High-Throughput 3D Bioprinted Organoids of Skin Cancer Utilized for Diagnosis and Personalized Therapy
Simple Summary
Abstract
1. Introduction
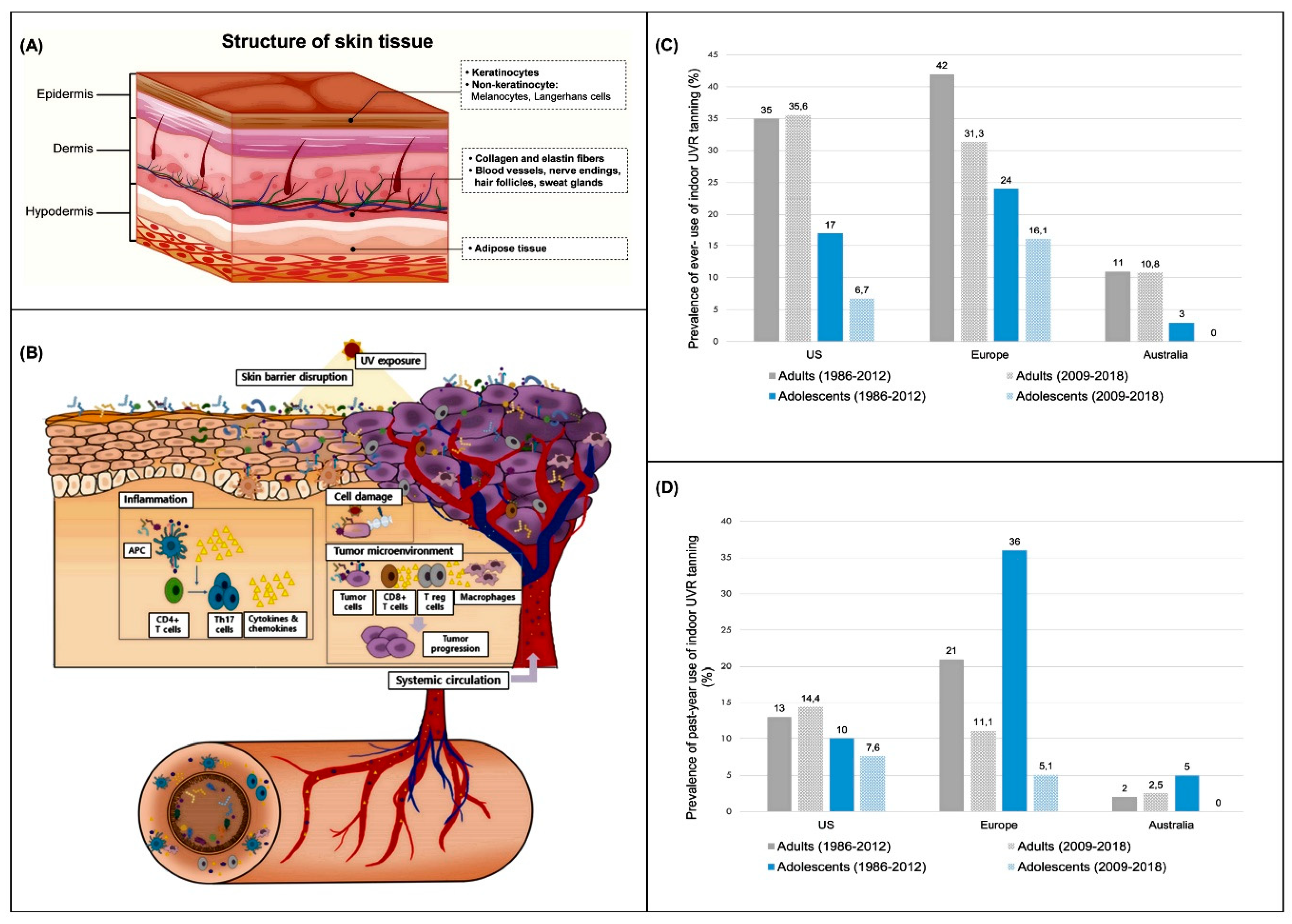
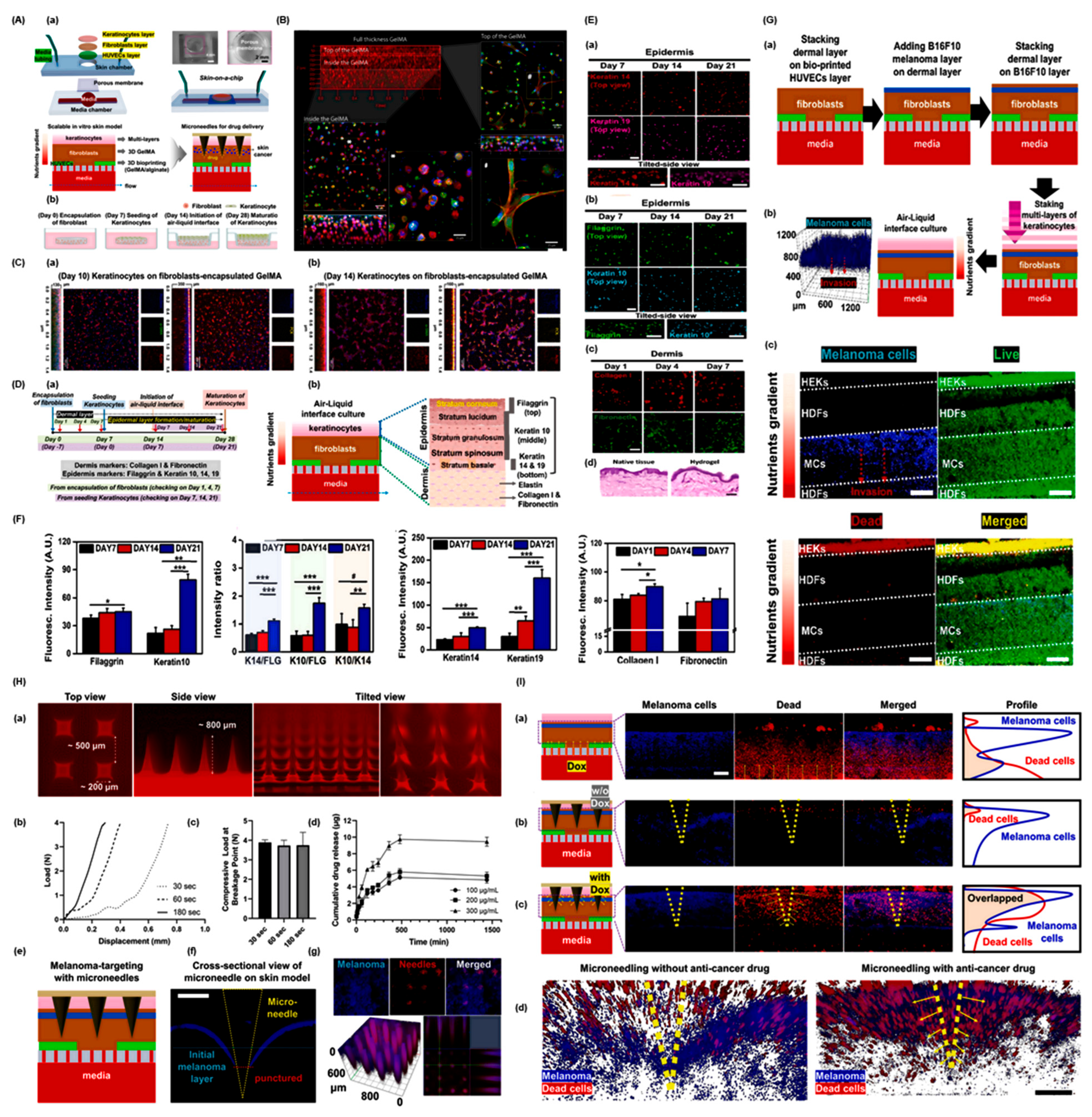
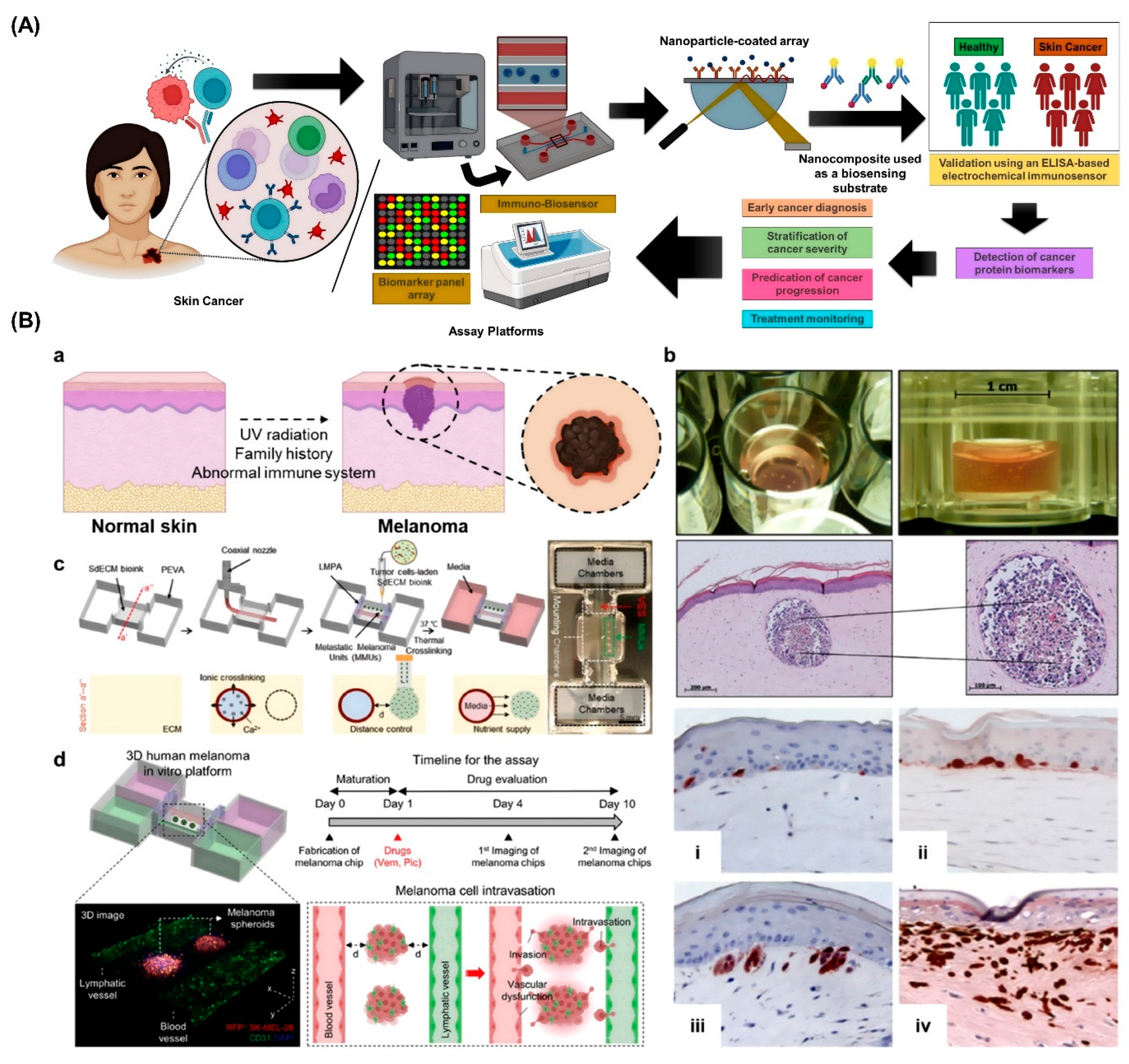
2. Advances in Skin Cancer Modeling
3. Bioprinting Technologies for Skin Cancer Organoids
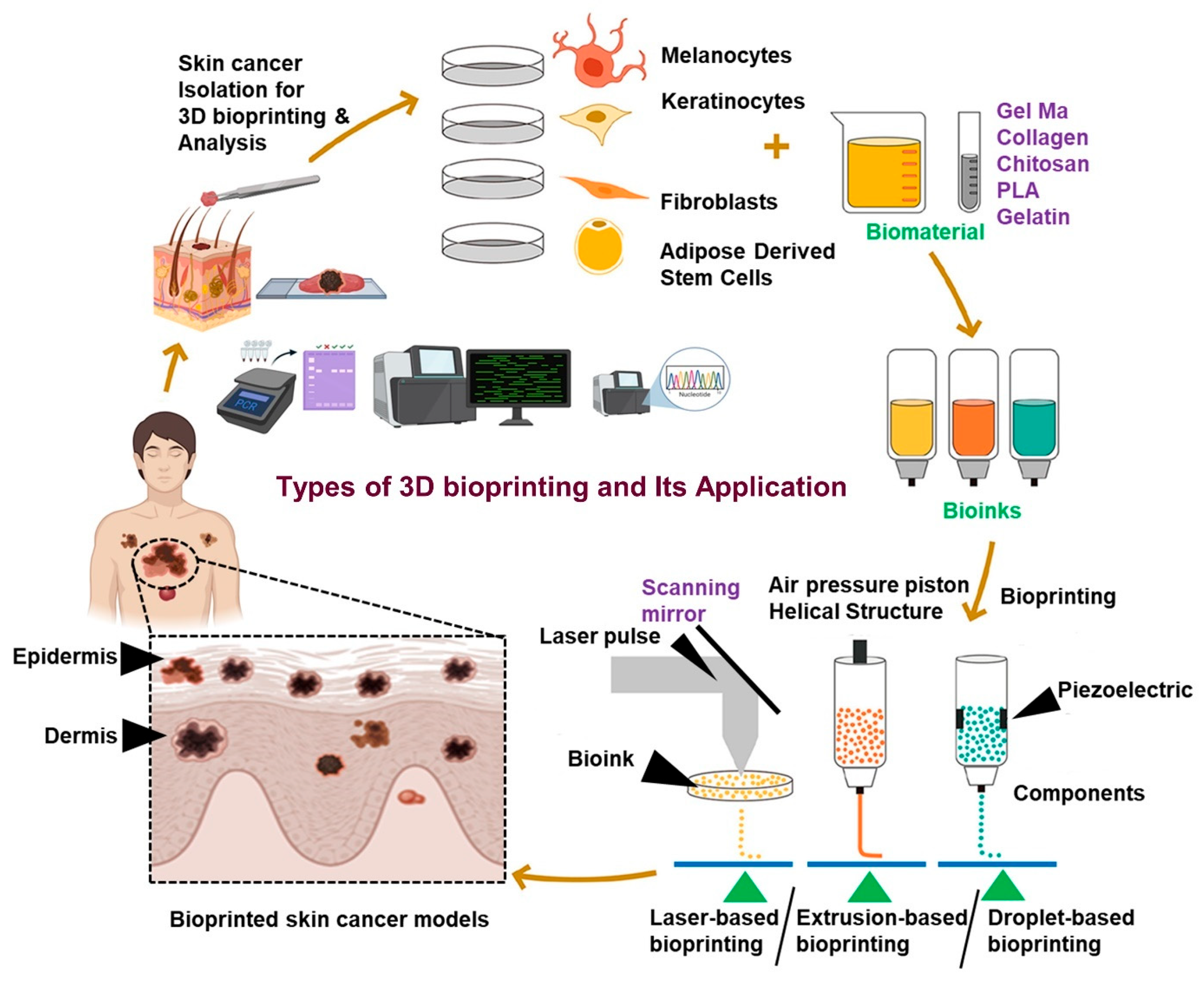
| Bioprinting Technique | Specific Bioinks Used | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Laser-based Bioprinting (e.g., Laser-Assisted, DLW, LIFT) |
|
|
| [91,92,93] |
| Extrusion-based Bioprinting |
|
|
| [94,95,96] |
| Droplet-based Bioprinting (e.g., Inkjet, Microvalve, EHD Jetting) |
|
|
| [97,98,99] |
| Platform | Advantages | Limitations | Translational Relevance | Ref. |
|---|---|---|---|---|
| Scaffold-based 3D Bioprinting | Provides structural support for complex tissue architecture; allows precise spatial positioning of multiple cell types; compatible with various biomaterials | Limited by potential cytotoxicity of crosslinkers; may not fully mimic native tissue stiffness or ECM dynamics | Useful for modeling tumor-stroma interactions and testing drug penetration in structured tissue environments | [81,100,101,102] |
| Scaffold-free Bioprinting/Spheroid-based | Cells self-assemble into microtissues/organoids; avoids biomaterial-induced artifacts; closely mimics native cell–cell interactions | Challenges in maintaining long-term structural integrity; limited scalability | Effective for high-throughput drug screening and patient-specific organoid generation | [103,104] |
| Microfluidic-integrated Bioprinting | Enables perfusion, nutrient delivery, and vascularization; allows dynamic modeling of tumor microenvironment | Complex fabrication; requires specialized equipment; challenging to standardize | Supports functional assays of tumor growth, angiogenesis, and drug response under physiologically relevant flow | [105,106] |
4. High-Throughput Strategies for Organoid Fabrication
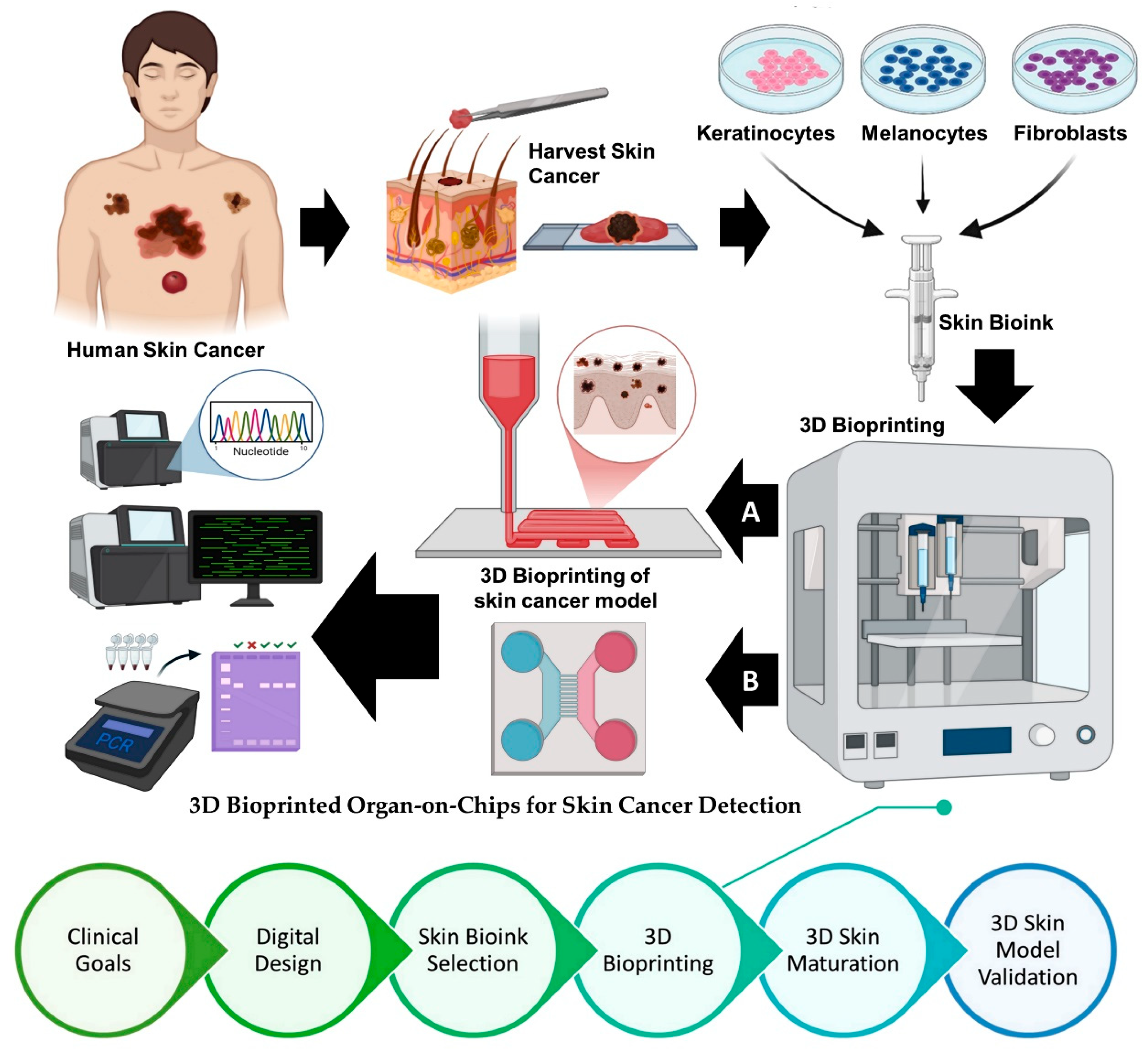
5. 3D Bioprinted Organ-on-Chips for Skin Cancer Detection: A Converging Platform for Precision Diagnostics
6. Applications in Diagnosis and Personalized Therapy
7. Future Perspectives
| Model Type | Method | Cell Composition | Matrix Used | Purpose | Limitations | Ref. |
|---|---|---|---|---|---|---|
| Spherical Melanoma Organoids | Co-culture with fibroblasts | Human skin fibroblasts; melanoma cell lines (WM1366/1205Lu) | Bovine Collagen I | Explore stromal influence on tumor development and resistance | Limited to a single healthy cell type; lacks layered skin structure | [156] |
| Co-culture with endothelial cells | HUVECs; melanoma cell lines (A375/M21) | - | Investigate tumor angiogenesis | Absence of healthy skin cells; non-functional capillary networks | [157] | |
| Three-cell model | Fibroblasts (CCD-1137Sk), keratinocytes (HaCaT), melanoma cells (SK-MEL-28) | Endogenous Collagen IV | Model early-stage melanoma, assess chemotherapy response | No cornified epidermal layer formed | [158] | |
| Five-cell model | Primary fibroblasts, keratinocytes, melanocytes, adipocytes; melanoma cells (SK-MEL-28) | - | Tumor-stroma crosstalk in melanoma | Does not replicate melanoma penetration | Unpublished | |
| Immune-Competent Melanoma Organoids | Air-liquid interface culture | Stromal and immune cells from tumor biopsies | Type I Collagen | Personalized immunotherapy | No healthy skin or epidermal stratification | [159,160,161] |
| Combined lymph node model | Melanoma tissue; lymph node-derived immune cells | Hyaluronic acid/Collagen hydrogel | Personalized treatment screening | Few patient samples; lacks full skin context | [162] | |
| Autologous lymphocyte co-culture | Melanoma tissue; peripheral lymphocytes | Matrigel | Candidate selection for immunotherapy | Limited patient scope; lacks full skin structure | [163] | |
| Melanoma on Planar Skin Constructs | Spheroid/Cell injection | Keratinocytes, fibroblasts; melanoma cell lines (WM35, SK-MEL-28, SBCL2, etc.) | DED, Collagen I, Alvetex scaffold | Study melanoma invasion and drug responses | Missing cell types like melanocytes, vasculature | [164,165,166] |
| Vascularized melanoma model | HMVECs, keratinocytes, fibroblasts; melanoma lines | - | Drug screening in vascularized environment | Time-consuming; low throughput | [167] | |
| Immunocompetent Planar Models | Activated immune cell addition | Keratinocytes; CD4+ T cells or Langerhans cells | DED, Collagen I | Psoriasis, allergy, drug testing | Donor mismatch and limited skin cell diversity | [168,169,170,171] |
| Bioprinted with macrophages | Keratinocytes, fibroblasts, macrophages (M1/M2) | Custom bioink with nanofibrillated cellulose, fibrinogen, etc. | Chronic inflammation (e.g., atopic dermatitis) | Lacks melanocytes, vasculature | [172] | |
| Bioprinted wound models | Keratinocytes, fibroblasts, HUVECs, macrophages | Collagen I + plasma-based fibrin bioink | Wound healing and inflammation | Missing melanocytes | [173] | |
| Immune-Competent Skin Constructs with Melanoma | Co-culture with melanoma and immune cells | Keratinocytes, fibroblasts, melanocytes, melanoma cells, dendritic or T cells | Collagen I, DED | Tumor-immune interaction, progression, and immunotherapy | No vasculature; no leukocyte extravasation | [174,175,176,177] |
| Melanoma-on-a-Chip Systems | Microfluidic integration | Keratinocytes, fibroblasts, melanoma cells (WM-115) | Collagen | Cell-cell crosstalk studies | No stratified skin architecture | [178] |
| Skin-on-chip with immune components | HaCaT, U937 or HL-60 cells, HUVECs | Collagen I | Allergy and immune response modeling | Lacks full dermal/immune composition | [179,180,181] | |
| Melanoma-immune chip systems | Melanoma spheroids, immune cells from biopsy | Collagen I | Immunotherapy and drug screening | No healthy skin structure; immune cell recruitment limited | [182,183] | |
| Vascularized chip with immune cells | HUVECs, melanoma cells (BLM), whole blood | Gelatin | Inflammation modeling | No skin layers included | [184] | |
| Circulating melanoma-neutrophil interactions | Melanoma A-375/A-375 MA2, neutrophils | Fibrin | Tumor cell extravasation, metastasis | Not representative of skin architecture | [185] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roky, A.H.; Islam, M.M.; Ahasan, A.M.F.; Mostaq, M.S.; Mahmud, M.Z.; Amin, M.N.; Mahmud, M.A. Overview of Skin Cancer Types and Prevalence Rates across Continents. Cancer Pathog. Ther. 2025, 3, 89–100. [Google Scholar] [CrossRef]
- Hasan, N.; Nadaf, A.; Imran, M.; Jiba, U.; Sheikh, A.; Almalki, W.H.; Almujri, S.S.; Mohammed, Y.H.; Kesharwani, P.; Ahmad, F.J. Skin Cancer: Understanding the Journey of Transformation from Conventional to Advanced Treatment Approaches. Mol. Cancer 2023, 22, 168. [Google Scholar] [CrossRef] [PubMed]
- Khayyati Kohnehshahri, M.; Sarkesh, A.; Mohamed Khosroshahi, L.; HajiEsmailPoor, Z.; Aghebati-Maleki, A.; Yousefi, M.; Aghebati-Maleki, L. Current Status of Skin Cancers with a Focus on Immunology and Immunotherapy. Cancer Cell Int. 2023, 23, 174. [Google Scholar] [CrossRef] [PubMed]
- Sorino, C.; Iezzi, S.; Ciuffreda, L.; Falcone, I. Immunotherapy in Melanoma: Advances, Pitfalls, and Future Perspectives. Front. Mol. Biosci. 2024, 11, 1403021. [Google Scholar] [CrossRef]
- He, G.; Li, Y.; Zeng, Y.; Zhang, Y.; Jiang, Q.; Zhang, Q.; Zhu, J.; Gong, J. Advancements in Melanoma Immunotherapy: The Emergence of Extracellular Vesicle Vaccines. Cell Death Discov. 2024, 10, 374. [Google Scholar] [CrossRef]
- Kolathur, K.K.; Nag, R.; Shenoy, P.V.; Malik, Y.; Varanasi, S.M.; Angom, R.S.; Mukhopadhyay, D. Molecular Susceptibility and Treatment Challenges in Melanoma. Cells 2024, 13, 1383. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Peng, S.; Liu, Q.; Chen, D.; He, Z.; Xiang, J.; Wang, B. Tumor Microenvironment: Nurturing Cancer Cells for Immunoevasion and Druggable Vulnerabilities for Cancer Immunotherapy. Cancer Lett. 2025, 611, 217385. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Hu, Q.; Zhu, Y.; Mei, J.; Liu, Y.; Zhou, G. Extracellular Matrix Dynamics in Tumor Immunoregulation: From Tumor Microenvironment to Immunotherapy. J. Hematol. Oncol. 2025, 18, 65. [Google Scholar] [CrossRef]
- Almawash, S. Revolutionary Cancer Therapy for Personalization and Improved Efficacy: Strategies to Overcome Resistance to Immune Checkpoint Inhibitor Therapy. Cancers 2025, 17, 880. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.K.; Ciążyńska, M.; Sulejczak, D.; Rutkowski, P.; Czarnecka, A.M. Mechanisms of Resistance to Anti-Pd-1 Immunotherapy in Melanoma and Strategies to Overcome It. Biomolecules 2025, 15, 269. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Zappasodi, R. A decade of Checkpoint Blockade Immunotherapy in Melanoma: Understanding the Molecular Basis for Immune Sensitivity and Resistance. Nat. Immunol. 2022, 23, 660–670. [Google Scholar] [CrossRef]
- Shukla, A.K.; Gao, G.; Kim, B.S. Applications of 3D Bioprinting Technology in Induced Pluripotent Stem Cells-Based Tissue Engineering. Micromachines 2022, 13, 155. [Google Scholar] [CrossRef]
- Shukla, A.K.; Ahn, M.J.; Lee, J.S.; Kim, B.S. In-Bath Bioprinting of Pre-Vascularized Skin Patches with Different Geometrical Patterns for Effective Skin Regeneration; Nurimedia (DBpia), 2023; Available online: https://www.dbpia.co.kr/Journal/articleDetail?nodeId=NODE11466839 (accessed on 3 November 2025).
- Wang, X.-Y.; Jia, Q.-N.; Li, J.; Zheng, H.-Y. Organoids as Tools for Investigating Skin Aging: Mechanisms, Applications, and Insights. Biomolecules 2024, 14, 1436. [Google Scholar] [CrossRef]
- Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. The Human Microbiota and Skin Cancer. Int. J. Mol. Sci. 2022, 23, 1813. [Google Scholar] [CrossRef]
- Dessinioti, C.; Stratigos, A.J. An Epidemiological Update on Indoor Tanning and the Risk of Skin Cancers. Curr. Oncol. 2022, 29, 8886–8903. [Google Scholar] [CrossRef]
- Wehner, M.R.; Chren, M.-M.; Nameth, D.; Choudhry, A.; Gaskins, M.; Nead, K.T.; Boscardin, W.J.; Linos, E. International Prevalence of Indoor Tanning: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2014, 150, 390–400. [Google Scholar] [CrossRef]
- Rodriguez-Acevedo, A.J.; Green, A.C.; Sinclair, C.; van Deventer, E.; Gordon, L.G. Indoor Tanning Prevalence after the International Agency for Research on Cancer Statement on Carcinogenicity of Artificial Tanning Devices: Systematic Review and Meta-Analysis. Br. J. Dermatol. 2020, 182, 849–859. [Google Scholar] [CrossRef]
- Ahn, M.; Shukla, A.; Cho, W.-W.; Cho, D.-W.; Kim, B. A Study on the Effect of Bioprinted Skin Patches with Various Vascular Patterns on Wound Healing; Nurimedia (DBpia), 2024; Available online: https://www.dbpia.co.kr/Journal/articleDetail?nodeId=NODE11798258 (accessed on 3 November 2025).
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Arora, S.; Singh, S.; Mittal, A.; Desai, N.; Khatri, D.K.; Gugulothu, D.; Lather, V.; Pandita, D.; Vora, L.K. Spheroids in Cancer Research: Recent Advances and Opportunities. J. Drug Deliv. Sci. Technol. 2024, 100, 106033. [Google Scholar] [CrossRef]
- Pipiya, V.V.; Gilazieva, Z.E.; Issa, S.S.; Rizvanov, A.A.; Solovyeva, V.V. Comparison of Primary and Passaged Tumor Cell Cultures and Their Application in Personalized Medicine. Explor. Target. Antitumor Ther. 2024, 5, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wen, J.; Yang, J.; Zhou, S.; Li, Y.; Xu, K.; Li, W.; Li, S. Tumor-Microenvironment-on-a-Chip: The Construction and Application. Cell Commun. Signal. 2024, 22, 515. [Google Scholar] [CrossRef]
- Mun, S.; Lee, H.J.; Kim, P. Rebuilding the Microenvironment of Primary Tumors in Humans: A Focus on Stroma. Exp. Mol. Med. 2024, 56, 527–548. [Google Scholar] [CrossRef]
- Dabaliz, A.; Al Hakawati, M.N.; Alrashdan, N.; Alrashdan, S.; Bakir, M.; Mohammad, K.S. Adipocyte–Tumor Interactions in the Bone Marrow Niche: Implications for Metastasis and Therapy. Int. J. Mol. Sci. 2025, 26, 9781. [Google Scholar] [CrossRef]
- Tan, L.; Huang, D.; Ge, H.; Fan, R.; Wei, X.; Feng, X.; Xu, C.; Zhou, W.; Qi, H. Safety Assessment of Drugs in Pregnancy: An Update of Pharmacological Models. Placenta 2025. [Google Scholar] [CrossRef]
- Budharaju, H.; Singh, R.K.; Kim, H.-W. Bioprinting for Drug Screening: A Path toward Reducing Animal Testing or Redefining Preclinical Research? Bioact. Mater. 2025, 51, 993–1017. [Google Scholar] [CrossRef]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent Progress in Translational Engineered in Vitro Models of the Central Nervous System. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef]
- Khalil, A.S.; Jaenisch, R.; Mooney, D.J. Engineered Tissues and Strategies to Overcome Challenges in Drug Development. Adv. Drug Deliv. Rev. 2020, 158, 116–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, P. Advances and Challenges in 3D Bioprinted cancer Models: Opportunities for Personalized Medicine and Tissue Engineering. Polymers 2025, 17, 948. [Google Scholar] [CrossRef]
- Shukla, A.K.; Yoon, S.; Oh, S.-O.; Lee, D.; Ahn, M.; Kim, B.S. Advancement in Cancer Vasculogenesis Modeling through 3D Bioprinting Technology. Biomimetics 2024, 9, 306. [Google Scholar] [CrossRef]
- Shukla, A.K. A Study on Angiogenic Potentials of Endothelial Cell-Patterned Skin Patches Fabricated by in-Bath 3D Bioprinting with Light-Activated Bioink for Improved Wound Healing. Available online: https://www.researchgate.net/profile/Arvind-Kumar-Shukla/publication/395494222_A_Study_on_Angiogenic_Potentials_of_Endothelial_Cell-Patterned_Skin_Patches_Fabricated_by_In-Bath_3D_Bioprinting_with_Light-ActivatedBioink_for_Improved_Wound_Healing/links/68c85f85f3032e2b4be1416c/A-Study-on-Angiogenic-Potentials-of-Endothelial-Cell-Patterned-Skin-Patches-Fabricated-by-In-Bath-3D-Bioprinting-with-Light-ActivatedBioink-for-Improved-Wound-Healing.pdf (accessed on 22 August 2025).
- Shukla, A.K.; Ahn, M.; Gao, J.; Lee, D.; Yoon, S.; Oh, S.-O.; Gao, G.; Cho, W.-W.; Kim, B.S. Exploring the Angiogenic Potential of Skin Patches with Endothelial Cell Patterns Fabricated via in-Bath 3D Bioprinting Using Light-Activated Bioink for Enhanced Wound Healing. Biomaterials 2025, 325, 123575. [Google Scholar] [CrossRef]
- Shukla, A.K.; Shukla, S.; Dutta, S.D.; Mongre, R.K. High-Throughput 3D Bioprinted Organoids of Skin Cancer Utilized for Diagnosis and Personalized Therapy. 2025. Available online: https://www.preprints.org/manuscript/202509.1577 (accessed on 18 September 2025).
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Mirshafiei, M.; Rashedi, H.; Yazdian, F.; Rahdar, A.; Baino, F. Advancements in Tissue and Organ 3D Bioprinting: Current Techniques, Applications, and Future Perspectives. Mater. Des. 2024, 240, 112853. [Google Scholar] [CrossRef]
- Ramadan, Q.; Zourob, M. 3D Bioprinting at the Frontier of Regenerative Medicine, Pharmaceutical, and Food Industries. Front. Med. Technol. 2020, 2, 607648. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Santos, J.D.; Atayde, L.; Alves, N.; Maurício, A.C. Three-Dimensional Printing/Bioprinting and Cellular Therapies for Regenerative Medicine: Current Advances. J. Funct. Biomater. 2025, 16, 28. [Google Scholar]
- Li, W.; Zhou, Z.; Zhou, X.; Khoo, B.L.; Gunawan, R.; Chin, Y.R.; Zhang, L.; Yi, C.; Guan, X.; Yang, M. 3D Biomimetic Models to Reconstitute Tumor Microenvironment in Vitro: Spheroids, Organoids, and Tumor-on-a-Chip. Adv. Health Mater. 2023, 12, e2202609. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, R.; Song, Y.; Wei, W.; Baek, D.; Gillin, M.; Kurabayashi, K.; Chen, W. Cancer-on-a-Chip for Precision Cancer Medicine. Lab Chip 2025, 25, 3314–3347. [Google Scholar]
- Cristini, N.; Tavakoli, M.; Sanati, M.; Yavari, S.A. Exploring Bone-Tumor Interactions through 3D in Vitro Models: Implications for Primary and Metastatic Cancers. J. Bone Oncol. 2025, 53, 100698. [Google Scholar] [CrossRef]
- Shukla, A.K.; Lee, D.; Yoon, S.; Ahn, M.; Kim, B.S. Vascularization Strategies for Human Skin Tissue Engineering via 3D Bioprinting. Int. J. Bioprinting 2024, 10, 1727. [Google Scholar] [CrossRef]
- Zhou, Z.; Cong, L.; Cong, X. Patient-Derived Organoids in Precision Medicine: Drug Screening, Organoid-on-a-Chip and Living Organoid Biobank. Front. Oncol. 2021, 11, 762184. [Google Scholar] [CrossRef]
- Jung, M.; Ghamrawi, S.; Du, E.Y.; Gooding, J.J.; Kavallaris, M. Advances in 3D Bioprinting for Cancer Biology and Precision Medicine: From Matrix Design to Application. Adv. Health Mater. 2022, 11, e2200690. [Google Scholar] [CrossRef]
- Puce, A.; Ferraresi, V.; Biagini, R.; Soddu, S.; Loria, R. Three-Dimensional Preclinical Models for Osteosarcoma: Advances and Translational Prospects. Biomed. Pharmacother. 2025, 191, 118471. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; D’Souza, G.G.M. Modeling Tumor Microenvironment Complexity in Vitro: Spheroids as Physiologically Relevant Tumor Models and Strategies for Their Analysis. Cells 2025, 14, 732. [Google Scholar] [CrossRef]
- Zambrano-Román, M.; Padilla-Gutiérrez, J.R.; Valle, Y.; Muñoz-Valle, J.F.; Valdés-Alvarado, E. Non-Melanoma Skin Cancer: A Genetic Update and Future Perspectives. Cancers 2022, 14, 2371. [Google Scholar] [CrossRef]
- Zhou, L.; Zhong, Y.; Han, L.; Xie, Y.; Wan, M. Global, Regional, and National Trends in the Burden of Melanoma and Non-Melanoma Skin Cancer: Insights from the Global Burden of Disease Study 1990–2021. Sci. Rep. 2025, 15, 5996. [Google Scholar] [CrossRef]
- Lang, C.M.R.; Chan, C.K.; Veltri, A.; Lien, W.H. Wnt Signaling Pathways in Keratinocyte Carcinomas. Cancers 2019, 11, 1216. [Google Scholar] [CrossRef]
- Riihilä, P.; Nissinen, L.; Kähäri, V.M. Matrix Metalloproteinases in Keratinocyte Carcinomas. Exp. Dermatol. 2021, 30, 50–61. [Google Scholar] [CrossRef]
- Dainese-Marque, O.; Garcia, V.; Andrieu-Abadie, N.; Riond, J. Contribution of Keratinocytes in Skin Cancer Initiation and Progression. Int. J. Mol. Sci. 2024, 25, 8813. [Google Scholar] [CrossRef]
- Arumugam, P.; Kaarthikeyan, G.; Eswaramoorthy, R. Three-Dimensional Bioprinting: The Ultimate Pinnacle of Tissue Engineering. Cureus 2024, 16, e58029. [Google Scholar] [CrossRef]
- Li, K.; He, Y.; Jin, X.; Jin, K.; Qian, J. Reproducible Extracellular Matrices for Tumor Organoid Culture: Challenges and Opportunities. J. Transl. Med. 2025, 23, 497. [Google Scholar] [CrossRef]
- Elango, J.; Zamora-Ledezma, C. Rheological, Structural, and Biological Trade-Offs in Bioink Design for 3D Bioprinting. Gels 2025, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D Bioprinting and Its Innovative Approach for Biomedical Applications. MedComm 2023, 4, e194. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D Bioprinting of Functional Tissue Models for Personalized Drug Screening and in Vitro Disease Modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Manduca, N.; Maccafeo, E.; De Maria, R.; Sistigu, A.; Musella, M. 3D Cancer Models: One Step Closer to in Vitro Human Studies. Front. Immunol. 2023, 14, 1175503. [Google Scholar] [CrossRef] [PubMed]
- Chaves, P.; Garrido, M.; Oliver, J.; Pérez-Ruiz, E.; Barragan, I.; Rueda-Domínguez, A. Preclinical Models in Head and Neck Squamous Cell Carcinoma. Br. J. Cancer 2023, 128, 1819–1827. [Google Scholar] [CrossRef]
- Ionita, I.; Malita, D.; Dehelean, C.; Olteanu, E.; Marcovici, I.; Geamantan, A.; Chiriac, S.; Roman, A.; Radu, D. Experimental Models for Rare Melanoma Research—The Niche That Needs to Be Addressed. Bioengineering 2023, 10, 673. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Wang, H.; Cheng, Y.; Fang, Y.; Wu, F.; Sun, G.; Sun, G.; Lv, C.; Hui, B. Application of Animal Models in Cancer Research: Recent Progress and Future Prospects. Cancer Manag. Res. 2021, 13, 2455–2475. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, Y.; Liu, S.; Yang, M. In Vitro and in Vivo Experimental Models for Cancer Immunotherapy Study. Curr. Res. Biotechnol. 2024, 7, 100210. [Google Scholar] [CrossRef]
- Wakefield, L.; Agarwal, S.; Tanner, K. Preclinical Models for Drug Discovery for Metastatic Disease. Cell 2023, 186, 1792–1813. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Cai, C.; Zhang, H.; Shen, H.; Han, Y. Patient-Derived Xenograft Models in Cancer Therapy: Technologies and Applications. Signal. Transduct. Target. Ther. 2023, 8, 160. [Google Scholar] [CrossRef]
- Jin, J.; Yoshimura, K.; Sewastjanow-Silva, M.; Song, S.; Ajani, J.A. Challenges and Prospects of Patient-Derived Xenografts for Cancer Research. Cancers 2023, 15, 4352. [Google Scholar] [CrossRef]
- Moro, M.; Bertolini, G.; Tortoreto, M.; Pastorino, U.; Sozzi, G.; Roz, L. Patient-Derived Xenografts of Non Small Cell Lung Cancer: Resurgence of an Old Model for Investigation of Modern Concepts of Tailored Therapy and Cancer Stem Cells. BioMed Res. Int. 2012, 2012, 568567. [Google Scholar] [CrossRef] [PubMed]
- Rafik, S.T.; Bakkalci, D.; MacRobert, A.J.; Cheema, U. Bioengineering Facets of the Tumor Microenvironment in 3D Tumor Models: Insights into Cellular, Biophysical and Biochemical Interactions. FEBS Open Bio 2025, 15, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Duchamp, M.; Oklu, R.; Ellisen, L.W.; Langer, R.; Khademhosseini, A. Bioprinting the Cancer Microenvironment. ACS Biomater. Sci. Eng. 2016, 2, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Biray Avci, C.; Goker Bagca, B.; Nikanfar, M.; Takanlou, L.S.; Takanlou, M.S.; Nourazarian, A. Tumor Microenvironment and Cancer Metastasis: Molecular Mechanisms and Therapeutic Implications. Front. Pharmacol. 2024, 15, 1442888. [Google Scholar] [CrossRef]
- Wright, K.; Ly, T.; Kriet, M.; Czirok, A.; Thomas, S.M. Cancer-Associated Fibroblasts: Master Tumor Microenvironment Modifiers. Cancers 2023, 15, 1899. [Google Scholar] [CrossRef]
- Lv, K.; He, T. Cancer-Associated Fibroblasts: Heterogeneity, Tumorigenicity and Therapeutic Targets. Mol. Biomed. 2024, 5, 70. [Google Scholar] [CrossRef]
- Finger, A.-M.; Hendley, A.M.; Figueroa, D.; Gonzalez, H.; Weaver, V.M. Tissue Mechanics in Tumor Heterogeneity and Aggression. Trends Cancer 2025, 11, 806–824. [Google Scholar] [CrossRef]
- Fiori, M.E.; Di Franco, S.; Villanova, L.; Bianca, P.; Stassi, G.; De Maria, R. Cancer-Associated Fibroblasts as Abettors of Tumor Progression at the Crossroads of Emt and Therapy Resistance. Mol. Cancer 2019, 18, 70. [Google Scholar] [CrossRef]
- Ahn, M.; Park, G.T.; Shukla, A.K.; Kwon, B.; Kim, J.H.; Sung, E.S.; Kim, B.S. 3D Bioprinting-Assisted Engineering of Stem Cell-Laden Hybrid Biopatches with Distinct Geometric Patterns Considering the Mechanical Characteristics of Regular and Irregular Connective Tissues. Adv. Healthc. Mater. 2025, 14, 2502763. [Google Scholar] [CrossRef]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted Cancer Models: Revolutionizing Personalized Cancer Therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef] [PubMed]
- Mazzaglia, C.; Shery Huang, Y.Y.; Shields, J.D. Advancing Tumor Microenvironment and Lymphoid Tissue Research through 3D Bioprinting and Biofabrication. Adv. Drug Deliv. Rev. 2025, 217, 115485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, C.; Li, Z.; Fu, X.; Huang, S. Advances in 3D Skin Bioprinting for Wound Healing and Disease Modeling. Regen. Biomater. 2023, 10, rbac105. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D Bioprinting: From Printing Methods to Biomedical Applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Budharaju, H.; Sundaramurthi, D.; Sethuraman, S. Embedded 3D Bioprinting—An Emerging Strategy to Fabricate Biomimetic & Large Vascularized Tissue Constructs. Bioact. Mater. 2024, 32, 356–384. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Fernandes, S.; Vyas, C.; Lim, P.; Pereira, R.F.; Virós, A.; Bártolo, P. 3D Bioprinting: An Enabling Technology to Understand Melanoma. Cancers 2022, 14, 3535. [Google Scholar] [CrossRef]
- Parodi, I.; Di Lisa, D.; Pastorino, L.; Scaglione, S.; Fato, M.M. 3D Bioprinting as a Powerful Technique for Recreating the Tumor Microenvironment. Gels 2023, 9, 482. [Google Scholar] [CrossRef]
- Lee, J.; Koehler, K.R. Skin Organoids: A New Human Model for Developmental and Translational Research. Exp. Dermatol. 2021, 30, 613–620. [Google Scholar] [CrossRef]
- Teertam, S.K.; Setaluri, V.; Ayuso, J.M. Advances in Microengineered Platforms for Skin Research. JID Innov. 2025, 5, 100315. [Google Scholar] [CrossRef]
- Hong, Z.X.; Zhu, S.T.; Li, H.; Luo, J.Z.; Yang, Y.; An, Y.; Wang, X.; Wang, K. Bioengineered Skin Organoids: From Development to Applications. Mil. Med. Res. 2023, 10, 40. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Ma, Y.; Wang, P.; Yao, R. Converging Bioprinting and Organoids to Better Recapitulate the Tumor Microenvironment. Trends Biotechnol. 2024, 42, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.G.; Choi, Y.M.; Jang, J. 3D Bioprinting-Based Vascularized Tissue Models Mimicking Tissue-Specific Architecture and Pathophysiology for in Vitro Studies. Front. Bioeng. Biotechnol. 2021, 9, 685507. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Chiang, Y.H.; Fernanda, M.S.; He, M. Using Spheroids as Building Blocks Towards 3D Bioprinting of Tumor Microenvironment. Int. J. Bioprint. 2021, 7, 444. [Google Scholar] [CrossRef]
- Fang, W.; Yang, M.; Wang, L.; Li, W.; Liu, M.; Jin, Y.; Wang, Y.; Yang, R.; Wang, Y.; Zhang, K.; et al. Hydrogels for 3D Bioprinting in Tissue Engineering and Regenerative Medicine: Current Progress and Challenges. Int. J. Bioprint. 2023, 9, 759. [Google Scholar] [CrossRef]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Mesbah Oskui, S.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective Bioprinting Resolution in Tissue Model Fabrication. Lab Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; Araujo, W.A.; Zorlutuna, P.; Vrana, N.E.; Ghaemmaghami, A.M.; Dokmeci, M.R.; et al. Direct-Write Bioprinting of Cell-Laden Methacrylated Gelatin Hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef]
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef]
- Asim, S.; Tabish, T.A.; Liaqat, U.; Ozbolat, I.T.; Rizwan, M. Advances in Gelatin Bioinks to Optimize Bioprinted Cell Functions. Adv. Health Mater. 2023, 12, e2203148. [Google Scholar] [CrossRef] [PubMed]
- Benwood, C.; Chrenek, J.; Kirsch, R.L.; Masri, N.Z.; Richards, H.; Teetzen, K.; Willerth, S.M. Natural Biomaterials and Their Use as Bioinks for Printing Tissues. Bioengineering 2021, 8, 27. [Google Scholar] [CrossRef]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprint. 2020, 6, 270. [Google Scholar] [CrossRef]
- Dell, A.C.; Wagner, G.; Own, J.; Geibel, J.P. 3D Bioprinting Using Hydrogels: Cell Inks and Tissue Engineering Applications. Pharmaceutics 2022, 14, 2596. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.; Agarwal, P.; Kasturi, M.; Srinivasan, V.; Seetharam, R.N.; Vasanthan, K.S. Innovative Bioinks for 3D Bioprinting: Exploring Technological Potential and Regulatory Challenges. J. Tissue Eng. 2025, 16. [Google Scholar] [CrossRef]
- Zoghi, S. Advancements in Tissue Engineering: A Review of Bioprinting Techniques, Scaffolds, and Bioinks. Biomed. Eng. Comput. Biol. 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-of-the-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.; Huang, Y.; Cheng, L.; Gu, Z. Applications and Recent Advances in 3D Bioprinting Sustainable Scaffolding Techniques. Molecules 2025, 30, 3027. [Google Scholar] [CrossRef]
- Murata, D.; Arai, K.; Nakayama, K. Scaffold-Free Bio-3D Printing Using Spheroids as “Bio-Inks” for Tissue (Re-)Construction and Drug Response Tests. Adv. Health Mater. 2020, 9, e1901831. [Google Scholar] [CrossRef]
- Liu, K.C.; Chen, Y.C.; Hsieh, C.F.; Wang, M.H.; Zhong, M.X.; Cheng, N.C. Scaffold-Free 3D Culture Systems for Stem Cell-Based Tissue Regeneration. APL Bioeng. 2024, 8, 041501. [Google Scholar] [CrossRef]
- Fang, L.; Liu, Y.; Qiu, J.; Wan, W. Bioprinting and its Use in Tumor-on-a-Chip Technology for Cancer Drug Screening: A Review. Int. J. Bioprint. 2022, 8, 603. [Google Scholar] [CrossRef]
- Ying-Jin, S.; Yuste, I.; González-Burgos, E.; Serrano, D.R. Fabrication of Organ-on-a-Chip Using Microfluidics. Bioprinting 2025, 46, e00394. [Google Scholar] [CrossRef]
- Zuo, J.; Fang, Y.; Wang, R.; Liang, S. High-Throughput Solutions in Tumor Organoids: From Culture to Drug Screening. Stem Cells 2025, 43, sxae070. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Jin, A.; Jiang, S.; Lei, L.; Liu, L. Biomaterial-Assisted Organoid Technology for Disease Modeling and Drug Screening. Mater. Today Bio 2025, 30, 101438. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Du, X.; Wang, M.; Su, J.; Wei, Y.; Xu, C. Construction of Tumor Organoids and Their Application to Cancer Research and Therapy. Theranostics 2024, 14, 1101–1125. [Google Scholar] [CrossRef]
- Dai, R.; Chen, W.; Chen, Y.; Jin, J.; Zhang, S.; Zhang, C.; Liu, J. 3D Bioprinting Platform Development for High-Throughput Cancer Organoid Models Construction and Drug Evaluation. Biofabrication 2024, 16, 035026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, X.; Fang, Y.; Xiong, Z.; Zhang, T. AI-Driven 3D Bioprinting for Regenerative Medicine: From Bench to Bedside. Bioact. Mater. 2025, 45, 201–230. [Google Scholar] [CrossRef]
- Tebon, P.J.; Wang, B.; Markowitz, A.L.; Davarifar, A.; Tsai, B.L.; Krawczuk, P.; Gonzalez, A.E.; Sartini, S.; Murray, G.F.; Nguyen, H.T.L.; et al. Drug Screening at Single-Organoid Resolution via Bioprinting and Interferometry. Nat. Commun. 2023, 14, 3168. [Google Scholar] [CrossRef]
- Morais, A.S.; Mendes, M.; Cordeiro, M.A.; Sousa, J.J.; Pais, A.C.; Mihăilă, S.M.; Vitorino, C. Organ-on-a-Chip: Ubi Sumus? Fundamentals and Design Aspects. Pharmaceutics 2024, 16, 615. [Google Scholar] [CrossRef]
- Ahmed, T. Organ-on-a-Chip Microengineering for Bio-Mimicking Disease Models and Revolutionizing Drug Discovery. Biosens. Bioelectron. X 2022, 11, 100194. [Google Scholar] [CrossRef]
- Regmi, S.; Poudel, C.; Adhikari, R.; Luo, K.Q. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors 2022, 12, 459. [Google Scholar] [CrossRef]
- Mierke, C.T. Bioprinting of Cells, Organoids and Organs-on-a-Chip Together with Hydrogels Improves Structural and Mechanical Cues. Cells 2024, 13, 1638. [Google Scholar] [CrossRef]
- Shukla, S.; Ray, N.; Shukla, A.K.; Upadhyay, A.M.; Mirone, G.; Mongre, R.K. Emerging Molecular and Clinical Challenges in Managing Lung Cancer Treatment during the Covid-19 Infection. J. Cancer Tumor Int. 2024, 14, 143–161. [Google Scholar] [CrossRef]
- Hsiung, N.; Ju, Y.; Yang, K.; Yang, P.; Zeng, W.; Zhao, H.; Zou, P.; Ye, J.; Yi, K.; Wang, X. Organoid-Based Tissue Engineering for Advanced Tissue Repair and Reconstruction. Mater. Today Bio 2025, 33, 102093. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Wang, Z.; Khutsishvili, D.; Tang, J.; Zhu, Y.; Cai, Y.; Dai, X.; Ma, S. Bridging the Organoid Translational Gap: Integrating Standardization and Micropatterning for Drug Screening in Clinical and Pharmaceutical Medicine. Life Med. 2024, 3, lnae016. [Google Scholar] [CrossRef]
- Roberto de Barros, N.; Wang, C.; Maity, S.; Peirsman, A.; Nasiri, R.; Herland, A.; Ermis, M.; Kawakita, S.; Gregatti Carvalho, B.; Hosseinzadeh Kouchehbaghi, N.; et al. Engineered Organoids for Biomedical Applications. Adv. Drug Deliv. Rev. 2023, 203, 115142. [Google Scholar] [CrossRef]
- Kim, D.; Lim, H.; Youn, J.; Park, T.-E.; Kim, D.S. Scalable Production of Uniform and Mature Organoids in a 3D Geometrically-Engineered Permeable Membrane. Nat. Commun. 2024, 15, 9420. [Google Scholar] [CrossRef]
- Barros, N.R.; Kang, R.; Kim, J.; Ermis, M.; Kim, H.-J.; Dokmeci, M.R.; Lee, J. A Human Skin-on-a-Chip Platform for Microneedling-Driven Skin Cancer Treatment. Mater. Today Bio 2025, 30, 101399. [Google Scholar] [CrossRef]
- Hwangbo, H.; Chae, S.; Kim, W.; Jo, S.; Kim, G.H. Tumor-on-a-Chip Models Combined with Mini-Tissues or Organoids for Engineering Tumor Tissues. Theranostics 2024, 14, 33–55. [Google Scholar] [CrossRef]
- Bosmans, C.; Ginés Rodriguez, N.; Karperien, M.; Malda, J.; Moreira Teixeira, L.; Levato, R.; Leijten, J. Towards Single-Cell Bioprinting: Micropatterning Tools for Organ-on-Chip Development. Trends Biotechnol. 2024, 42, 739–759. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Rezapour Sarabi, M.; Birtek, M.T.; Mustafaoglu, N.; Zhang, Y.S.; Tasoglu, S. 3D Bioprinted Organ-on-Chips. Aggregate 2023, 4, e197. [Google Scholar] [CrossRef]
- Gaebler, D.; Hachey, S.J.; Hughes, C.C.W. Improving Tumor Microenvironment Assessment in Chip Systems through Next-Generation Technology Integration. Front. Bioeng. Biotechnol. 2024, 12, 1462293. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Peluzzi, V.; Raniolo, S.; Roscilli, G.; Trombetta, M.; Mozetic, P.; Rainer, A. On-Chip Recapitulation of the Tumor Microenvironment: A Decade of Progress. Biomaterials 2024, 306, 122482. [Google Scholar] [CrossRef]
- Li, C.; Holman, J.B.; Shi, Z.; Qiu, B.; Ding, W. On-Chip Modeling of Tumor Evolution: Advances, Challenges and Opportunities. Mater. Today Bio 2023, 21, 100724. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, G.; Javed, B.; Singh, B.; Tian, F. Biosensor-Enhanced Organ-on-a-Chip Models for Investigating Glioblastoma Tumor Microenvironment Dynamics. Sensors 2024, 24, 2865. [Google Scholar] [CrossRef]
- Moses, S.R.; Adorno, J.J.; Palmer, A.F.; Song, J.W. Vessel-on-a-Chip Models for Studying Microvascular Physiology, Transport, and Function in Vitro. Am. J. Physiol. Cell Physiol. 2021, 320, C92–C105. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yuan, H.; Hay, D.C.; Hu, H.; Wang, C. Revolutionizing Drug Discovery: The Impact of Distinct Designs and Biosensor Integration in Microfluidics-Based Organ-on-a-Chip Technology. Biosensors 2024, 14, 425. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, Z.; Wolter, T.; Womelsdorf, J.; Somers, A.; Feng, Y.; Nuutila, K.; Tian, Z.; Chen, J.; Tamayol, A.; et al. Engineering Microneedles for Biosensing and Drug Delivery. Bioact. Mater. 2025, 52, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.R.; Maia, R.F.; Ciardulli, M.C.; Santos, H.A.; Sarmento, B. Organ-on-Chip Platforms for Nanoparticle Toxicity and Efficacy Assessment: Advancing Beyond Traditional in Vitro and in Vivo Models. Mater. Today Bio 2025, 33, 102053. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Liu, Y.; Liu, Y. Organ-on-a-Chip Applications in Microfluidic Platforms. Micromachines 2025, 16, 201. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Shukla, S.; Shukla, A.K.; Ray, N.; Upadhyay, A.M.; Mirone, G.; Mongre, R.K. Long-Lasting Response of Human Circulating T-Follicular Helper Cells (cTfh) To Post SARS-CoV-2 mRNA Immunization. Asian J. Immunol. 2024, 7, 228–246. [Google Scholar] [CrossRef]
- Ahn, M.; Cho, W.-W.; Park, W.; Lee, J.-S.; Choi, M.-J.; Gao, Q.; Gao, G.; Cho, D.-W.; Kim, B.S. 3D Biofabrication of Diseased Human Skin Models in Vitro. Biomater. Res. 2023, 27, 80. [Google Scholar] [CrossRef]
- Yao, Q.; Cheng, S.; Pan, Q.; Yu, J.; Cao, G.; Li, L.; Cao, H. Organoids: Development and Applications in Disease Models, Drug Discovery, Precision Medicine, and Regenerative Medicine. MedComm 2024, 5, e735. [Google Scholar] [CrossRef] [PubMed]
- Heinzelmann, E.; Piraino, F.; Costa, M.; Roch, A.; Norkin, M.; Garnier, V.; Homicsko, K.; Brandenberg, N. iPSC-Derived and Patient-Derived Organoids: Applications and Challenges in Scalability and Reproducibility as Pre-Clinical Models. Curr. Res. Toxicol. 2024, 7, 100197. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; You, X.; Zhao, G. Harnessing the Power of Artificial Intelligence for Human Living Organoid Research. Bioact. Mater. 2024, 42, 140–164. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Mostafa, A.M.R.H.; Morton, J.P.; Hawinkels, L.J.A.C.; Prakash, J. Translating Complexity and Heterogeneity of Pancreatic Tumor: 3D in Vitro to in Vivo Models. Adv. Drug Deliv. Rev. 2021, 174, 265–293. [Google Scholar] [CrossRef]
- Phan, N.; Hong, J.J.; Tofig, B.; Mapua, M.; Elashoff, D.; Moatamed, N.A.; Huang, J.; Memarzadeh, S.; Damoiseaux, R.; Soragni, A. A Simple High-Throughput Approach Identifies Actionable Drug Sensitivities in Patient-Derived Tumor Organoids. Commun. Biol. 2019, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Skala, M.C.; Deming, D.A.; Kratz, J.D. Technologies to Assess Drug Response and Heterogeneity in Patient-Derived Cancer Organoids. Annu. Rev. Biomed. Eng. 2022, 24, 157–177. [Google Scholar] [CrossRef]
- Al-Kabani, A.; Huda, B.; Haddad, J.; Yousuf, M.; Bhurka, F.; Ajaz, F.; Patnaik, R.; Jannati, S.; Banerjee, Y. Exploring Experimental Models of Colorectal Cancer: A Critical Appraisal from 2D Cell Systems to Organoids, Humanized Mouse Avatars, Organ-on-Chip, Crispr Engineering, and Ai-Driven Platforms—Challenges and Opportunities for Translational Precision Oncology. Cancers 2025, 17, 2163. [Google Scholar]
- Makesh, K.Y.; Navaneethan, A.; Ajay, M.; Munuswamy-Ramanujam, G.; Chinnasamy, A.; Gnanasampanthapandian, D.; Palaniyandi, K. A Concise Review of Organoid Tissue Engineering: Regenerative Applications and Precision Medicine. Organoids 2025, 4, 16. [Google Scholar] [CrossRef]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, R. Transforming Clinical Research: The Power of High-Throughput Omics Integration. Proteomes 2024, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huang, M.; Ma, Y.; Zhang, Y.; Shi, C. Novel Research Model for in Vitro Immunotherapy: Co-Culturing Tumor Organoids with Peripheral Blood Mononuclear Cells. Cancer Cell Int. 2024, 24, 438. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, N.J.; Nicholls, C.; Templeton, A.R.; Perera, M.P.; Jeffery, P.L.; Zimmermann, K.; Kulasinghe, A.; Kenna, T.J.; Vela, I.; Williams, E.D.; et al. Modelling the Tumor Immune Microenvironment for Precision Immunotherapy. Clin. Transl. Immunol. 2022, 11, e1400. [Google Scholar] [CrossRef]
- Noorintan, S.T.; Angelius, C.; Torizal, F.G. Organoid Models in Cancer Immunotherapy: Bioengineering Approach for Personalized Treatment. Immuno 2024, 4, 312–324. [Google Scholar] [CrossRef]
- Cui, X.; Jiao, J.; Yang, L.; Wang, Y.; Jiang, W.; Yu, T.; Li, M.; Zhang, H.; Chao, B.; Wang, Z.; et al. Advanced Tumor Organoid Bioprinting Strategy for Oncology Research. Mater. Today Bio 2024, 28, 101198. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Wu, R.; He, Y.; Zhao, Y.; Wang, W.; Gao, X.; Wang, D.; Zhao, L.; Li, W. 3D Bioprinting in Cancer Modeling and Biomedicine: From Print Categories to Biological Applications. ACS Omega 2024, 9, 44076–44100. [Google Scholar] [CrossRef]
- Yang, Q.; Li, M.; Yang, X.; Xiao, Z.; Tong, X.; Tuerdi, A.; Li, S.; Lei, L. Flourishing Tumor Organoids: History, Emerging Technology, and Application. Bioeng. Transl. Med. 2023, 8, e10559. [Google Scholar] [CrossRef]
- Duarte, A.C.; Costa, E.C.; Filipe, H.A.L.; Saraiva, S.M.; Jacinto, T.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Animal-Derived Products in Science and Current Alternatives. Biomater. Adv. 2023, 151, 213428. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Stabrauskiene, J.; Kazlauskaite, J.A.; Bernatonyte, U.; Kopustinskiene, D.M. The Future of Medicine: How 3D Printing Is Transforming Pharmaceuticals. Pharmaceutics 2025, 17, 390. [Google Scholar] [CrossRef]
- Flach, E.H.; Rebecca, V.W.; Herlyn, M.; Smalley, K.S.; Anderson, A.R. Fibroblasts Contribute to Melanoma Tumor Growth and Drug Resistance. Mol. Pharm. 2011, 8, 2039–2049. [Google Scholar] [CrossRef]
- Shoval, H.; Karsch-Bluman, A.; Brill-Karniely, Y.; Stern, T.; Zamir, G.; Hubert, A.; Benny, O. Tumor Cells and Their Crosstalk with Endothelial Cells in 3D Spheroids. Sci. Rep. 2017, 7, 10428. [Google Scholar] [CrossRef]
- Klicks, J.; Maßlo, C.; Kluth, A.; Rudolf, R.; Hafner, M. A Novel Spheroid-Based Co-Culture Model Mimics Loss of Keratinocyte Differentiation, Melanoma Cell Invasion, and Drug-Induced Selection of Abcb5-Expressing Cells. BMC Cancer 2019, 19, 402. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988. [Google Scholar] [CrossRef] [PubMed]
- Powley, I.R.; Patel, M.; Miles, G.; Pringle, H.; Howells, L.; Thomas, A.; Kettleborough, C.; Bryans, J.; Hammonds, T.; MacFarlane, M. Patient-Derived Explants (Pdes) as a Powerful Preclinical Platform for Anti-Cancer Drug and Biomarker Discovery. Br. J. Cancer 2020, 122, 735–744. [Google Scholar] [CrossRef]
- Yin, Q.; Yu, W.; Grzeskowiak, C.L.; Li, J.; Huang, H.; Guo, J.; Chen, L.; Wang, F.; Zhao, F.; von Boehmer, L. Nanoparticle-Enabled Innate Immune Stimulation Activates Endogenous Tumor-Infiltrating T Cells with Broad Antigen Specificities. Proc. Natl. Acad. Sci. USA 2021, 118, e2016168118. [Google Scholar] [CrossRef]
- Votanopoulos, K.I.; Forsythe, S.; Sivakumar, H.; Mazzocchi, A.; Aleman, J.; Miller, L.; Levine, E.; Triozzi, P.; Skardal, A. Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann. Surg. Oncol. 2020, 27, 1956–1967. [Google Scholar] [CrossRef]
- Troiani, T.; Giunta, E.F.; Tufano, M.; Vigorito, V.; Arrigo, P.D.; Argenziano, G.; Ciardiello, F.; Romano, M.F.; Romano, S. Alternative Macrophage Polarisation Associated with Resistance to Anti-Pd1 Blockade Is Possibly Supported by the Splicing of Fkbp51 Immunophilin in Melanoma Patients. Br. J. Cancer 2020, 122, 1782–1790. [Google Scholar] [CrossRef]
- Haridas, P.; McGovern, J.A.; McElwain, S.D.; Simpson, M.J. Quantitative Comparison of the Spreading and Invasion of Radial Growth Phase and Metastatic Melanoma Cells in a Three-Dimensional Human Skin Equivalent Model. PeerJ 2017, 5, e3754. [Google Scholar] [CrossRef]
- Vörsmann, H.; Groeber, F.; Walles, H.; Busch, S.; Beissert, S.; Walczak, H.; Kulms, D. Development of a Human Three-Dimensional Organotypic Skin-Melanoma Spheroid Model for in Vitro Drug Testing. Cell Death Dis. 2013, 4, e719. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.S.; Robinson, N.D.; Caley, M.P.; Chen, M.; O’Toole, E.A.; Armstrong, J.L.; Przyborski, S.; Lovat, P.E. A Novel Fully Humanized 3D Skin Equivalent to Model Early Melanoma Invasion. Mol. Cancer Ther. 2015, 14, 2665–2673. [Google Scholar] [CrossRef]
- Bourland, J.; Fradette, J.; Auger, F.A. Tissue-Engineered 3D Melanoma Model with Blood and Lymphatic Capillaries for Drug Development. Sci. Rep. 2018, 8, 13191. [Google Scholar] [CrossRef]
- Shin, J.U.; Abaci, H.E.; Herron, L.; Guo, Z.; Sallee, B.; Pappalardo, A.; Jackow, J.; Wang, E.H.C.; Doucet, Y.; Christiano, A.M. Recapitulating T Cell Infiltration in 3D Psoriatic Skin Models for Patient-Specific Drug Testing. Sci. Rep. 2020, 10, 4123. [Google Scholar] [CrossRef]
- Wallmeyer, L.; Dietert, K.; Sochorová, M.; Gruber, A.D.; Kleuser, B.; Vávrová, K.; Hedtrich, S. TSLP Is a Direct Trigger for T Cell Migration in Filaggrin-Deficient Skin Equivalents. Sci. Rep. 2017, 7, 774. [Google Scholar] [CrossRef]
- Kosten, I.J.; Spiekstra, S.W.; de Gruijl, T.D.; Gibbs, S. MUTZ-3 Derived Langerhans Cells in Human Skin Equivalents Show Differential Migration and Phenotypic Plasticity after Allergen or Irritant Exposure. Toxicol. Appl. Pharmacol. 2015, 287, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bogaard, E.H.; Tjabringa, G.S.; Joosten, I.; Vonk-Bergers, M.; Van Rijssen, E.; Tijssen, H.J.; Erkens, M.; Schalkwijk, J.; Koenen, H.J. Crosstalk between Keratinocytes and T Cells in a 3D Microenvironment: A Model to Study Inflammatory Skin Diseases. J. Investig. Dermatol. 2014, 134, 719–727. [Google Scholar] [CrossRef]
- Lègues, M.; Milet, C.; Forraz, N.; Berthelemy, N.; Pain, S.; André-Frei, V.; Cadau, S.; McGuckin, C. The World’s First 3D Bioprinted Immune Skin Model Suitable for Screening Drugs and Ingredients for Normal and Inflamed Skin. IFSCC Mag. 2020, 4, 8–12. [Google Scholar]
- Jara, C.P.; Catarino, C.M.; Lei, Y.; Velloso, L.A.; Karande, P.; Velander, W.H.; de Araujo, E.P. Demonstration of Re-Epithelialization in a Bioprinted Human Skin Equivalent Wound Model. Bioprinting 2021, 24, e00102. [Google Scholar] [CrossRef]
- Michielon, E.; López González, M.; Burm, J.L.; Waaijman, T.; Jordanova, E.S.; de Gruijl, T.D.; Gibbs, S. Micro-Environmental Cross-Talk in an Organotypic Human Melanoma-in-Skin Model Directs M2-like Monocyte Differentiation via IL-10. Cancer Immunol. Immunother. 2020, 69, 2319–2331. [Google Scholar] [CrossRef]
- Michielon, E.; López González, M.; Stolk, D.A.; Stolwijk, J.G.; Roffel, S.; Waaijman, T.; Lougheed, S.M.; de Gruijl, T.D.; Gibbs, S. A Reconstructed Human Melanoma-in-Skin Model to Study Immune Modulatory and Angiogenic Mechanisms Facilitating Initial Melanoma Growth and Invasion. Cancers 2023, 15, 2849. [Google Scholar] [CrossRef]
- Di Blasio, S.; van Wigcheren, G.F.; Becker, A.; van Duffelen, A.; Gorris, M.; Verrijp, K.; Stefanini, I.; Bakker, G.-J.; Bloemendal, M.; Halilovic, A. The Tumour Microenvironment Shapes Dendritic Cell Plasticity in a Human Organotypic Melanoma Culture. Nat. Commun. 2020, 11, 2749. [Google Scholar] [CrossRef]
- Kaur, A.; Ecker, B.L.; Douglass, S.M.; Kugel III, C.H.; Webster, M.R.; Almeida, F.V.; Somasundaram, R.; Hayden, J.; Ban, E.; Ahmadzadeh, H. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov. 2019, 9, 64–81. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Sadangi, S.; Lares, M.; Rehman, S.; Humayun, M.; Denecke, K.M.; Skala, M.C.; Beebe, D.J.; Setaluri, V. Microfluidic Model with Air-Walls Reveals Fibroblasts and Keratinocytes Modulate Melanoma Cell Phenotype, Migration, and Metabolism. Lab Chip 2021, 21, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Getschman, A.E.; Hwang, S.; Volkman, B.F.; Klonisch, T.; Levin, D.; Zhao, M.; Santos, S.; Liu, S.; Cheng, J. Investigations on T Cell Transmigration in a Human Skin-on-Chip (Soc) Model. Lab Chip 2021, 21, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Q.; Ting, F.C.W. In Vitro Micro-Physiological Immune-Competent Model of the Human Skin. Lab Chip 2016, 16, 1899–1908. [Google Scholar] [CrossRef]
- Kwak, B.S.; Jin, S.P.; Kim, S.J.; Kim, E.J.; Chung, J.H.; Sung, J.H. Microfluidic Skin Chip with Vasculature for Recapitulating the Immune Response of the Skin Tissue. Biotechnol. Bioeng. 2020, 117, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Aref, A.R.; Lizotte, P.H.; Ivanova, E.; Stinson, S.; Zhou, C.W.; Bowden, M.; Deng, J.; Liu, H.; Miao, D. Ex Vivo Profiling of Pd-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov. 2018, 8, 196–215. [Google Scholar] [CrossRef]
- Sun, Y.; Revach, O.-y.; Anderson, S.; Kessler, E.A.; Wolfe, C.H.; Jenney, A.; Mills, C.E.; Robitschek, E.J.; Davis, T.G.; Kim, S. Targeting Tbk1 to Overcome Resistance to Cancer Immunotherapy. Nature 2023, 615, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Sanchez, N.A.; Goette, M. Role of Cell Surface Proteoglycans in Cancer Immunotherapy. Semin. Cancer Biol. 2020, 62, 48–67. [Google Scholar] [CrossRef]
- Chen, M.B.; Hajal, C.; Benjamin, D.C.; Yu, C.; Azizgolshani, H.; Hynes, R.O.; Kamm, R.D. Inflamed Neutrophils Sequestered at Entrapped Tumor Cells via Chemotactic Confinement Promote Tumor Cell Extravasation. Proc. Natl. Acad. Sci. USA 2018, 115, 7022–7027. [Google Scholar] [CrossRef]
- Mendes, M.; Morais, A.S.; Carlos, A.; Sousa, J.J.; Pais, A.C.; Mihăilă, S.M.; Vitorino, C. Organ-on-a-Chip: Quo Vademus? Applications and Regulatory Status. Colloids Surf. B Biointerfaces 2025, 249, 114507. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Zhang, L.; Wang, Q. Integrated Applications of Microfluidics, Organoids, and 3D Bioprinting in in Vitro 3D Biomimetic Models. IJB 2025, 11, 115–153. [Google Scholar] [CrossRef]
- Deng, S.; Li, C.; Cao, J.; Cui, Z.; Du, J.; Fu, Z.; Yang, H.; Chen, P. Organ-on-a-Chip Meets Artificial Intelligence in Drug Evaluation. Theranostics 2023, 13, 4526–4558. [Google Scholar] [CrossRef]
- Wang, X.; Lin, D.; Feng, N. Harnessing Organoid Technology in Urological Cancer: Advances and Applications in Urinary System Tumors. World J. Surg. Oncol. 2025, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Dai, Y.; Zhao, Z.; Zhu, J.; Guo, H.; Yang, R. Single-Cell Sequencing to Multi-Omics: Technologies and Applications. Biomark. Res. 2024, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Y.; Yuan, K.; Yang, S.; Zhang, S.; Li, H.; Tang, T. Multi-Omics Analysis Based on 3D-Bioprinted Models Innovates Therapeutic Target Discovery of Osteosarcoma. Bioact. Mater. 2022, 18, 459–470. [Google Scholar] [CrossRef]
- Kang, M.S.; Jang, J.; Jo, H.J.; Kim, W.-H.; Kim, B.; Chun, H.-J.; Lim, D.; Han, D.-W. Advances and Innovations of 3D Bioprinting Skin. Biomolecules 2023, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Ruchika; Bhardwaj, N.; Yadav, S.K.; Saneja, A. Recent Advances in 3D Bioprinting for Cancer Research: From Precision Models to Personalized Therapies. Drug Discov. Today 2024, 29, 103924. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, K.; Zhang, C.; Zhao, Y.; Guo, Y.; He, J.; Chang, S.; Fang, X.; Liu, K.; Zhu, P.; et al. Bioprinted Organoids: An Innovative Engine in Biomedicine. Adv. Sci. 2025, 12, e07317. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Gibelli, F.; Sirignano, A. Three-Dimensional Bioprinting of Human Organs and Tissues: Bioethical and Medico-Legal Implications Examined through a Scoping Review. Bioengineering 2023, 10, 1052. [Google Scholar] [CrossRef]
- Mallya, D.; Gadre, M.A.; Varadharajan, S.; Vasanthan, K.S. 3D bioprinting for the Construction of Drug Testing Models-Development Strategies and Regulatory Concerns. Front. Bioeng. Biotechnol. 2025, 13, 1457872. [Google Scholar] [CrossRef]
- Loukelis, K.; Koutsomarkos, N.; Mikos, A.G.; Chatzinikolaidou, M. Advances in 3D Bioprinting for Regenerative Medicine Applications. Regen. Biomater. 2024, 11, rbae033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, A.K.; Shukla, S.; Suryawanshi, S.P.; Mahendra Upadhyay, A.; Ray, N.; Thiruppathi, G.; Dutta, S.D.; Mongre, R.K. High-Throughput 3D Bioprinted Organoids of Skin Cancer Utilized for Diagnosis and Personalized Therapy. Curr. Oncol. 2025, 32, 653. https://doi.org/10.3390/curroncol32120653
Shukla AK, Shukla S, Suryawanshi SP, Mahendra Upadhyay A, Ray N, Thiruppathi G, Dutta SD, Mongre RK. High-Throughput 3D Bioprinted Organoids of Skin Cancer Utilized for Diagnosis and Personalized Therapy. Current Oncology. 2025; 32(12):653. https://doi.org/10.3390/curroncol32120653
Chicago/Turabian StyleShukla, Arvind Kumar, Sandhya Shukla, Sonali Pradeep Suryawanshi, Adarsha Mahendra Upadhyay, Navin Ray, Govindhan Thiruppathi, Sayan Deb Dutta, and Raj Kumar Mongre. 2025. "High-Throughput 3D Bioprinted Organoids of Skin Cancer Utilized for Diagnosis and Personalized Therapy" Current Oncology 32, no. 12: 653. https://doi.org/10.3390/curroncol32120653
APA StyleShukla, A. K., Shukla, S., Suryawanshi, S. P., Mahendra Upadhyay, A., Ray, N., Thiruppathi, G., Dutta, S. D., & Mongre, R. K. (2025). High-Throughput 3D Bioprinted Organoids of Skin Cancer Utilized for Diagnosis and Personalized Therapy. Current Oncology, 32(12), 653. https://doi.org/10.3390/curroncol32120653









