Efficacy of Anti-VEGF and Anti-EGFRs in Microsatellite Instable (MSI-H) Metastatic Colorectal Cancer in a Turkish Oncology Group (TOG) Cohort Study †
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Variable Measurement and Definition
2.3. Statistical Analysis
3. Results
| N (%) | ||
| 132 (100) | ||
| Age (median and range) in years | 60 (23–82) | |
| Sex | ||
| Female | 56 (43.2) | |
| Male | 67 (50.8) | |
| Comorbid disease | ||
| Yes | 62 (45.5) | |
| No | 60 (47.0) | |
| ECOG PS | ||
| 0–1 | 124 (93.9) | |
| ≥2 | 8 (6.1) | |
| Secondary Malignancy | ||
| Yes | 16 (12.1) | |
| No | 106 (80.3) | |
| Lynch Syndrome | ||
| Yes | 15 (11.4) | |
| No | 34 (25.8) | |
| Missing data | 83 (62.8) | |
| Primary tumor location | ||
| Right * | 81 (61.4) | |
| Left ** | 33 (25) | |
| Rectum | 18 (13.6) | |
| Advanced stage | ||
| De novo metastatic | 72 (54.5) | |
| Recurrence disease | 60 (45.5) | |
| MLH1 | ||
| Wild | 54 (40.9) | |
| Mutant | 78 (59.1) | |
| MSH2 | ||
| Wild | 74 (56.1) | |
| Mutant | 58 (43.9) | |
| MSH6 | ||
| Wild | 75 (56.8) | |
| Mutant | 55 (41.7) | |
| PMS2 | ||
| Wild | 61 (46.2) | |
| Mutant | 68 (51.5) | |
| Pan-Ras mutation | ||
| Wild | 80 (60.0) | |
| Mutant | 47 (35.6) | |
| BRAF mutation | ||
| Wild | 116 (87.9) | |
| Mutant | 16 (12.1) | |
| Surgery | ||
| No | 24 (18.2) | |
| Primary tumor | 82 (62.1) | |
| Primary tumor and metastasis | 26 (19.7) | |
| Systemic treatment after metastasectomy | ||
| No | 98 (74.2) | |
| Yes | 29 (22.0) | |
| First-Line Therapy | ||
| Xelox & + bevacizumab | 6 (4.5) | |
| Folfox && | 32 (24.2) | |
| Folfox && + bevacizumab | 38 (28.7) | |
| Folfox && + cetuximab/panitumumab | 2015.1) | |
| Folfiri + | 2 (1.5) | |
| Folfiri + + bevacizumab | 28 (21.2) | |
| Folfiri + + cetuximab/panitumumab | 6 (4.5) | |
| Immunotherapy as subsequent line | ||
| Yes | 14 (10.6) | |
| No | 118 (89.4) | |
| Second-line immunotherapy agent | ||
| Nivolumab | 6 (4.5) | |
| Pembrolizumab | 5 (3.7) | |
| Third-line immunotherapy agent | ||
| Nivolumab | 3 (2.2) | |
| ECOG PS: Eastern Cooperative Oncology Group Performance Score | ||
| PFS (Months) | OS (Months) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||||
| Median (Months) | %95 CI | p-Value | Median (Months) | %95 CI | p-Value | %95 CI | HR | p-Value | ||
| Age (year) | 0.86 | 0.62 | ||||||||
| <60 | 9.9 | 8.4–11.3 | 44.9 | 25.0–64.7 | ||||||
| ≥60 | 12.5 | 9.8–15.1 | 44.6 | 17.4–71.8 | ||||||
| Sex | 0.55 | |||||||||
| Female | 11.1 | 7.5–14.7 | 32.6 | 6.7–58.5 | 0.59 | |||||
| Male | 9.9 | 7.5–12.4 | 44.6 | 26.9–62.3 | ||||||
| Primary tumor location | 0.045 | 0.036 | ||||||||
| Right | 9.3 | 7.9–10.7 | 55.2 | 24.7–85.6 | ||||||
| Left | 12.5 | 9.1–15.8 | 44.9 | 16.0–73.7 | ||||||
| Rectum | 9.6 | 9.2–12.6 | 29.9 | 15.9–44.0 | ||||||
| De novo metastasis | 0.55 | 0.04 | ||||||||
| Yes | 10.9 | 9.2–12.6 | 72.2 | 21.1–68.1 | ||||||
| No | 10.3 | 61.1–14.4 | 54.5 | 11.8–53.9 | ||||||
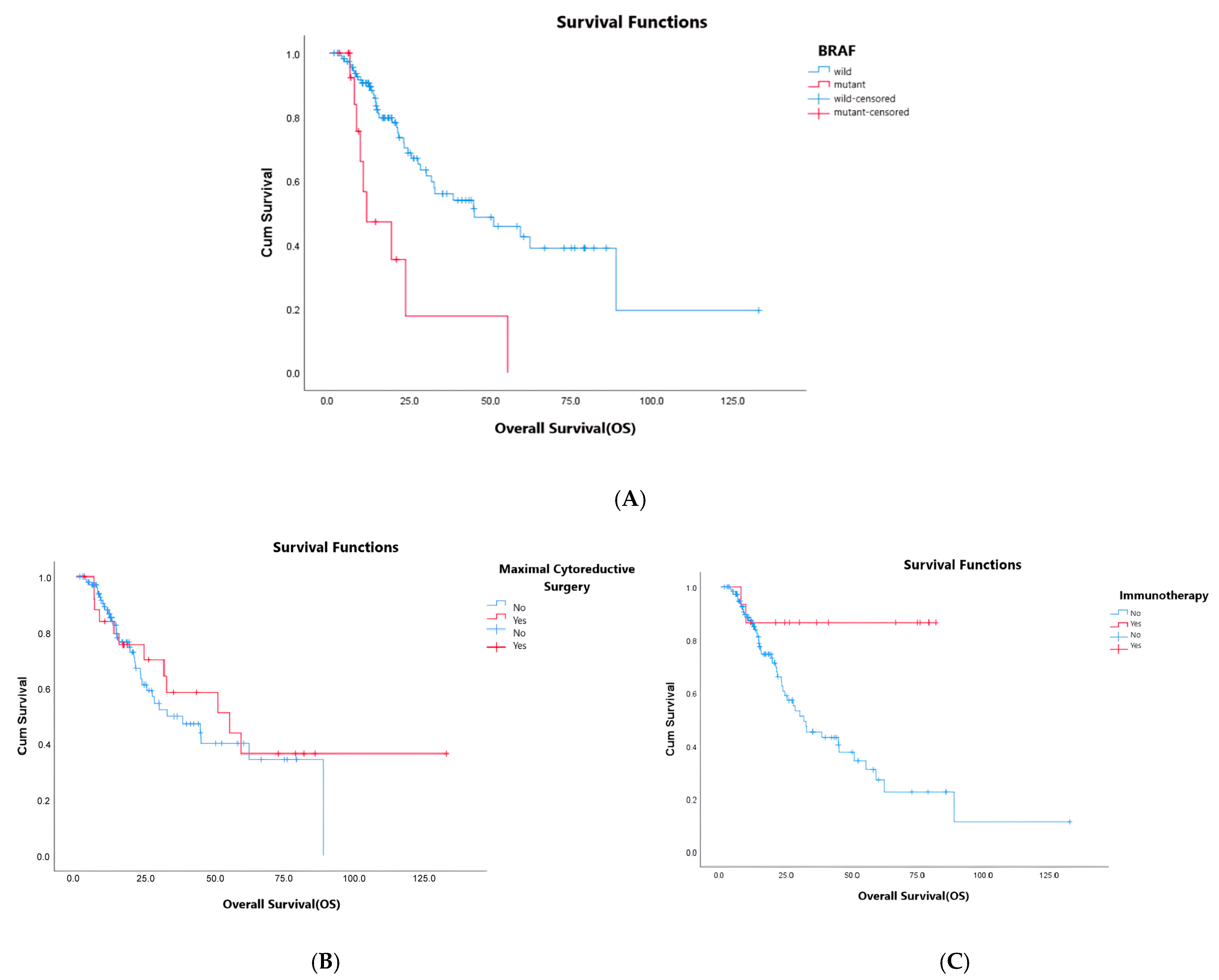
| Mutation status | 0.037 | <0.01 | 2.72–20.05 | 7.9 | <0.01 | |||||
| KRAS/NRAS mutant | 11.5 | 6.5–16.4 | 50.8 | 20.4–69.3 | ||||||
| BRAF mutant | 8.9 | 5.7–12.0 | 11.5 | 0.0–23.5 | ||||||
| RAS/BRAF wild | 10.9 | 9.3–12.4 | 38.3 | 21.7–43.0 | ||||||
| Site of metastasis | 0.092 | 0.053 | ||||||||
| Liver | 11.1 | 8.4–13.8 | 50.8 | 24.6–77.1 | ||||||
| Peritoneum | 9.3 | 8.5–10.1 | 32.4 | 32.5–59.4 | ||||||
| Others * | 11.3 | 2.5–20.1 | 31.5 | 18.5–44.5 | ||||||
| Biological treatment | 0.089 | |||||||||
| No | 7.9 | 4.6–11.3 | ||||||||
| Bevacizumab | 13.4 | 9.7–17.1 | ||||||||
| Cetuximab | 12.5 | 10.2–14.7 | ||||||||
| Panitumumab | 11.3 | 2.7–19.9 | ||||||||
| Maintenance treatment | 0.007 | 0.14 | 0.20–1.07 | 0.46 | 0.72 | |||||
| Yes | 14.0 | 12.4–15.5 | 55.2 | 29.9–80.4 | ||||||
| No | 8.7 | 7.1–10.3 | 31.5 | 8.9–54.2 | ||||||
| Maximal cytoreductive surgery | 0.89 | 0.030 | 0.15–0.86 | 0.36 | 0.022 | |||||
| Yes | 8.9 | 6.8–11.0 | 55.2 | 15.0–50.2 | ||||||
| No | 11.1 | 9.3–13.0 | 32.6 | 17.9–92.4 | ||||||
| İmmunotherapy in subsequent treatment | 0.005 | 0.28–0.51 | 0.12 | 0.005 | ||||||
| Yes | NR | |||||||||
| No | 31.5 | 20.2–42.8 | ||||||||
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Breast Cancer Initiative Implementation Framework: Assessing, Strengthening and Scaling-Up of Services for the Early Detection and Management of Breast Cancer; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Laiyemo, A.O.; Major, J.M.; Schootman, M.; Lian, M.; Park, Y.; Graubard, B.I.; Hollenbeck, A.R.; Sinha, R. Socioeconomic status and the risk of colorectal cancer: An analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer 2012, 118, 3636–3644. [Google Scholar] [CrossRef]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Sargent, D.J. Molecular pathways: Microsatellite instability in colorectal cancer: Prognostic, predictive, and therapeutic implications. Clin. Cancer Res. 2012, 18, 1506–1512. [Google Scholar] [CrossRef]
- Schwitalle, Y.; Kloor, M.; Eiermann, S.; Linnebacher, M.; Kienle, P.; Knaebel, H.P.; Tariverdian, M.; Benner, A.; Doeberitz, M.K. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 2008, 134, 988–997. [Google Scholar] [CrossRef]
- Sargent, D.J.; Marsoni, S.; Monges, G.; Thibodeau, S.N.; Labianca, R.; Hamilton, S.R.; French, A.J.; Kabat, B.; Foster, N.R.; Torri, V.; et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010, 28, 3219–3226. [Google Scholar] [CrossRef]
- Cohen, R.; Taieb, J.; Fiskum, J.; Yothers, G.; Goldberg, R.; Yoshino, T.; Alberts, S.; Allegra, C.; Gramont, A.; Seitz, J.F.; et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: An ACCENT pooled analysis of 12 adjuvant trials. J. Clin. Oncol. 2021, 39, 642–651. [Google Scholar] [CrossRef]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Siegmund, K.D.; Campan, M.; Young, J.; Long, T.I.; Faasse, M.A.; Kang, G.H.; Widschwendter, M.; Weener, D.; Buchanan, D.; et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006, 38, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Carbonero, R.G.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Berlin, J.; Posey, J.; Tchekmedyian, S.; Hu, E.; Chan, D.; Malik, I.; Yang, L.; Amado, R.G.; Hecht, J.R. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin. Color. Cancer 2009, 8, 9–14. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Rivera, F.; Berry, S.; Kretzschmar, A.; Michael, M.; DiBartolomeo, M.; Mazier, M.A.; Canon, J.L.; Georgoulias, V.; Peeters, M.; et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: The BEAT study. Ann. Oncol. 2009, 20, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Available online: https://www.equator-network.org/reporting-guidelines/strobe (accessed on 10 November 2025).
- Innocenti, F.; Ou, F.S.; Qu, X.; Zemla, T.J.; Niedzwiecki, D.; Tam, R.; Mahajan, S.; Goldberg, R.M.; Bertagnolli, M.M.; Blanke, C.D.; et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J. Clin. Oncol. 2019, 37, 1217–1227. [Google Scholar] [CrossRef]
- Gonsalves, W.I.; Mahoney, M.R.; Sargent, D.J.; Nelson, G.D.; Alberts, S.R.; Sinicrope, F.A.; Goldberg, R.M.; Limburg, P.J.; Thibodeau, S.N.; Grothey, A.; et al. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J. Natl. Cancer Inst. 2014, 106, dju106. [Google Scholar] [CrossRef]
- Modest, D.P.; Ricard, I.; Heinemann, V.; Hegewisch-Becker, S.; Schmiegel, W.; Porschen, R.; Stintzing, S.; Graeven, U.; Arnold, D.; Weikersthal, L.F.; et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: Pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann. Oncol. 2016, 27, 1746–1753. [Google Scholar] [CrossRef]
- Yokota, T.; Ura, T.; Shibata, N.; Takahari, D.; Shitara, K.; Nomura, M.; Kondo, C.; Mizota, A.; Utsunomiya, S.; Muro, K.; et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br. J. Cancer 2011, 104, 856–862. [Google Scholar] [CrossRef]
- Barras, D. BRAF mutation in colorectal cancer: An update: Supplementary issue: Biomarkers for colon cancer. Biomark. Cancer 2015, 7, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Santorsola, M.; Circelli, L.; Ianniello, M.; Casillo, M.; Petrillo, N.; Sabbatino, F.; Cascella, M.; Perri, F.; Capuozzo, M.; et al. BRAF p. V600E mutation as a molecular boundary between genuine oligo-repeated and poly-metastatic disease in colorectal cancer. Neoplasia 2023, 44, 100930. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.W.; Ahn, M.S.; Jeong, G.S.; Lee, H.W.; Jeong, S.H.; Kang, S.Y.; Park, J.S.; Choi, J.H.; Son, S.Y.; Hur, H.; et al. The role of surgical resection before palliative chemotherapy in advanced gastric cancer. Sci. Rep. 2019, 9, 4136. [Google Scholar] [CrossRef]
- Soran, A.; Ozmen, V.; Ozbas, S.; Karanlik, H.; Muslumanoglu, M.; Igci, A.; Canturk, N.Z.; Utkan, Z.; Evrensel, T.; Sezgin, E. Primary surgery with systemic therapy in patients with de novo stage IV breast cancer: 10-year follow-up; protocol MF07-01 randomized clinical trial. J. Am. Coll. Surg. 2021, 233, 742–751.e5. [Google Scholar] [CrossRef]
- Khan, S.A.; Zhao, F.; Goldstein, L.J.; Cella, D.; Basik, M.; Golshan, M.; Julian, T.B.; Pockaj, B.A.; Lee, C.A.; Razaq, W.; et al. Early local therapy for the primary site in de novo stage IV breast cancer: Results of a randomized clinical trial (E2108). J. Clin. Oncol. 2022, 40, 978–987. [Google Scholar] [CrossRef]
- Bakkerus, L.; Buffart, L.M.; Buffart, T.E.; Meyer, Y.M.; Zonderhuis, B.M.; Haasbeek, C.J.A.; Versteeg, K.S.; Loosveld, O.J.L.; Groot, J.W.B.; Hendriks, M.P.; et al. Health-Related Quality of Life in Patients with Metastatic Colorectal Cancer Undergoing Systemic Therapy with or Without Maximal Tumor Debulking. J. Natl. Compr. Cancer Netw. 2023, 21, 1059–1066.e5. [Google Scholar] [CrossRef]
- Badwe, R.; Hawaldar, R.; Nair, N.; Kaushik, R.; Parmar, V.; Siddique, S.; Budrukkar, A.; Mittra, I.; Gupta, S. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: An open-label randomised controlled trial. Lancet Oncol. 2015, 16, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Covens, A.L. A Critique of Surgical Cytoreduction in Advanced Ovarian Cancer. Gynecol. Oncol. 2000, 78, 269–274. [Google Scholar] [CrossRef]
- Cirocchi, R.; Trastulli, S.; Abraha, I.; Vettoretto, N.; Boselli, C.; Montedori, A.; Parisi, A.; Noya, G.; Platell, C. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst. Rev. 2012, Cd008997. [Google Scholar] [CrossRef]
- Ahmed, S.; Leis, A.; Fields, A.; Chandra-Kanthan, S.; Haider, K.; Alvi, R.; Reeder, B.; Pahwa, P. Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: Results from a large population-based cohort study. Cancer 2014, 120, 683–691. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Lenz, H.J.; Van Cutsem, E.; Luisa Limon, M.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Elez, E.; Van Cutsem, E.; Jensen, L.H.; Bennouna, J.; Mendez, G.; Schenker, M.; Fouchardiere, C.; Limon, M.L.; Yoshino, T.; et al. Nivolumab (NIVO) plus ipilimumab (IPI) vs. chemotherapy (chemo) as first-line (1L) treatment for microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): First results of the CheckMate 8HW study. Am. Soc. Clin. Oncol. 2024, 42, LBA768. [Google Scholar] [CrossRef]
- Deliktaş Onur, İ.; Dogan, M.; Ozturk, M.A.; Sahin, T.K.; Kiracı, M.; Arslan, A.M.; Karapelit Agitoğlu, E.; Karaoğlan, B.B.; Majidova, N.; Sahin, E.; et al. Efficacy of anti-VEGF & anti-EGFRs in microsatellite instable (MSI-H) metastatic colorectal cancer, Turkish Oncology Group (TOG) study. In Proceedings of the ASCO 2025, Chicago, IL, USA, 30 May–3 June 2025. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deliktaş Onur, İ.; Doğan, M.; Öztürk, M.A.; Sahin, T.K.; Kiracı, M.; Arslan, A.M.; Karapelit, E.; Karaoğlan, B.B.; Majidova, N.; Şahin, E.; et al. Efficacy of Anti-VEGF and Anti-EGFRs in Microsatellite Instable (MSI-H) Metastatic Colorectal Cancer in a Turkish Oncology Group (TOG) Cohort Study. Curr. Oncol. 2025, 32, 639. https://doi.org/10.3390/curroncol32110639
Deliktaş Onur İ, Doğan M, Öztürk MA, Sahin TK, Kiracı M, Arslan AM, Karapelit E, Karaoğlan BB, Majidova N, Şahin E, et al. Efficacy of Anti-VEGF and Anti-EGFRs in Microsatellite Instable (MSI-H) Metastatic Colorectal Cancer in a Turkish Oncology Group (TOG) Cohort Study. Current Oncology. 2025; 32(11):639. https://doi.org/10.3390/curroncol32110639
Chicago/Turabian StyleDeliktaş Onur, İlknur, Mutlu Doğan, Mehmet Akif Öztürk, Taha Koray Sahin, Murat Kiracı, Ahmet Melih Arslan, Eda Karapelit, Bahar Beliz Karaoğlan, Nargiz Majidova, Elif Şahin, and et al. 2025. "Efficacy of Anti-VEGF and Anti-EGFRs in Microsatellite Instable (MSI-H) Metastatic Colorectal Cancer in a Turkish Oncology Group (TOG) Cohort Study" Current Oncology 32, no. 11: 639. https://doi.org/10.3390/curroncol32110639
APA StyleDeliktaş Onur, İ., Doğan, M., Öztürk, M. A., Sahin, T. K., Kiracı, M., Arslan, A. M., Karapelit, E., Karaoğlan, B. B., Majidova, N., Şahin, E., Göktaş, S., Sakin, A., Oğul, A., Türkmen, E., Başkurt, K., Yüksel Yaşar, Z., Ergün, Y., Türkmen Bekmez, E., Yıldırım Dişli, Ş., ... Ateş, Ö. (2025). Efficacy of Anti-VEGF and Anti-EGFRs in Microsatellite Instable (MSI-H) Metastatic Colorectal Cancer in a Turkish Oncology Group (TOG) Cohort Study. Current Oncology, 32(11), 639. https://doi.org/10.3390/curroncol32110639





