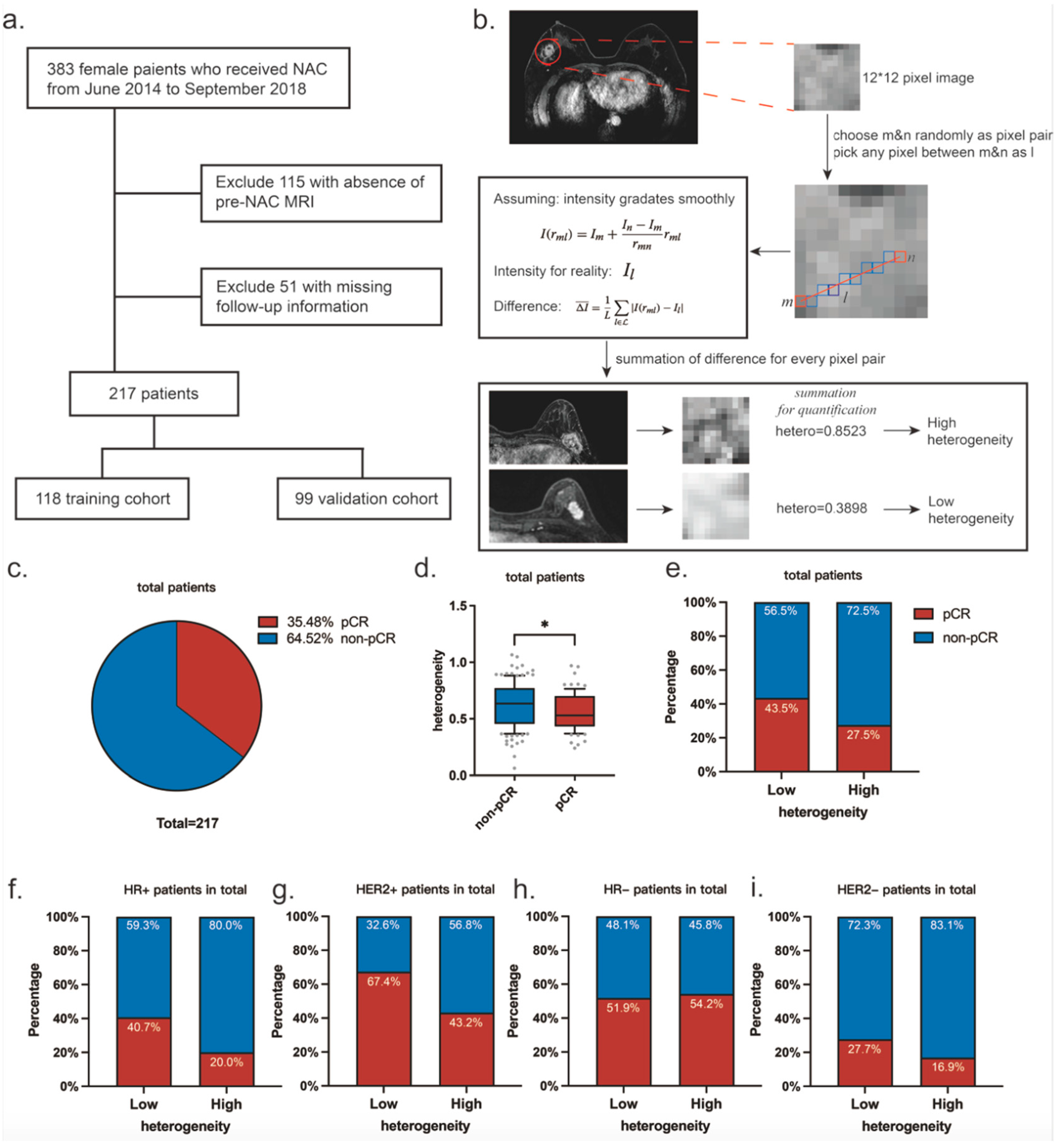
2.1. Patient Group
2.2. MRI Acquisition
2.3. MR Image Processing and Heterogeneity Definition
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
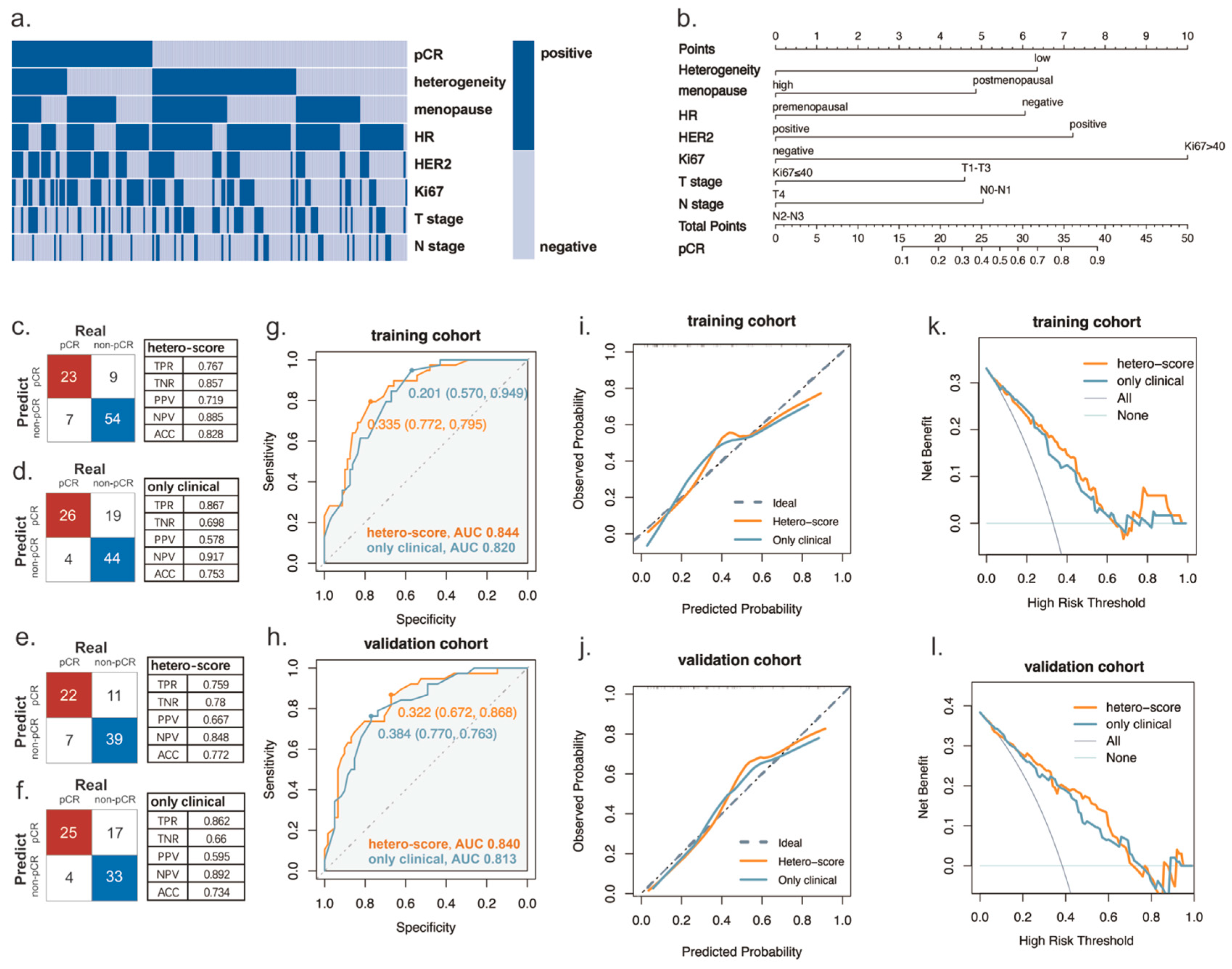
3.2. Variables Associated with pCR Rates
3.3. Prediction pCR by Using a Multivariate Model
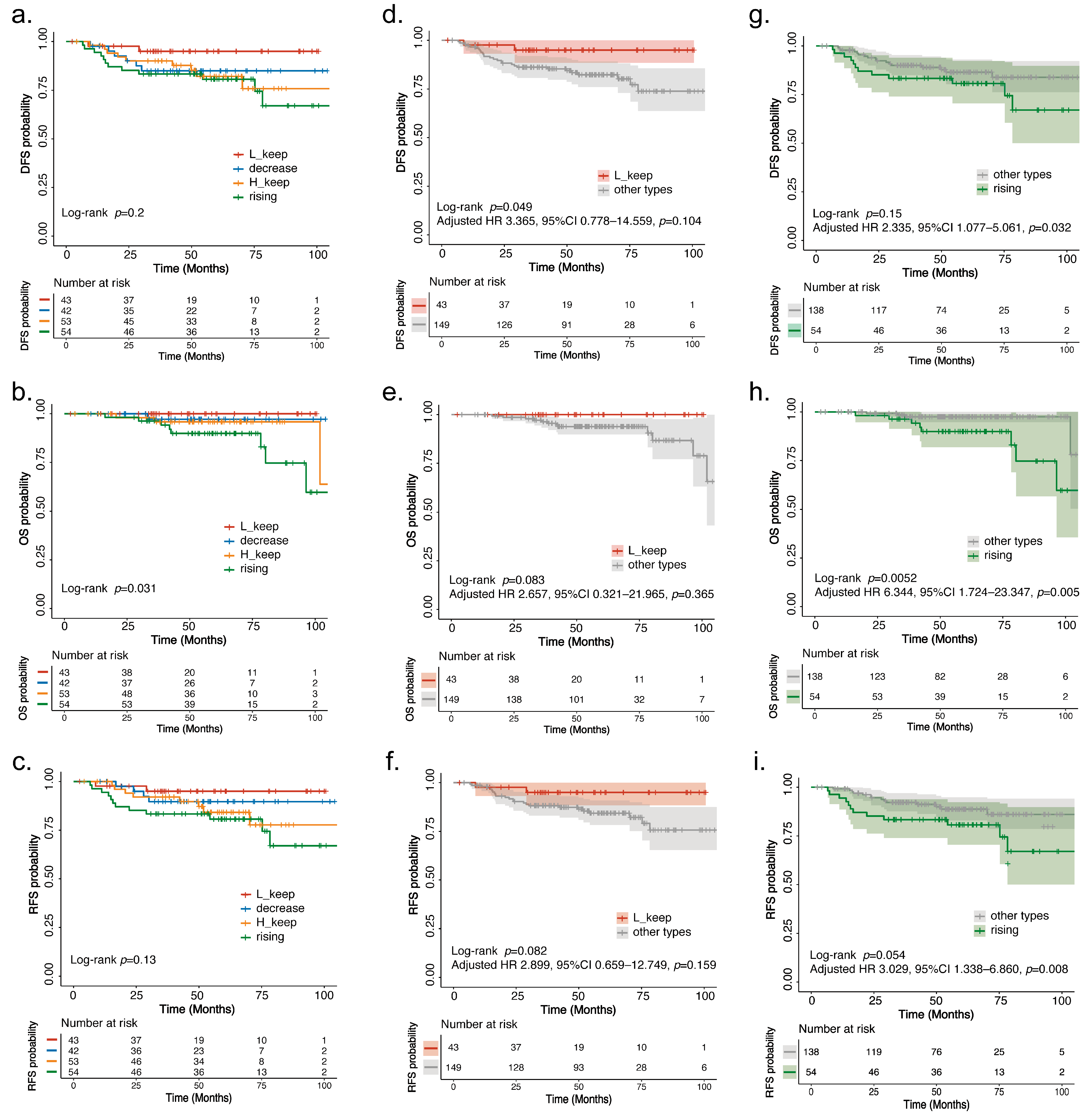
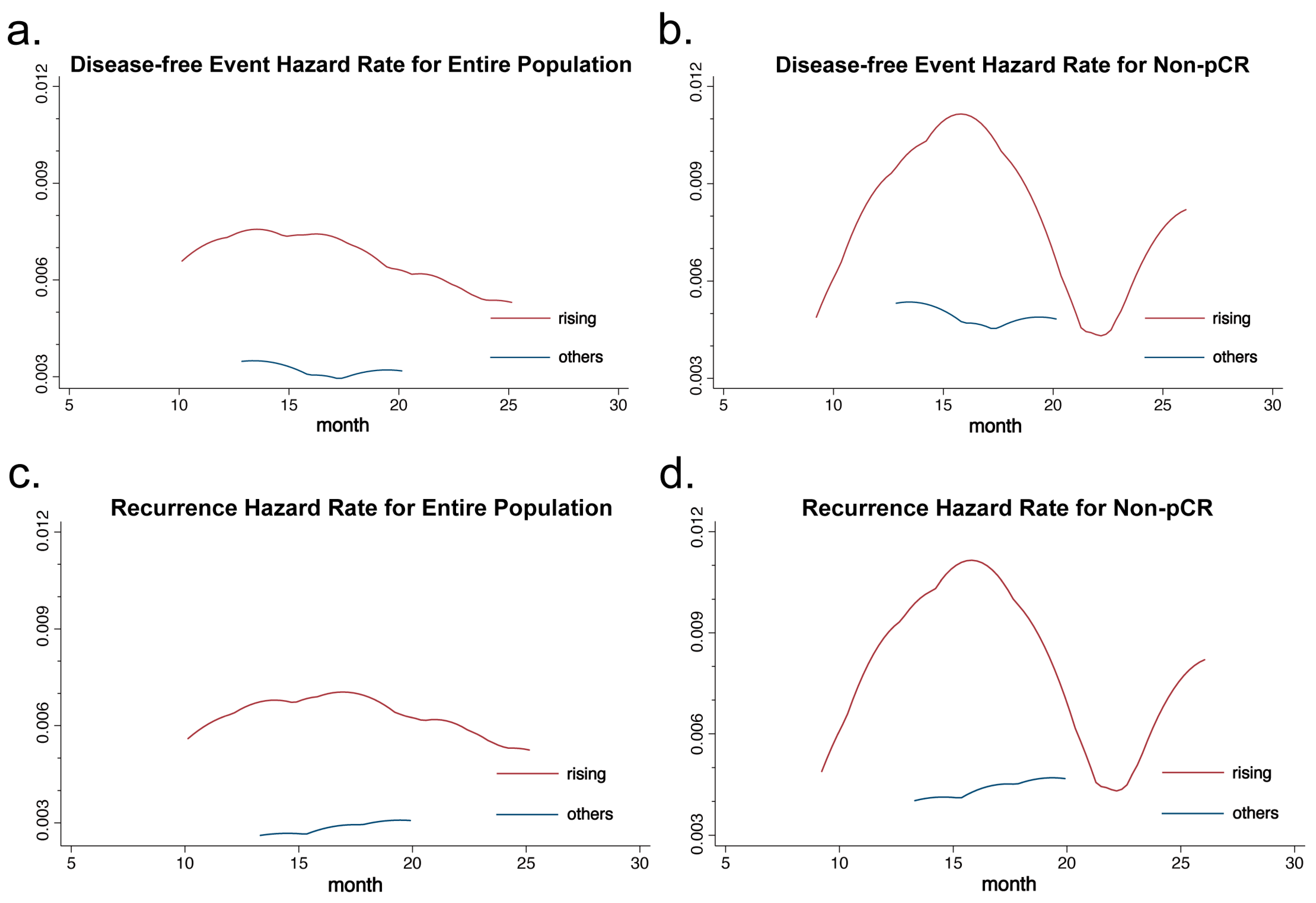
3.4. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Accuracy |
| AUC | Area under the curve |
| CI | Confidence interval |
| DFS | Disease-free survival |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hormone receptor |
| NAC | Neoadjuvant chemotherapy |
| NPV | Negative predictive value |
| OR | Odds ratio |
| OS | Overall survival |
| pCR | Pathologic complete response |
| PPV | Positive predictive value |
| RFS | Relapse-free survival |
| TNR | True negative rate |
| TPR | True positive rate |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Charalampoudis, P.; Karakatsanis, A. Neoadjuvant chemotherapy for early breast cancer. Lancet Oncol. 2018, 19, e128. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tong, X.; Hu, P.; Invernizzi, M.; Lai, L.; Wang, L.V. Photoacoustic Computed Tomography of Breast Cancer in Response to Neoadjuvant Chemotherapy. Adv. Sci. 2021, 8, 2003396. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 367, 976. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lashen, A.G.; Wahab, N.; Miligy, I.M.; Jahanifar, M.; Toss, M.; Graham, S.; Bilal, M.; Bhalerao, A.; Atallah, N.M.; et al. AI-based intra-tumor heterogeneity score of Ki67 expression as a prognostic marker for early-stage ER+/HER2− breast cancer. J. Pathol. Clin. Res. 2024, 10, e346. [Google Scholar] [CrossRef]
- Tinterri, C.; Fernandes, B.; Zambelli, A.; Sagona, A.; Barbieri, E.; Di Maria Grimaldi, S.; Darwish, S.S.; Jacobs, F.; De Carlo, C.; Iuzzolino, M.; et al. The Impact of Different Patterns of Residual Disease on Long-Term Oncological Outcomes in Breast Cancer Patients Treated with Neo-Adjuvant Chemotherapy. Cancers 2024, 16, 376. [Google Scholar] [CrossRef]
- Woodhams, R.; Kakita, S.; Hata, H.; Iwabuchi, K.; Kuranami, M.; Gautam, S.; Hatabu, H.; Kan, S.; Mountford, C. Identification of Residual Breast Carcinoma Following Neoadjuvant Chemotherapy: Diffusion-weighted Imaging—Comparison with Contrast-enhanced MR Imaging and Pathologic Findings. Radiology 2010, 254, 357–366. [Google Scholar] [CrossRef]
- Runge, V.M.; Richter, J.K.; Heverhagen, J.T. Speed in Clinical Magnetic Resonance. Investig. Radiol. 2017, 52, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.L.; Cowin, G.; Galloway, G. Magnetic resonance imaging: The underlying principles. J. Orthop. Sports Phys. Ther. 2011, 41, 806–819. [Google Scholar] [CrossRef]
- Brooks, F.J.; Grigsby, P.W. Quantification of heterogeneity observed in medical images. BMC Med. Imaging 2013, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef]
- Yoshida, G.J. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 2015, 34, 111. [Google Scholar] [CrossRef]
- Schwill, M.; Tamaskovic, R.; Gajadhar, A.S.; Kast, F.; White, F.M.; Plückthun, A. Systemic analysis of tyrosine kinase signaling reveals a common adaptive response program in a HER2-positive breast cancer. Sci. Signal. 2019, 12, eaau2875. [Google Scholar] [CrossRef] [PubMed]
- Robertson-Tessi, M.; Gillies, R.J.; Gatenby, R.A.; Anderson, A.R. Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res. 2015, 75, 1567–1579. [Google Scholar] [CrossRef]
- Swanton, C. Intratumor heterogeneity: Evolution through space and time. Cancer Res. 2012, 72, 4875–4882. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Quintanal-Villalonga, A.; Chan, J.M.; Yu, H.A.; Pe’er, D.; Sawyers, C.L.; Sen, T.; Rudin, C.M. Lineage plasticity in cancer: A shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 2020, 17, 360–371. [Google Scholar] [CrossRef] [PubMed]
| Variables | Training Cohort | Validation Cohort | Total | ||||
|---|---|---|---|---|---|---|---|
| High Heterogeneity = 61 | Low Heterogeneity = 57 | p | High Heterogeneity = 48 | Low Heterogeneity = 51 | p | ||
| Median age, years (range) | 0.87 | 0.705 | |||||
| 51.2 (28–69) | 50.9 (23–71) | 51.3 (30–69) | 50.5 (26–68) | 51.0 (23–71) | |||
| Menopausal status, n (%) | 0.681 | 0.499 | |||||
| Premenopausal | 28 (45.9%) | 24 (42.1%) | 24 (50.0%) | 22 (43.1%) | 98 (45.2%) | ||
| Postmenopausal | 33 (54.1%) | 33 (57.9%) | 24 (50.0%) | 29 (56.9%) | 119 (54.8%) | ||
| Hormone receptor status, n (%) | 0.527 | 0.943 | |||||
| Positive | 48 (78.7%) | 42 (73.7%) | 37 (77.1%) | 39 (76.5%) | 166 (76.5%) | ||
| Negative | 13 (21.3%) | 15 (26.3%) | 11 (22.9%) | 12 (23.5%) | 51 (23.5%) | ||
| HER2 status, n (%) | 0.501 | 0.395 | |||||
| Positive | 23 (37.7%) | 25 (43.9%) | 21 (43.8%) | 18 (35.3%) | 87 (40.1%) | ||
| Negative | 38 (62.3%) | 32 (56.1%) | 27 (56.2%) | 33 (64.7%) | 130 (59.9%) | ||
| Ki67 score, n (%) | 0.417 | 0.98 | |||||
| Ki67 ≤ 40 | 34 (55.7%) | 36 (63.2%) | 30 (62.5%) | 32 (62.7%) | 132 (60.8%) | ||
| Ki67 > 40 | 27 (44.3%) | 21 (36.8%) | 18 (37.5%) | 19 (37.3%) | 85 (39.2%) | ||
| T stage, n (%) | 0.199 | 0.844 | |||||
| T1–T3 | 37 (60.7%) | 41 (71.9%) | 33 (68.8%) | 36 (70.6%) | 147 (67.7%) | ||
| T4 | 24 (39.3%) | 16 (28.1%) | 15 (31.2%) | 15 (29.4%) | 70 (32.3%) | ||
| N stage, n (%) | 0.357 | 0.73 | |||||
| N0–N1 | 51 (83.6%) | 51 (89.5%) | 39 (81.3%) | 40 (78.4%) | 181 (83.4%) | ||
| N2–N3 | 10 (16.4%) | 6 (10.5%) | 9 (18.7%) | 11 (21.6%) | 36 (16.6%) | ||
| Variables | Comparison | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95%CI | p Value | OR | 95%CI | p Value | ||
| Menopausal status | Post vs. Pre | 1.065 | 0.609–1.863 | 0.825 | 0.711 | 0.357–1.415 | 0.331 |
| HR | Positive vs. Negative | 0.383 | 0.202–0.728 | 0.003 | 0.387 | 0.185–0.810 | 0.012 |
| HER2 | Positive vs. Negative | 4.284 | 2.374–7.739 | <0.001 | 5.298 | 2.649–10.595 | <0.001 |
| Ki67 | >40 vs. ≤40 | 2.707 | 1.524–4.809 | 0.001 | 2.927 | 1.521–5.630 | 0.001 |
| T stage | T4 vs. T1–T3 | 0.632 | 0.341–1.169 | 0.143 | 0.45 | 0.264–1.591 | 0.344 |
| N stage | N2–N3 vs. N0–N1 | 0.554 | 0.246–1.248 | 0.154 | 0.648 | 0.330–2.188 | 0.737 |
| Heterogeneity | High vs. Low | 0.493 | 0.280–0.869 | 0.014 | 0.401 | 0.208–0.775 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Wu, Q.; Geng, X.; Xie, H.; Wang, Y.; Wu, Z.; Lin, Y.; Zhou, L.; Xu, S.; Ye, Y.; et al. Measurement of Intratumor Heterogeneity and Its Changing Pattern to Predict Response and Recurrence Risk After Neoadjuvant Chemotherapy in Breast Cancer. Curr. Oncol. 2025, 32, 93. https://doi.org/10.3390/curroncol32020093
Zhu M, Wu Q, Geng X, Xie H, Wang Y, Wu Z, Lin Y, Zhou L, Xu S, Ye Y, et al. Measurement of Intratumor Heterogeneity and Its Changing Pattern to Predict Response and Recurrence Risk After Neoadjuvant Chemotherapy in Breast Cancer. Current Oncology. 2025; 32(2):93. https://doi.org/10.3390/curroncol32020093
Chicago/Turabian StyleZhu, Mingxi, Qiong Wu, Xiaochuan Geng, Huaying Xie, Yan Wang, Ziping Wu, Yanping Lin, Liheng Zhou, Shuguang Xu, Yumei Ye, and et al. 2025. "Measurement of Intratumor Heterogeneity and Its Changing Pattern to Predict Response and Recurrence Risk After Neoadjuvant Chemotherapy in Breast Cancer" Current Oncology 32, no. 2: 93. https://doi.org/10.3390/curroncol32020093
APA StyleZhu, M., Wu, Q., Geng, X., Xie, H., Wang, Y., Wu, Z., Lin, Y., Zhou, L., Xu, S., Ye, Y., Yin, W., Hua, J., Lu, J., & Wang, Y. (2025). Measurement of Intratumor Heterogeneity and Its Changing Pattern to Predict Response and Recurrence Risk After Neoadjuvant Chemotherapy in Breast Cancer. Current Oncology, 32(2), 93. https://doi.org/10.3390/curroncol32020093





