Molecular Tumor Board-Guided Targeted Treatments for Biliary Tract Cancers in a Publicly Funded Healthcare System
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Whole-Genome and Transcriptome Sequencing
2.3. Molecular Tumor Boards
2.4. Statistical Analysis
3. Results
3.1. Cohort Profile
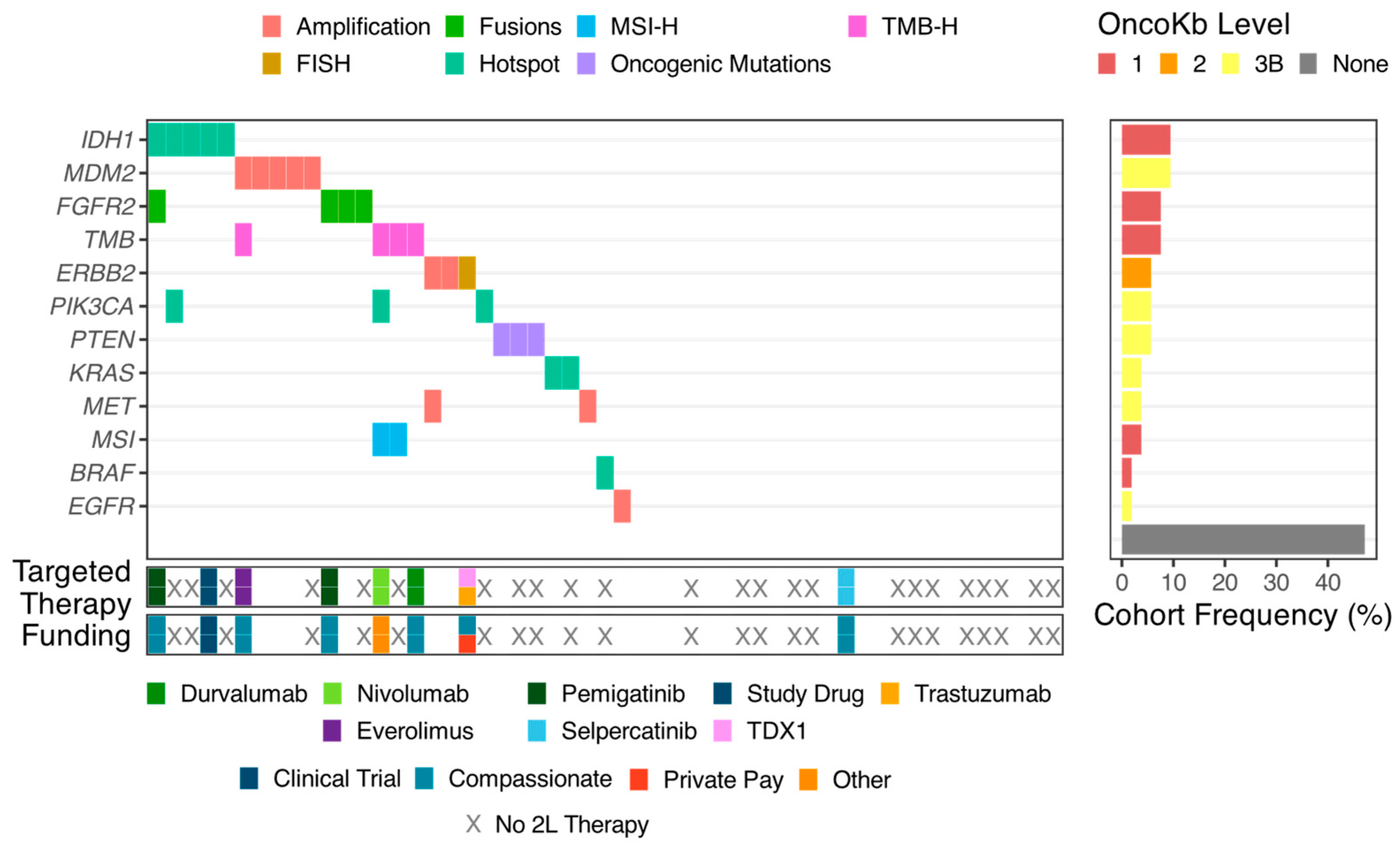
3.2. Targetable Alterations
3.3. Molecular Tumor Board
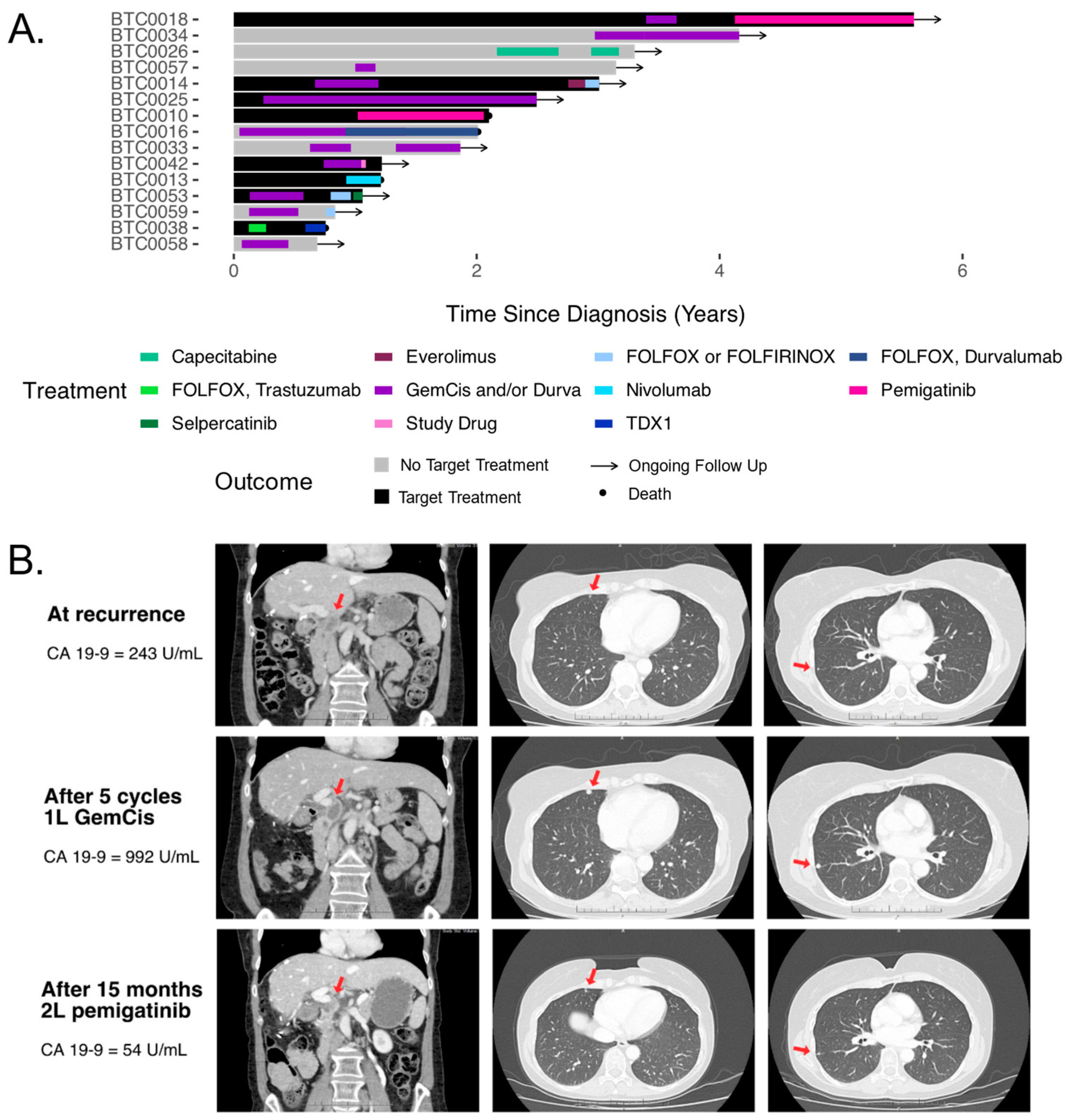
3.4. Treatments and Outcomes
3.5. Funding of Therapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 98–107. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Évid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Ozaka, M.; Verslype, C.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.; Klümpen, H.; Malka, D.; Primrose, J.; Rimassa, L.; Stenzinger, A.; Valle, J.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Ni Huang, M.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, J.; Sellers, C.M.; Cha, C.; Khan, S.A.; Lacy, J.; Stein, S.M.; Kim, H.S. Intrahepatic Cholangiocarcinoma: Socioeconomic Discrepancies, Contemporary Treatment Approaches and Survival Trends from the National Cancer Database. Ann. Surg. Oncol. 2019, 26, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Ramjeesingh, R.; Chaudhury, P.; Tam, V.C.; Roberge, D.; Lim, H.J.; Knox, J.J.; Asselah, J.; Doucette, S.; Chhiber, N.; Goodwin, R. A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada. Curr. Oncol. 2023, 30, 7132–7150. [Google Scholar] [CrossRef]
- Lamarca, A.; Vogel, A. Futibatinib: Second EMA approval for FGFR inhibitor in cholangiocarcinoma. ESMO Open 2023, 8, 102049. [Google Scholar] [CrossRef] [PubMed]
- Chan-Seng-Yue, M.; Kim, J.C.; Wilson, G.W.; Ng, K.; Figueroa, E.F.; O’Kane, G.M.; Connor, A.A.; Denroche, R.E.; Grant, R.C.; McLeod, J.; et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 2020, 52, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Cibulskis, K.; Lawrence, M.S.; Carter, S.L.; Sivachenko, A.; Jaffe, D.; Sougnez, C.; Gabriel, S.; Meyerson, M.; Lander, E.S.; Getz, G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 2013, 31, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Scheffler, K.; Halpern, A.L.; Bekritsky, M.A.; Noh, E.; Källberg, M.; Chen, X.; Kim, Y.; Beyter, D.; Krusche, P.; et al. Strelka2: Fast and accurate calling of germline and somatic variants. Nat. Methods 2018, 15, 591–594. [Google Scholar] [CrossRef]
- Wala, J.A.; Bandopadhayay, P.; Greenwald, N.F.; O’Rourke, R.; Sharpe, T.; Stewart, C.; Schumacher, S.; Li, Y.; Weischenfeldt, J.; Yao, X.; et al. SvABA: Genome-wide detection of structural variants and indels by local assembly. Genome Res. 2018, 28, 581–591. [Google Scholar] [CrossRef]
- Chen, X.; Schulz-Trieglaff, O.; Shaw, R.; Barnes, B.; Schlesinger, F.; Källberg, M.; Cox, A.J.; Kruglyak, S.; Saunders, C.T. Manta: Rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 2016, 32, 1220–1222. [Google Scholar] [CrossRef]
- Rausch, T.; Zichner, T.; Schlattl, A.; Stütz, A.M.; Benes, V.; Korbel, J.O. DELLY: Structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 2012, 28, i333–i339. [Google Scholar] [CrossRef]
- Notta, F.; Chan-Seng-Yue, M.; Lemire, M.; Li, Y.; Wilson, G.W.; Connor, A.A.; Denroche, R.E.; Liang, S.-B.; Brown, A.M.K.; Kim, J.C.; et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 2016, 538, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Golan, T.; O’kane, G.M.; Denroche, R.E.; Raitses-Gurevich, M.; Grant, R.C.; Holter, S.; Wang, Y.; Zhang, A.; Jang, G.H.; Stossel, C.; et al. Genomic Features and Classification of Homologous Recombination Deficient Pancreatic Ductal Adenocarcinoma. Gastroenterology 2021, 160, 2119–2132.e9. [Google Scholar] [CrossRef] [PubMed]
- Bancarz, I.; Beaudry, F.; Alam, A.; Fortuna, A.; Pugh, T. Djerba: A modular system to generate clinical genome interpretation reports for cancer. Cancer Genet. 2023, 278, 6. [Google Scholar] [CrossRef]
- Chen, M.-H.; Li, W.-S.; Liao, B.-C.; Wu, C.-E.; Li, C.-F.; Hsieh, C.-H.; Kuan, F.-C.; Tzeng, H.-E.; Hang, J.-F.; Chiang, N.-J.; et al. Expert Consensus on Molecular Tumor Boards in Taiwan: Joint Position Paper by the Taiwan Oncology Society and the Taiwan Society of Pathology. J. Cancer Res. Pract. 2024, 11, 18–27. [Google Scholar] [CrossRef]
- Luchini, C.; Lawlor, R.T.; Milella, M.; Scarpa, A. Molecular Tumor Boards in Clinical Practice. Trends Cancer 2020, 6, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Kendre, G.; Murugesan, K.; Brummer, T.; Segatto, O.; Saborowski, A.; Vogel, A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J. Hepatol. 2022, 78, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-Line Pemigatinib vs Gemcitabine plus Cisplatin for Advanced Cholangiocarcinoma with FGFR2 Rearrangements. Future Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. JCO 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and Trastuzumab for HER2-Positive, Metastatic Biliary Tract Cancer (MyPathway): A Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Kawamoto, Y.; Komatsu, Y.; Ueno, M.; Kobayashi, S.; Ikeda, M.; Sasaki, M.; Furuse, J.; Okano, N.; et al. Trastuzumab Deruxtecan in Human Epidermal Growth Factor Receptor 2–Expressing Biliary Tract Cancer (HERB; NCCH1805): A Multicenter, Single-Arm, Phase II Trial. JCO 2024, 42, 3207–3217. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Gazzah, A.; Lassen, U.; Stein, A.; Wen, P.Y.; Dietrich, S.; de Jonge, M.J.A.; Blay, J.-Y.; et al. Dabrafenib plus Trametinib in BRAFV600E-Mutated Rare Cancers: The Phase 2 ROAR Trial. Nat. Med. 2023, 29, 1103–1112. [Google Scholar] [CrossRef]
- Rose, A.A.N.; Maxwell, J.; Rousselle, E.; Riaud, M.; Tobin, C.; Mcguire, M.; King, I.; Zhang, T.; Saeed Kamil, Z.; Pugh, T.J.; et al. Binimetinib and Encorafenib for the Treatment of Advanced Solid Tumors with Non-V600E BRAF Mutations (Mts): Final Results of the Investigator-Initiated Phase II BEAVER Trial. JCO 2024, 42 (16_suppl), 3104. [Google Scholar] [CrossRef]
- Macarulla, T.; Yamamoto, N.; Tolcher, A.W.; Hafez, N.; Lugowska, I.; Ramlau, R.; Geng, J.; Li, J.; Teufel, M.; Maerten, A.; et al. Efficacy and Safety of Brigimadlin (BI 907828), an MDM2–P53 Antagonist, in Patients (Pts) with Advanced Biliary Tract Cancer: Data from Two Phase Ia/Ib Dose-Escalation/Expansion Trials. JCO 2024, 42 (3_suppl), 487. [Google Scholar] [CrossRef]
- Pant, S.; Yaeger, R.; Spira, A.I.; Pelster, M.; Sabari, J.K.; Hafez, N.; Barve, M.A.; Velastegui, K.; Yan, X.; Der-Torossian, H.; et al. KRYSTAL-1: Activity and Safety of Adagrasib (MRTX849) in Patients with Advanced Solid Tumors Harboring a KRASG12C Mutation. JCO 2023, 41 (36_suppl), 425082. [Google Scholar] [CrossRef]
- Sacher, A.G.; Villalona-Calero, M.; O’Neil, B.H.; Rodon, J.; Doi, T.; Postel-Vinay, S.; Ghiringhelli, F.; Yamamoto, N.; Wahlroos, S.; Villaruz, L.; et al. 604O Phase I Dose Escalation and Initial Dose Expansion Results of AMG 193: A MTA-Cooperative PRMT5 Inhibitor, in Patients (Pts) with MTAP-Deleted Solid Tumors. Ann. Oncol. 2024, 35, S484–S485. [Google Scholar] [CrossRef]
- Golan, T.; Raitses-Gurevich, M.; Kelley, R.K.; Bocobo, A.G.; Borgida, A.; Shroff, R.T.; Holter, S.; Gallinger, S.; Ahn, D.H.; Aderka, D.; et al. Overall Survival and Clinical Characteristics of BRCA-Associated Cholangiocarcinoma: A Multicenter Retrospective Study. Oncologist 2017, 22, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Jenei, K.; Gentilini, A.; Haslam, A.; Prasad, V. Clinical Benefit, Reimbursement Outcomes, and Prices of FDA-Approved Cancer Drugs Reviewed through Project Orbis in the USA, Canada, England, and Scotland: A Retrospective, Comparative Analysis. Lancet Oncol. 2024, 25, 979–988. [Google Scholar] [CrossRef]
- Hernando-Calvo, A.; Nguyen, P.; Bedard, P.L.; Chan, K.K.W.; Saleh, R.R.; Weymann, D.; Yu, C.; Amir, E.; Regier, D.A.; Gyawali, B.; et al. Impact on Costs and Outcomes of Multi-Gene Panel Testing for Advanced Solid Malignancies: A Cost-Consequence Analysis Using Linked Administrative Data. eClinicalMedicine 2024, 69, 102443. [Google Scholar] [CrossRef] [PubMed]
- Arriola, E.; Bernabé, R.; Campelo, R.G.; Biscuola, M.; Enguita, A.B.; López-Ríos, F.; Martínez, R.; Mezquita, L.; Palanca, S.; Pareja, M.J.; et al. Cost-Effectiveness of Next-Generation Sequencing Versus Single-Gene Testing for the Molecular Diagnosis of Patients With Metastatic Non–Small-Cell Lung Cancer From the Perspective of Spanish Reference Centers. JCO Precis. Oncol. 2023, 7, e2200546. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Goerke, L.; Anderson, A.; Wilsdon, T. Assessing the Cost-Effectiveness of Next-Generation Sequencing as a Biomarker Testing Approach in Oncology and Policy Implications: A Literature Review. Value Health 2024, 27, 1300–1309. [Google Scholar] [CrossRef]
- Zou, D.; Ye, W.; Hess, L.M.; Bhandari, N.R.; Ale-Ali, A.; Foster, J.; Quon, P.; Harris, M. Diagnostic Value and Cost-Effectiveness of Next-Generation Sequencing-Based Testing for Treatment of Patients with Advanced/Metastatic Non-Squamous Non-Small-Cell Lung Cancer in the United States. J. Mol. Diagn. 2022, 24, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Christofyllakis, K.; Bittenbring, J.T.; Thurner, L.; Ahlgrimm, M.; Stilgenbauer, S.; Bewarder, M.; Kaddu-Mulindwa, D. Cost-Effectiveness of Precision Cancer Medicine-Current Challenges in the Use of next Generation Sequencing for Comprehensive Tumour Genomic Profiling and the Role of Clinical Utility Frameworks (Review). Mol. Clin. Oncol. 2022, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- pemigatinib | CDA-AMC. Available online: https://www.cda-amc.ca/pemigatinib (accessed on 22 January 2025).
- Chueh, C.-H.; Tsai, Y.-W.; Chen, Z.-R.; Shiu, M.-N.; Wen, Y.-W.; Chiang, N.-J. Cost-Effectiveness Analysis of a New Second-Line Treatment Regimen for Advanced Intrahepatic Cholangiocarcinoma: Biomarker-Driven Targeted Therapy of Pemigatinib Versus 5-FU Chemotherapy. PharmacoEconomics 2023, 41, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-R.; Chueh, C.-H.; Chiang, N.-J.; Tsai, Y.-W. Cost-Effectiveness of Pemigatinib as a Second-Line Treatment for Advanced Intrahepatic Cholangiocarcinoma with Fibroblast Growth Factor Receptor 2 Fusions in Taiwan: From the Evidence of the Phase II Trial and the Perspective of Taiwan’s National Health Insurance Administration. Cost Eff. Resour. Alloc. 2023, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-A.; Huang, W.-M.; Chen, E.Y.-T.; Ho, P.-K.; Chueh, C.-H.; Wen, Y.-W.; Chen, M.-H.; Chiang, N.-J.; Tsai, Y.-W. Cost-Effectiveness of Ivosidenib versus Chemotherapy for Previously Treated IDH1-Mutant Advanced Intrahepatic Cholangiocarcinoma in Taiwan. BMC Cancer 2024, 24, 622. [Google Scholar] [CrossRef]
- Love, T.M.; Anaya, D.A.; Prime, M.S.; Ardolino, L.; Ekinci, O. Development and Validation of ACTE-MTB: A Tool to Systematically Assess the Maturity of Molecular Tumor Boards. PLoS ONE 2022, 17, e0268477. [Google Scholar] [CrossRef] [PubMed]

| Overall, n = 55 | |
|---|---|
| Male sex (male, n, [%]) | 33 (60.0) |
| Age at diagnosis, years (IQR) | 53 (45–69) |
| Body mass index, kg/m2 (median, IQR) | 25.2 (22.9–30.1) |
| Ethnicity (n, [%]) | |
| Caucasian | 34 (61.8) |
| Asian | 10 (18.2) |
| Black/African Canadian | 5 (9.1) |
| Unknown | 6 (10.9) |
| Anatomic origin of the cancer (n, [%]) | |
| Intrahepatic | 38 (69.1) |
| Perihilar | 7 (12.7) |
| Distal | 4 (7.3) |
| Gallbladder | 6 (10.9) |
| Cancer stage, AJCC 8th (n, [%]) | |
| I | 4 (7.3) |
| II | 16 (29.1) |
| IIIA | 4 (7.3) |
| IIIB | 7 (12.7) |
| IV | 23 (41.8) |
| CA-19-9 at diagnosis (U/mL, IQR) | 46 (21–282) |
| Metastasis at diagnosis (n, [%]) | 20 (37.7) |
| Surgical resection (n, %) | 35 (63.6) |
| Adjuvant chemotherapy (n/35, [%]) | |
| Capecitabine | 17 (48.6) |
| Gemcitabine and cisplatin | 4 (11.4) |
| Gemcitabine | 1 (2.9) |
| None | 13 (37.1) |
| Recurrence after surgery (n/35, [%]) | 18 (51.4) |
| First-line systemic therapy (n/37, [%]) | |
| Gemcitabine, cisplatin, and durvalumab | 25 (67.6) |
| Gemcitabine and cisplatin | 6 (16.2) |
| Gemcitabine, cisplatin, and nab-paclitaxel | 1 (2.7) |
| Capecitabine | 2 (5.4) |
| FOLFIRINOX | 1 (2.7) |
| FOLFOX | 1 (2.7) |
| Pemigatinib | 1 (2.7) |
| Second-line systemic therapy (n/19, [%]) | |
| FOLFOX | 11 (57.9) |
| Capecitabine | 1 (5.3) |
| Pemigatinib | 1 (5.3) |
| Everolimus | 1 (5.3) |
| Trastuzumab | 1 (5.3) |
| Nivolumab | 1 (5.3) |
| AMG 193 | 1 (5.3) |
| Selpercatinib | 1 (5.3) |
| Clinical trial | 1 (5.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaudry, F.E.G.; Li, Z.; Borgida, A.; Zorigtbaatar, A.; Wang, X.; Hildebrand, M.; Hamza, O.; Jang, G.H.; Bucur, R.; Dodd, A.; et al. Molecular Tumor Board-Guided Targeted Treatments for Biliary Tract Cancers in a Publicly Funded Healthcare System. Curr. Oncol. 2025, 32, 80. https://doi.org/10.3390/curroncol32020080
Beaudry FEG, Li Z, Borgida A, Zorigtbaatar A, Wang X, Hildebrand M, Hamza O, Jang GH, Bucur R, Dodd A, et al. Molecular Tumor Board-Guided Targeted Treatments for Biliary Tract Cancers in a Publicly Funded Healthcare System. Current Oncology. 2025; 32(2):80. https://doi.org/10.3390/curroncol32020080
Chicago/Turabian StyleBeaudry, Felix E. G., Zhihao Li, Ayelet Borgida, Anudari Zorigtbaatar, Xin Wang, Maggie Hildebrand, Oumaima Hamza, Gun Ho Jang, Roxana Bucur, Anna Dodd, and et al. 2025. "Molecular Tumor Board-Guided Targeted Treatments for Biliary Tract Cancers in a Publicly Funded Healthcare System" Current Oncology 32, no. 2: 80. https://doi.org/10.3390/curroncol32020080
APA StyleBeaudry, F. E. G., Li, Z., Borgida, A., Zorigtbaatar, A., Wang, X., Hildebrand, M., Hamza, O., Jang, G. H., Bucur, R., Dodd, A., Wilson, J., Auer, R. C., Saibil, S., Tsang, E. S., Vogel, A., O’Kane, G. M., Gallinger, S., Knox, J. J., Notta, F., ... Grant, R. C. (2025). Molecular Tumor Board-Guided Targeted Treatments for Biliary Tract Cancers in a Publicly Funded Healthcare System. Current Oncology, 32(2), 80. https://doi.org/10.3390/curroncol32020080





