A Rare Case of Metastatic Urethral Squamous Cell Carcinoma Presenting with Paraneoplastic Sweet Syndrome and Treated with Pembrolizumab
Simple Summary
Abstract
1. Introduction
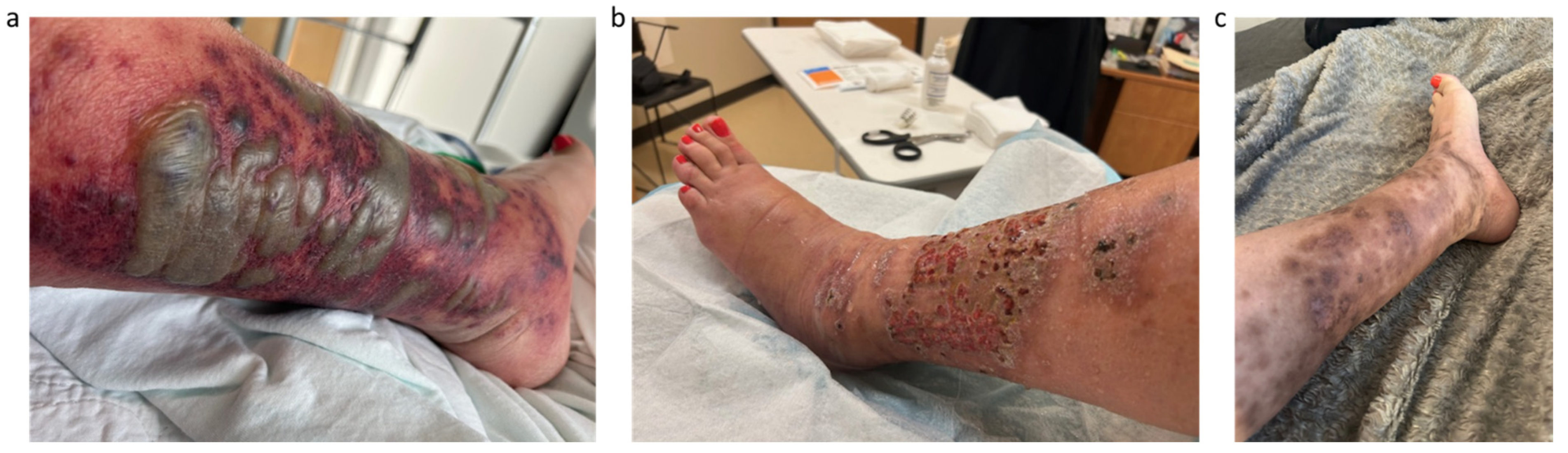
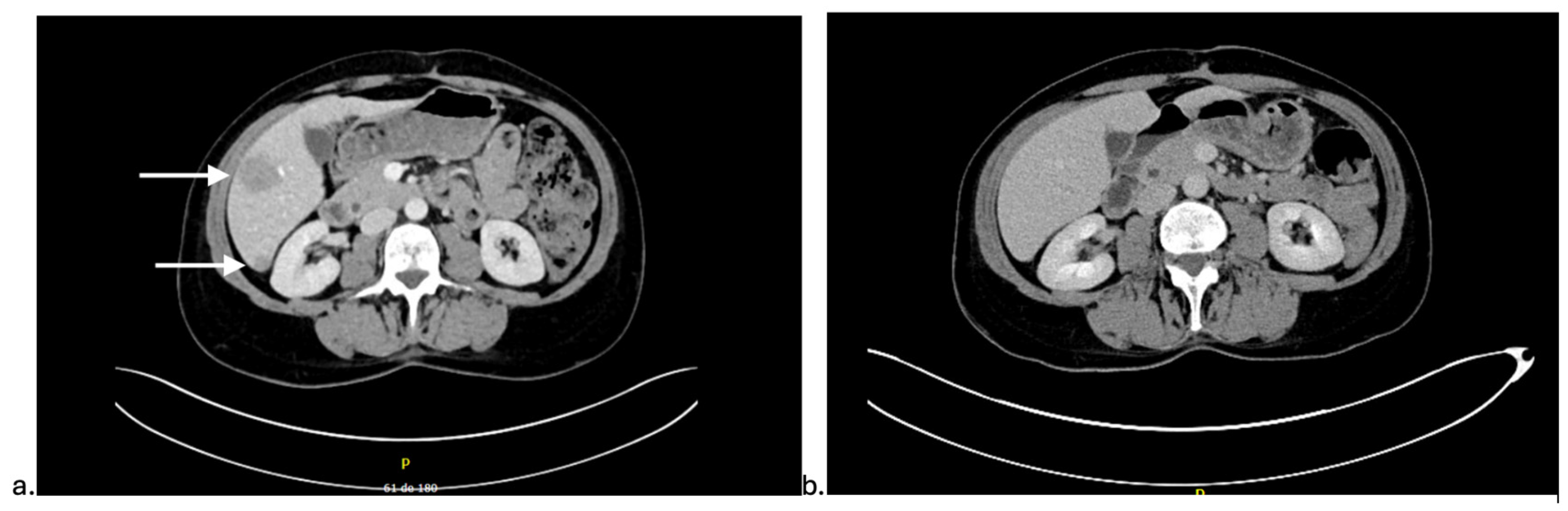
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, H.; Zhu, Y.; Ye, D. A brief review on the diagnostic and therapeutic principles of primary urethral cancer. Asian J. Urol. 2022, 9, 423–429. [Google Scholar] [CrossRef]
- Aleksic, I.; Rais-Bahrami, S.; Daugherty, M.; Agarwal, P.K.; Vourganti, S.; Bratslavsky, G. Primary urethral carcinoma: A Surveillance, Epidemiology, and End Results data analysis identifying predictors of cancer-specific survival. Urol. Ann. 2018, 10, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perdomo, H.A.; Davila-Raigoza, A.M.; Summers, E.; Billingham, L.; Necchi, A.; Griffiths, G.; Spiess, P.E. Urethral cancer: A comprehensive review endorsed by the Global Society of Rare Genitourinary Tumours. BJU Int. 2024, 134, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guan, Y.; Li, S. Programmed death receptor (PD-)1/PD-ligand (L)1 in urological cancers: The “all-around warrior” in immunotherapy. Mol. Cancer 2024, 23, 183. [Google Scholar] [CrossRef]
- Yilmaz, E.; Ismaila, N.; Dabney, R.; Saba, N.F.; Mell, L.K. Immunotherapy and Biomarker Testing in Recurrent and Metastatic Head and Neck Cancers: ASCO Guideline Q and A. JCO Oncol. Pract. 2023, 19, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Usta, E.H.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Clancy, K.; Hamill, C.S.; O’Neill, W.Q.; Vu, B.; Thuener, J.; Gui, S.; Li, S.; Fowler, N.; Rezaee, R.; Lavertu, P.; et al. Impact of p16 Status and Anatomical Site in Anti-PD-1 Immunotherapy-Treated Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma Patients. Cancers 2021, 13, 4861. [Google Scholar] [CrossRef]
- Ulas, E.B.; Hashemi, S.M.S.; Houda, I.; Kaynak, A.; Veltman, J.D.; Fransen, M.F.; Radonic, T.; Bahce, I. Predictive Value of Combined Positive Score and Tumor Proportion Score for Immunotherapy Response in Advanced NSCLC. JTO Clin. Res. Rep. 2023, 4, 100532. [Google Scholar] [CrossRef]
- Raza, S.; Kirkland, R.S.; Patel, A.A.; Shortridge, J.R.; Freter, C. Insight into Sweet’s syndrome and associated-malignancy: A review of the current literature. Int. J. Oncol. 2013, 42, 1516–1522. [Google Scholar] [CrossRef]
- Gakis, G.; Morgan, T.M.; Daneshmand, S.; Keegan, K.A.; Todenhofer, T.; Mischinger, J.; Schubert, T.; Zaid, H.B.; Hrbacek, J.; Ali-El-Dein, B.; et al. Impact of perioperative chemotherapy on survival in patients with advanced primary urethral cancer: Results of the international collaboration on primary urethral carcinoma. Ann. Oncol. 2015, 26, 1754–1759. [Google Scholar] [CrossRef]
- Trabulsi, E.J.; Hoffman-Censits, J. Chemotherapy for penile and urethral carcinoma. Urol. Clin. North Am. 2010, 37, 467–474. [Google Scholar] [CrossRef]
- Eng, T.Y.; Chen, T.W.; Patel, A.J.; Vincent, J.N.; Ha, C.S. Treatment and Outcomes of Primary Urethra Cancer. Am. J. Clin. Oncol. 2018, 41, 905–908. [Google Scholar] [CrossRef]
- Wenzel, M.; Deuker, M.; Nocera, L.; Ruvolo, C.C.; Tian, Z.; Shariat, S.F.; Saad, F.; Briganti, A.; Becker, A.; Kluth, L.A.; et al. Comparison Between Urothelial and Non-Urothelial Urethral Cancer. Front. Oncol. 2020, 10, 629692. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Zeng, Q.; Guo, X.J.; Wang, H.; Liu, H.H.; Dong, Z.-Y. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Rep. 2019, 9, 13404. [Google Scholar] [CrossRef]
- Bai, H.; Han, H.; Wang, F.; Shi, H. Chemotherapy combined with immunotherapy in primary female urethral squamous cell carcinoma: A case report. J. Int. Med. Res. 2022, 50, 3000605221132418. [Google Scholar] [CrossRef]
- Cohen, P.R.; Holder, W.R.; Tucker, S.B.; Kono, S.; Kurzrock, R. Sweet syndrome in patients with solid tumors. Cancer 1993, 72, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.P.; Friske, S.K.; Hsiou, D.A.; Duvic, M. New Practical Aspects of Sweet Syndrome. Am. J. Clin. Dermatol. 2022, 23, 301–318. [Google Scholar] [CrossRef]
- Jung, E.H.; Park, J.H.; Kim, K.H.; Kim, J.S.; Choi, I.S.; Byun, J.M.; Koh, Y.; Shin, D.-Y.; Hong, J.; Yoon, S.-S.; et al. Characteristics of Sweet syndrome in patients with or without malignancy. Ann. Hematol. 2022, 101, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Chandler, W.; Long, P.R.; Palmer, M.L.; Fadugba, O. Dupilumab-Associated Sweet Syndrome. Cutis 2023, 111, E7–E9. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Mariano, A.T.; Joshi, D.; Andrews, T.E.; Liu, S.; Basha, A.; Huynh, N. A Rare Case of Drug-Induced Sweet Syndrome After Pembrolizumab Therapy. Cureus 2024, 16, e62027. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.K.; Chapman, M.S.; Carter, J.B.; Samie, F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr. Probl. Cancer 2017, 41, 125–128. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.-T.C.; Hamilou, Z.; Bonnardeaux, E.; Blais, N.; de Vries-Brilland, M. A Rare Case of Metastatic Urethral Squamous Cell Carcinoma Presenting with Paraneoplastic Sweet Syndrome and Treated with Pembrolizumab. Curr. Oncol. 2025, 32, 683. https://doi.org/10.3390/curroncol32120683
Nguyen D-TC, Hamilou Z, Bonnardeaux E, Blais N, de Vries-Brilland M. A Rare Case of Metastatic Urethral Squamous Cell Carcinoma Presenting with Paraneoplastic Sweet Syndrome and Treated with Pembrolizumab. Current Oncology. 2025; 32(12):683. https://doi.org/10.3390/curroncol32120683
Chicago/Turabian StyleNguyen, Dan-Thanh Christine, Zineb Hamilou, Evelyne Bonnardeaux, Normand Blais, and Manon de Vries-Brilland. 2025. "A Rare Case of Metastatic Urethral Squamous Cell Carcinoma Presenting with Paraneoplastic Sweet Syndrome and Treated with Pembrolizumab" Current Oncology 32, no. 12: 683. https://doi.org/10.3390/curroncol32120683
APA StyleNguyen, D.-T. C., Hamilou, Z., Bonnardeaux, E., Blais, N., & de Vries-Brilland, M. (2025). A Rare Case of Metastatic Urethral Squamous Cell Carcinoma Presenting with Paraneoplastic Sweet Syndrome and Treated with Pembrolizumab. Current Oncology, 32(12), 683. https://doi.org/10.3390/curroncol32120683





