Von Hippel–Lindau Disease-Associated Endolymphatic Sac Tumours: Seven Cases and Genotype–Phenotype Features
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Audiological, Radiological and Surgical Evaluation
2.2. Next-Generation Sequencing
2.3. Literature Review and Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Radiological Characteristics
3.3. Surgical Approaches and Outcomes
3.4. Genotype-Phenotype Features
4. Discussion
4.1. Demographic and Clinicopathological Characteristics
4.2. Clinical Presentation and Diagnostic Challenges
4.3. Pathological Manifestation
4.4. Therapeutic Considerations and Outcomes
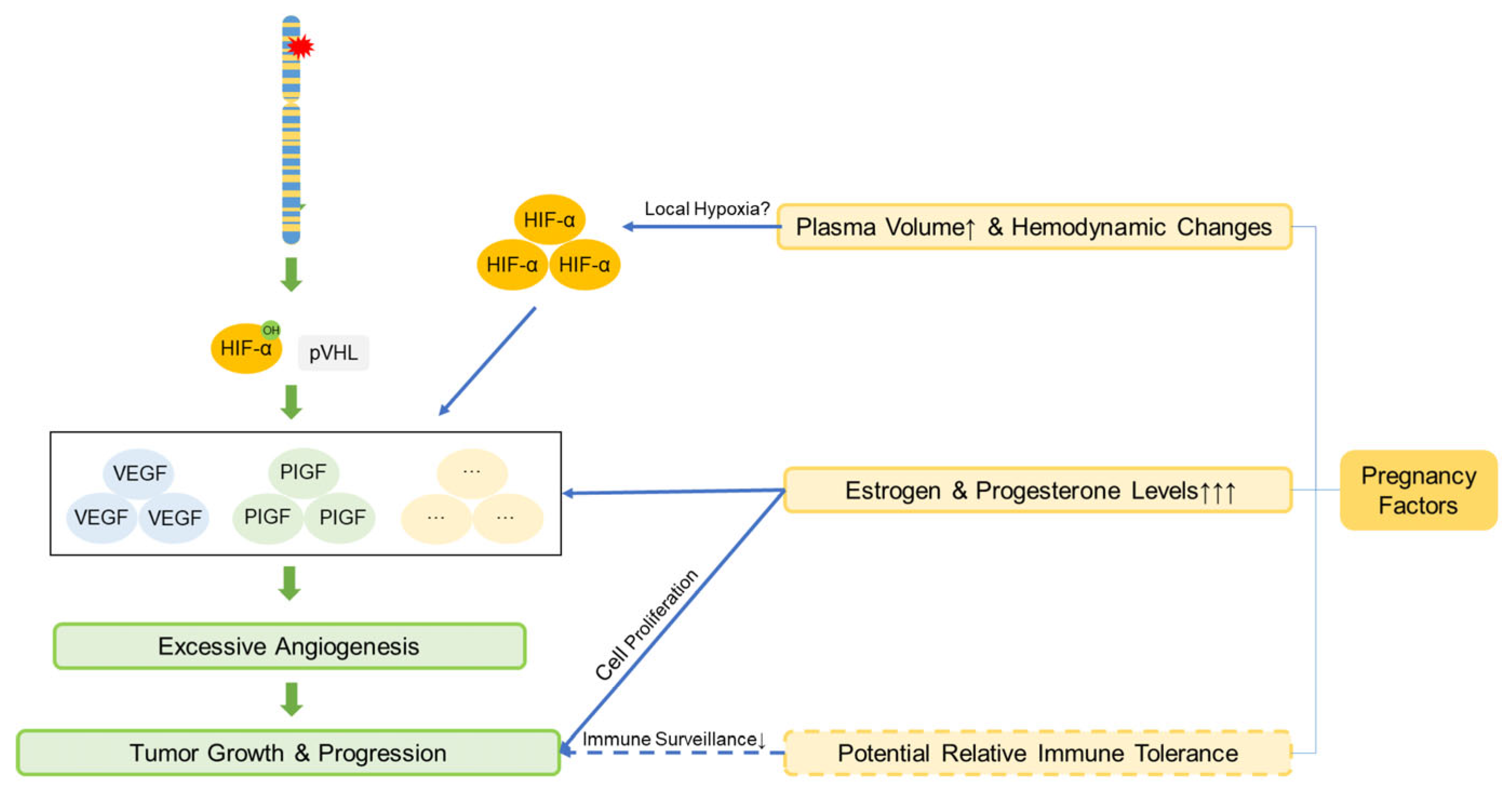
4.5. Genetic and Pregnancy-Related Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VHL | Von Hippel–Lindau |
| ELST | Endolymphatic sac tumor |
| ELS | Endolymphatic sac |
| MDT | Multidisciplinary team |
| FN | Facial nerve |
| HIFs | Hypoxia-inducible factors |
| RCC | Renal cell carcinoma |
| PTA | Pure-tone audiometry |
| HRCT | High-resolution computed tomography |
| MRI | Magnetic resonance imaging |
| DSA | Digital subtraction angiography |
| SNHL | Sensorineural hearing loss |
| IAC | Internal acoustic canal |
| CPA | Cerebellopontine angle |
| H&E | Haematoxylin and eosin |
| PVA | Polyvinyl-alcohol |
| PNEN | Pancreatic neuroendocrine neoplasm |
| CI | cochlear implantation |
| CNS | Central Nervous System |
References
- Melmon, K.L.; Rosen, S.W. Lindau’s Disease. Review of the Literature and Study of a Large Kindred. Am. J. Med. 1964, 36, 595–617. [Google Scholar]
- Talukdar, R.; Epari, S.; Sahay, A.; Choudhari, A.; Dasgupta, A.; Chatterjee, A.; Gupta, T. Endolymphatic sac tumor: Single-institution series of seven cases with updated review of literature. Eur. Arch. Otorhinolaryngol. 2022, 279, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer 2002, 2, 673–682. [Google Scholar] [CrossRef]
- Li, C.; Fu, C.; Zhou, W.; Li, H.; Liu, Z.; Wu, G.; He, T.; Shen, M.; Liu, H. Lactylation modification of HIF-1α enhances its stability by blocking VHL recognition. Cell Commun. Signal. 2025, 23, 364. [Google Scholar] [CrossRef] [PubMed]
- Zanoletti, E.; Girasoli, L.; Borsetto, D.; Opocher, G.; Mazzoni, A.; Martini, A. Endolymphatic sac tumour in von Hippel-Lindau disease: Management strategies. Acta Otorhinolaryngol. Ital. 2017, 37, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Chittiboina, P.; Lonser, R.R. Von Hippel-Lindau Disease. Handb. Clin. Neurol. 2015, 132, 139–156. [Google Scholar]
- Manski, T.J.; Heffner, D.K.; Glenn, G.M.; Patronas, N.J.; Pikus, A.T.; Katz, D.; Lebovics, R.; Sledjeski, K.; Choyke, P.L.; Zbar, B.; et al. Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA 1997, 277, 1461–1466. [Google Scholar] [CrossRef]
- Chadha, S.; Kamenov, K.; Cieza, A. The world report on hearing, 2021. Bull. World Health Organ. 2021, 99, 242–242A. [Google Scholar] [CrossRef]
- Wu, N.; Ma, X.; Shen, W.; Hou, Z.; Han, W.; Dai, P.; Zhao, H.; Huang, D.; Han, D.; Yang, S. Surgical management of endolymphatic sac tumor: Classification, outcomes and strategy. A single institution’s experience. Eur. Arch. Otorhinolaryngol. 2023, 280, 69–76. [Google Scholar] [CrossRef]
- Buckley, M.; Terwagne, C.; Ganner, A.; Cubitt, L.; Brewer, R.; Kim, D.K.; Kajba, C.M.; Forrester, N.; Dace, P.; De Jonghe, J.; et al. Saturation genome editing maps the functional spectrum of pathogenic VHL alleles. Nat. Genet. 2024, 56, 1446–1455. [Google Scholar] [CrossRef]
- Skalova, A.; Síma, R.; Bohus, P.; Curík, R.; Lukás, J.; Michal, M. Endolymphatic sac tumor (aggressive papillary tumor of middle ear and temporal bone): Report of two cases with analysis of the VHL gene. Pathol. Res. Pract. 2008, 204, 599–606. [Google Scholar] [CrossRef]
- Rao, Q.; Zhou, J.; Wang, J.D.; Jin, X.Z.; Ma, H.H.; Lu, Z.F.; Zhou, X.J. Endolymphatic sac tumor with von Hippel-Lindau disease: Report of a case with analysis of von Hippel-Lindau gene and review. Ann. Diagn. Pathol. 2010, 14, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.-S.; Fang, Y.; Zhang, X.-H.; Zhang, Y.-K. Endolymphatic sac tumor with von Hippel-Lindau disease: Report of a case with atypical pathology of endolymphatic sac tumor. Int. J. Clin. Exp. Pathol. 2014, 7, 2609–2614. [Google Scholar] [PubMed]
- de Minteguiaga, C.; García Ibáñez, L.; Tran Ba Huy, P. Endolymphatic sac tumor and von Hippel-Lindau disease. Review of the literature. Acta Otorrinolaringol. Esp. 2002, 53, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Lodi, M.; Marrazzo, A.; Cacchione, A.; Macchiaiolo, M.; Romanzo, A.; Mastronardi, L.; Diomedi-Camassei, F.; Carboni, A.; Carai, A.; Gandolfo, C.; et al. Synchronous Presentation of Rare Brain Tumors in Von Hippel-Lindau Syndrome. Diagnostics 2021, 11, 1005. [Google Scholar] [CrossRef]
- Jensen, R.L.; Gillespie, D.; House, P.; Layfield, L.; Shelton, C. Endolymphatic sac tumors in patients with and without von Hippel-Lindau disease: The role of genetic mutation, von Hippel-Lindau protein, and hypoxia inducible factor-1alpha expression. J. Neurosurg. 2004, 100, 488–497. [Google Scholar] [CrossRef]
- Codreanu, C.; Tran Ba Huy, P. Isolate vertigo crisis revealing an endolymphatic sac tumor. Rom. J. Morphol. Embryol. 2010, 51, 387–389. [Google Scholar]
- Priesemann, M.; Davies, K.M.; Perry, L.A.; Drake, W.M.; Chew, S.L.; Monson, J.P.; Savage, M.O.; Johnston, L.B. Benefits of screening in von Hippel-Lindau disease--comparison of morbidity associated with initial tumours in affected parents and children. Horm. Res. 2006, 66, 1–5. [Google Scholar] [CrossRef]
- Kawahara, N.; Kume, H.; Ueki, K.; Mishima, K.; Sasaki, T.; Kirino, T. VHL gene inactivation in an endolymphatic sac tumor associated with von Hippel-Lindau disease. Neurology 1999, 53, 208–210. [Google Scholar] [CrossRef]
- Tsoli, M.; Panagaki, M.; Tasouli, E.; Kolomodi, D.; Kaltsas, G. New Developments in VHL-Associated Neuroendocrine Neoplasms. Curr. Oncol. Rep. 2025, 27, 59–67. [Google Scholar] [CrossRef]
- Butman, J.A.; Kim, H.J.; Baggenstos, M.; Ammerman, J.M.; Dambrosia, J.; Patsalides, A.; Patronas, N.J.; Oldfield, E.H.; Lonser, R.R. Mechanisms of morbid hearing loss associated with tumors of the endolymphatic sac in von Hippel-Lindau disease. JAMA 2007, 298, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, L.; Thierfelder, F.; Thomas, C.; Soschinski, P.; Kim, H.Y.; Jödicke, R.; Woltering, N.; Förster, A.; Teichmann, D.; Siewert, C.; et al. Molecular characterisation of sporadic endolymphatic sac tumours and comparison to von Hippel-Lindau disease-related tumours. Neuropathol. Appl. Neurobiol. 2021, 47, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Bausch, B.; Wellner, U.; Peyre, M.; Boedeker, C.C.; Hes, F.J.; Anglani, M.; de Campos, J.M.; Kanno, H.; Maher, E.R.; Krauss, T.; et al. Characterization of endolymphatic sac tumors and von Hippel-Lindau disease in the International Endolymphatic Sac Tumor Registry. Head. Neck. 2016, 38 (Suppl. 1), E673–E679. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hagan, M.; Butman, J.A.; Baggenstos, M.; Brewer, C.; Zalewski, C.; Linehan, W.M.; Lonser, R.R. Surgical resection of endolymphatic sac tumors in von Hippel-Lindau disease: Findings, results, and indications. Laryngoscope 2013, 123, 477–483. [Google Scholar] [CrossRef]
- Gioacchini, F.M.; Kaleci, S.; Chiarella, G.; Viola, P.; Pisani, D.; Scarpa, A.; Tulli, M.; Pace, A.; Iannella, G.; Re, M. Symptoms and clinical features in patients affected by endolymphatic sac tumor: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2022, 279, 5081–5088. [Google Scholar] [CrossRef]
- Su, Y.; Shen, W.D.; Wang, C.C.; Han, W.J.; Liu, J.; Hou, Z.H.; Song, Z.G.; Huang, D.L.; Han, D.Y.; Yang, S.M. Endolymphatic sac tumor with von Hippel-Lindau disease: Report of two cases with testing of von Hippel-Lindau gene. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013, 48, 913–918. [Google Scholar]
- Fatih Turgut, N.; Crunkhorn, R.; Iqbal, J.; Dasgupta, S. Vestibular Function in Children with Von Hippel-Lindau Disease. J. Int. Adv. Otol. 2021, 17, 361–367. [Google Scholar] [CrossRef]
- Tan, D.; Fujiwara, R.J.T.; Tan, C.; Isaacson, B.; Hunter, J.B. Endolymphatic Sac Tumors Associated with von Hippel-Lindau: A Case Report Highlighting Opportunity for Novel Orphan Drug Therapy. Otol. Neurotol. 2024, 45, e644–e646. [Google Scholar] [CrossRef]
- Bambakidis, N.C.; Megerian, C.A.; Ratcheson, R.A. Differential grading of endolymphatic sac tumor extension by virtue of von Hippel-Lindau disease status. Otol. Neurotol. 2004, 25, 773–781. [Google Scholar] [CrossRef]
- Schipper, J.; Maier, W.; Rosahl, S.K.; van Velthoven, V.; Berlis, A.; Boedeker, C.C.; Laszig, R.; Teszler, C.B.; Ridder, G.J. Endolymphatic sac tumours: Surgical management. J. Otolaryngol. 2006, 35, 387–394. [Google Scholar] [CrossRef]
- Friedman, R.A.; Hoa, M.; Brackmann, D.E. Surgical management of endolymphatic sac tumors. J. Neurol. Surg. B Skull Base 2013, 74, 12–19. [Google Scholar]
- Sinclair, G.; Al-Saffar, Y.; Brigui, M.; Martin, H.; Bystam, J.; Benmakhlouf, H.; Shamikh, A.; Dodoo, E. Gamma knife radiosurgery in the management of endolymphatic sac tumors. Surg. Neurol. Int. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B.; Lonser, R.R.; Ondos, J.; Oldfield, E.H.; Camphausen, K.; Simone, N.L. Infratentorial craniospinal irradiation for von Hippel-Lindau: A retrospective study supporting a new treatment for patients with CNS hemangioblastomas. Neuro Oncol. 2011, 13, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Lonser, R.R.; Butman, J.A.; Huntoon, K.; Asthagiri, A.R.; Wu, T.; Bakhtian, K.D.; Chew, E.Y.; Zhuang, Z.; Linehan, W.M.; Oldfield, E.H. Prospective natural history study of central nervous system hemangioblastomas in von Hippel-Lindau disease. J. Neurosurg. 2014, 120, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.L.; Zhao, Y.H.; Ma, D.L.; Liu, H.G. Expression of VHL, VEGF and HIF-1α in endolymphatic sac tumors. Zhonghua Bing Li Xue Za Zhi 2021, 50, 1228–1233. [Google Scholar]
- Xu, Z.; Liu, L.; Jiang, W.; Qiu, Y.; Zhang, B.; Cheng, J.; Luo, J.; Guo, J.; Xu, J. VHL missense mutation delineate aggressive clear cell renal cell carcinoma subtype with favorable immunotherapeutic response. J. Immunother. Cancer 2024, 12, e009963. [Google Scholar] [CrossRef]
- Binderup, M.L.; Bisgaard, M.L.; Harbud, V.; Møller, H.U.; Gimsing, S.; Friis-Hansen, L.; Hansen, T.v.O.; Bagi, P.; Knigge, U.; Kosteljanetz, M.; et al. Von Hippel-Lindau disease (vHL). National clinical guideline for diagnosis and surveillance in Denmark. 3rd edition. Dan. Med. J. 2013, 60, B4763. [Google Scholar]
- Binderup, M.L.M.; Jensen, A.M.; Budtz-Jørgensen, E.; Bisgaard, M.L. Survival and causes of death in patients with von Hippel-Lindau disease. J. Med. Genet. 2017, 54, 11–18. [Google Scholar] [CrossRef]
- Wang, J.Y.; Peng, S.H.; Li, T.; Ning, X.H.; Liu, S.J.; Hong, B.A.; Liu, J.Y.; Wu, P.J.; Zhou, B.W.; Zhou, J.C.; et al. Risk factors for survival in patients with von Hippel-Lindau disease. J. Med. Genet. 2018, 55, 322–328. [Google Scholar] [CrossRef]
- Ye, D.Y.; Bakhtian, K.D.; Asthagiri, A.R.; Lonser, R.R. Effect of pregnancy on hemangioblastoma development and progression in von Hippel-Lindau disease. J. Neurosurg. 2012, 117, 818–824. [Google Scholar] [CrossRef]
- Binderup, M.L.M.; Budtz-Jørgensen, E.; Bisgaard, M.L. New von Hippel-Lindau manifestations develop at the same or decreased rates in pregnancy. Neurology 2015, 85, 1500–1503. [Google Scholar] [CrossRef]
- Frantzen, C.; Kruizinga, R.C.; van Asselt, S.J.; Zonnenberg, B.A.; Lenders, J.W.; de Herder, W.W.; Walenkamp, A.M.; Giles, R.H.; Hes, F.J.; Sluiter, W.J.; et al. Pregnancy-related hemangioblastoma progression and complications in von Hippel-Lindau disease. Neurology 2012, 79, 793–796. [Google Scholar] [CrossRef]
- da Mota Silveira Rodrigues, A.; Simões Fernandes, F.; Farage, L.; Almeida Prado Franceschi, L.E.; de Fátima Brito Vogt, M.; Zaconeta, A.M. Pregnancy-induced growth of a spinal hemangioblastoma: Presumed mechanisms and their implications for therapeutic approaches. Int. J. Womens Health 2018, 10, 325–328. [Google Scholar] [CrossRef]
- Laviv, Y.; Wang, J.L.; Anderson, M.P.; Kasper, E.M. Accelerated growth of hemangioblastoma in pregnancy: The role of proangiogenic factors and upregulation of hypoxia-inducible factor (HIF) in a non-oxygen-dependent pathway. Neurosurg. Rev. 2019, 42, 209–226. [Google Scholar] [CrossRef]
- Rajakumar, A.; Michael, H.M.; Daftary, A.; Jeyabalan, A.; Gilmour, C.; Conrad, K.P. Proteasomal activity in placentas from women with preeclampsia and intrauterine growth restriction: Implications for expression of HIF-alpha proteins. Placenta 2008, 29, 290–299. [Google Scholar] [CrossRef]
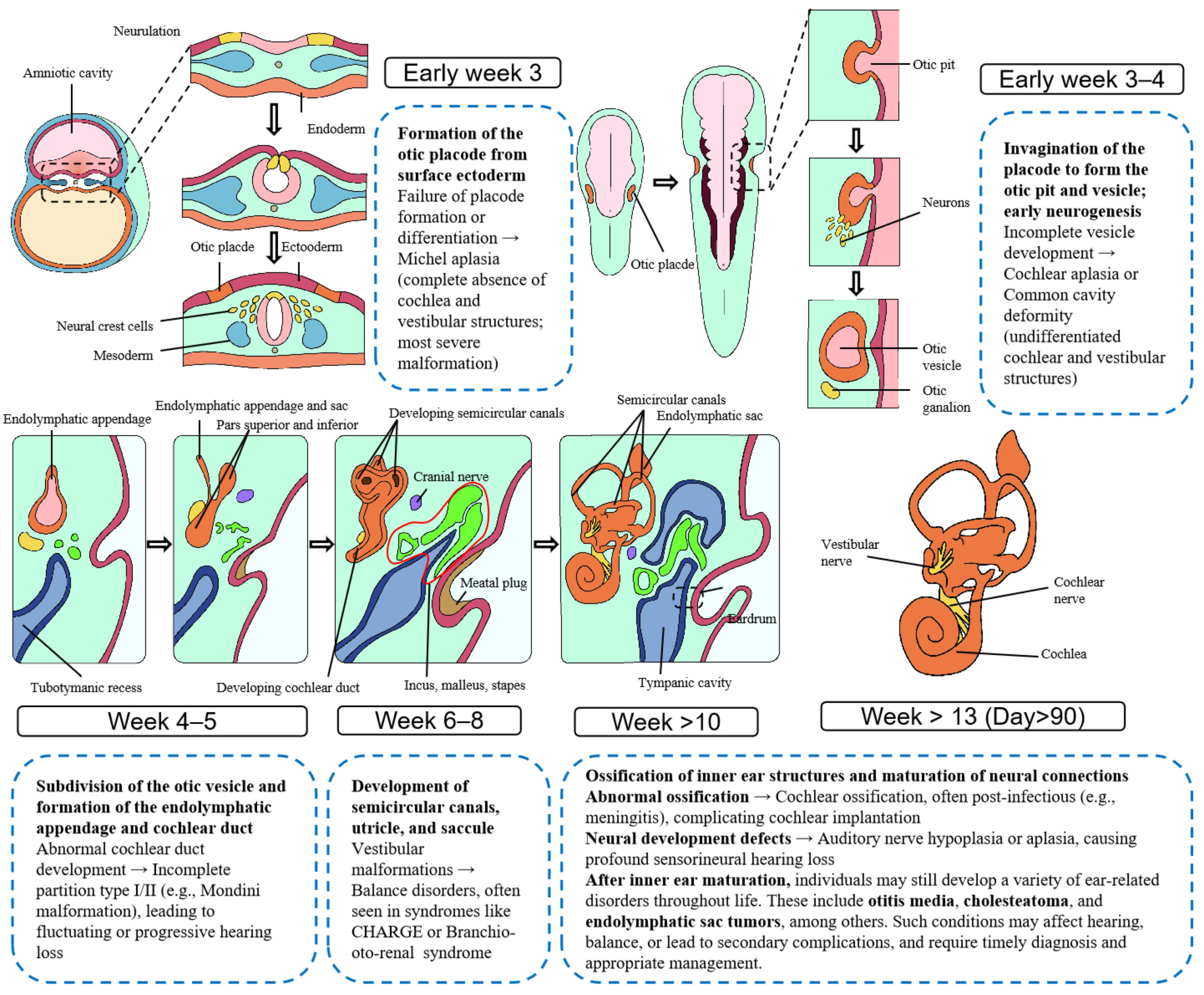
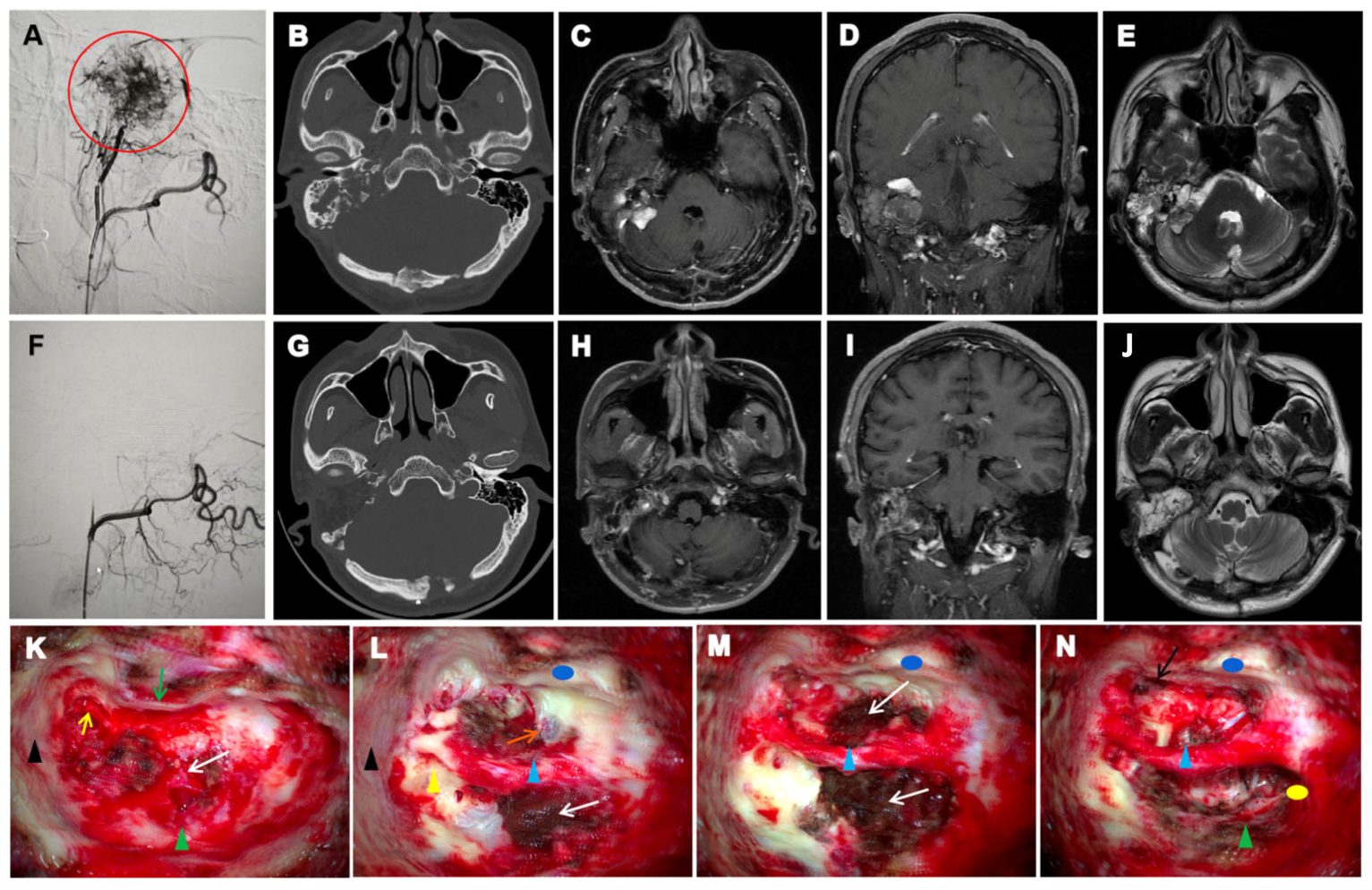
| Imaging Modality | Primary Indications | Typical Findings | Clinical Utility |
|---|---|---|---|
| HRCT | Assess bony erosion, tumor boundaries, and preoperative planning | ipsilateral temporal bone “moth-eaten” or honeycomb-like osteolytic destruction with crest-like/granular calcifications and residual bone formation | CT localization typically revealed tumour centres in the vestibular aqueduct operculum region, with early-stage lesions showing periaqueductal osteolysis and preserved surrounding architecture that facilitated origin identification. In advanced cases, the jugular foramen or subdural extension often obscured primary site determination. |
| MRI | Early screening, Lesion localization and classification, tumor relationship with the inner ear, brainstem, and cranial nerves, postoperative follow-up and long-term surveillance | hyperintense peripheral tumour margins and flow voids on T1-weighted imaging; and heterogeneous signal intensity on T2-weighted imaging. | Superior soft tissue contrast allows early detection of subclinical lesions, precise delineation of tumor extent, and assessment of involvement of critical neurovascular structures. Essential for surgical planning and long-term surveillance, especially in bilateral or recurrent VHL-associated cases. |
| DSA | Assess vascular supply and guide preoperative embolization | Tumor blush from external carotid artery branches; occasionally supplied by internal carotid or vertebral artery branches | Defines feeding arteries; essential for embolization planning and minimizing intraoperative bleeding risk |
| Patient | Age of Onset (Year) | Sex | Otologic Symptoms and H-B Grade (Pre/Post) | Family History and Mutation | Abnormal Findings |
|---|---|---|---|---|---|
| 1 | 27 | M | Otorrhea, FN paralysis, Tinnitus, Complete deafness; H-B III/H-B III (R) | N; c.485G>T | Brain haemangioblastoma, multiple hepatic haemangiomas; Pancreatic cysts; Parotid nodules; Thyroid nodules (Bethesda II), Right vocal cord fixation; Left retinal haemangioblastoma, Left Renal cyst |
| 2 | 27 | M | Vertigo, Tinnitus, Otalgia, FN paralysis (R), Complete deafness (B); H-B VI/H-B V (R) | P; Not tested | Bilateral vestibular schwannomas, cranial hemangioblastomas, Pancreatic cystadenoma; Renal mass; Hyperechoic nodule in the left hepatic lobe |
| 3 | 29 | F | Vertigo, Tinnitus, Otalgia, FN paralysis, Complete deafness; H-B II/H-B II (L) | P; Not tested | Hepatic haemangiomas, Pancreatic cysts, right RCC (Fuhrman I-II), Left renal mass, Bilateral renal cysts |
| 4 | 10 | F | vertigo, Tinnitus, Complete deafness; H-B I/H-B I (B) | P; Not tested | Bilateral renal cysts, Pancreatic tail cyst; right retinal haemangioblastoma, brain haemangioblastoma |
| 5 | 33 | F | Headache, Vertigo, FN paralysis, Complete deafness; H-B IV/H-B IV (L) | N; Not tested | Cerebellar haemangioblastoma |
| 6 | 17 | F | Tinnitus, Otorrhea, FN paralysis, Complete deafness H-B VI/H-B IV (L) | P; c.499C>T | Right retinal haemangioblastoma |
| 7 | 14 | F | Otalgia, Otorrhea, Vertigo, FN paralysis, Complete deafness; H-B VI/H-B IV (L) | P; c.194C>G | Right retinal haemangioblastoma, T1 intramedullary haemangioblastoma, Cerebellar vermis haemangioblastoma |
| Patient | Tumor Blood Supply | Immunohistochemistry | Surgical History and Outcome |
|---|---|---|---|
| 1 | Right external carotid artery occipital branch | CK (+), CD31(+), CD34(+), D2-40(−), Ki-67(+3%) | Neurosurgery (2005, 2013, 2024; Other hospital): Brain haemangioblastoma resection ENT (2024, our centre): Subtotal temporal bone resection, FN rerouting (R) Neurosurgery (2025, our centre): Cerebellar haemangioblastoma resection Follow up (2025.8, our centre): No evidence of ELST recurrence. |
| 2 | Right external carotid artery | TTF-1 (−), TG (−), CD34 (+), CK7 (+), CK (+), S-100 (−), P63 (−), Ki-67 (+3%), Syn (−), CgA (−), EMA (+), GFAP (−) | Radiation Oncology (2007, Other hospital): Bilateral vestibular schwannoma treated with Gamma Knife ENT (2013, our centre): CI (R) Neurosurgery (2016, 2017, Other hospital): Resection of cranial hemangioblastomas ENT (2019, our centre): Subtotal temporal bone resection, hypoglossal-FN anastomosis (R), cochlear implant explantation (R) ENT (2021, our centre): CI and cochlear implant explantation (L) ENT (2023, our centre): Subtotal temporal bone resection (R) Follow up (2025.8, our centre): No evidence of ELST recurrence. Underwent Phase III clinical trial for RCC (Other hospital). |
| 3 | Left carotid artery posterior auricular branch; Sella turcica area shows round tumor-like staining | Microscopy: Partial papillary structures, cystic wall lined by monolayer cuboidal epithelium | Became pregnant in 2001, 2007 ENT (2013, our centre): Subtotal temporal bone resection (L) Urology (2014, our centre): Right nephrectomy Follow up (2025.8, our centre): No evidence of ELST recurrence. |
| 4 | - | GFAP (+), CK (+) | ENT (2013, our centre): Extended retrolabyrinthine approach + CI (R) Neurosurgery (2014, Other hospital): hemangioblastoma resection + radiotherapy Got pregnant in 2016 (hemangioblastoma enlargement to 10 cm within 1 year) ENT (2016, our centre): cochlear implant explantation (R) Neurosurgery (2016, Other hospital): Craniospinal hemangioblastoma resection Follow up (2025.8, our centre): No evidence of ELST recurrence. |
| 5 | - | TTF-1 (−), S-100 (scattered cells +), Syn (−), CK5 (+), Ki-67 (+1%), TG (−), PAX-8 (partial), CD56 (+), Vimentin (+) | Became pregnant in 2005, 2016 Neurosurgery (2016&2017, Other hospital): Cerebral haemangioblastoma resection ENT (2019, our centre): Subtotal temporal bone resection (L) Neurosurgery (2024, our centre): Midline approach cerebellar haemangioblastoma resection Follow up (2025.8, our centre): No evidence of ELST recurrence. |
| 6 | Left external carotid artery; Intracranial presence of 3 aneurysms | TTF-1 (−), Syn (−), CK (+), Ki-67 (+<5%), TG (−), CD56 (+), Vimentin (+), GFAP (−), PAS (+) | ENT (2010, Other hospital): Left mastoidectomy ENT (2011, our centre): Extended retrolabyrinthine approach, great auricular nerve grafting (L) Follow up (2012.10, our centre): MRI revealed residual cerebellopontine angle tumour Follow up (2025.8, our centre): Lost to follow-up |
| 7 | Left external carotid artery posterior auricular branch | TTF-1 (−), S-100 (+), Syn (−), CK (+), Ki-67 (−), CD56 (+), Vimentin (−), EMA (−), CgA (−) | Neurosurgery (2011, our centre): T1 intramedullary haemangioblastoma resection ENT (2011, our centre): Subtotal temporal bone resection, great auricular nerve grafting (L) Neurosurgery (2013, our centre): Cerebellar vermis haemangioblastoma resection Follow up (2025.8, our centre): Lost to follow-up |
| Dimension | Case 1 | Case 2 | Clinical Implication |
|---|---|---|---|
| Age/Sex | 27/Male | 27/Male | Comparable demographic background |
| Otologic Symptoms and H-B Grade | Otorrhea, facial nerve paralysis, tinnitus, complete deafness (right); H-B III/III | Vertigo, otalgia, tinnitus, facial nerve paralysis (right), complete bilateral deafness; H-B VI/V | Case 2 presents with more severe bilateral auditory and FN involvement |
| Family History/Genetic Testing | No family history; VHL gene mutation confirmed (c.485G>T, exon 3) | Positive family history: genetic testing not performed | Case 1 is genetically confirmed VHL; Case 2 is clinically suspected but genetically unverified |
| Vestibular Schwannomas | Absent | Bilateral vestibular schwannomas (treated with Gamma Knife) | Case 2 shows broader cranial nerve involvement, possibly indicating NF2-like overlap or VHL variant |
| Renal Findings | Left renal cyst | Renal mass enrolled in Phase III carcinoma trial | Case 1 presents with a benign renal cyst, which may progress to clear cell renal cell carcinoma (ccRCC) over time; Case 2 has confirmed renal malignancy requiring systemic therapy |
| Pancreatic Lesions | Pancreatic cysts | Pancreatic cystadenoma | Cystadenoma in Case 2 may carry higher neoplastic potential. However, the cystic lesion in Case 1 carries an estimated 8–17% risk of progression to pancreatic neuroendocrine neoplasm (PNEN) [20]. |
| Other Systemic Findings | Parotid and thyroid nodules (Bethesda II), left retinal hemangioblastoma, right vocal cord fixation | Hyperechoic hepatic nodule | Case 1 shows multi-organ benign involvement; Case 2 has fewer benign lesions but higher malignant risk |
| Histopathology | Papillary and glandular epithelial architecture consistent with ELST | Papillary epithelial architecture consistent with ELST | Both cases are histologically consistent with ELST |
| Immunohistochemistry | CK(+), CD31(+), CD34(+), D2-40(−), Ki-67(+3%) → vascular phenotype | CK(+), CK7(+), EMA(+), CD34(+), Ki-67(+3%); TTF-1(−), TG(−), Syn(−), CgA(−), S-100(−), P63(−), GFAP(−) → epithelial phenotype | Case 1 shows vascular markers possibly reflecting stromal components; Case 2 demonstrates classic epithelial differentiation, resembling ccRCC |
| Tumor Growth Risk | Low Ki-67; no ELST recurrence; recurrent CNS hemangioblastomas; renal cyst requires long-term surveillance | Low Ki-67; no ELST recurrence; renal carcinoma under active treatment → high systemic risk | Case 1 may develop renal malignancy over time; Case 2 is undergoing systemic therapy |
| Therapeutic Strategy | Requires comprehensive systemic surveillance; prompt multidisciplinary intervention upon new findings; previously underwent CNS hemangioblastoma resection and ELST surgery | Requires systemic therapy and coordinated multidisciplinary management, including ELST surgery and renal carcinoma treatment | Case 1 emphasizes preventive monitoring and individualized intervention; Case 2 necessitates active systemic treatment and cross-specialty collaboration |
| Exon | Sex | Age (Years) | Tumor Side | Family History and Mutation | Symptoms | Hemangioblastoma | Other Diseases | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 14 | L | P c.194C>G | Otalgia, Otorrhea, Vertigo, FN paralysis, HL | Spinal, Retinal | - | 6 months after ELST resection: No evidence of ELST recurrence 14 years after ELST resection: Lost to follow-up | Our research |
| F | 24 | Not mentioned | P c.194C>T | HL, middle ear mass | Cranial | - | 26 months after ELST resection: recurrent destructive ELST of the middle ear appeared | Skalova et al. [11] | |
| F | 32 | R | P IVS1 +1G>A | HL, vertigo, headache | Cranial | - | No evidence of ELST recurrence was noted, although the exact timing of follow-up was not specified. | Rao et al. [12] | |
| F | 42 | R | N c.291-292insGCCGCAGCCC | Headache, dizziness | Cranial | - | No evidence of ELST recurrence was noted, although the exact timing of follow-up was not specified. | Yang et al. [13] | |
| F | 34 | R | N Uncertain | otalgia, hypoacusia | Cranial | Pancreatic Cyst | 14 months after ELST resection: No evidence of tumor recurrence | Minteguiaga et al. [14] | |
| 2 | M | 16 | L | N c.394delC | HL, visual impairment, diplopia, headache.” | Cranial, Retinal | Multiple pancreatic cysts | ELST recurrence was noted once, followed by reoperation; no further ELST recurrence has been observed, although the timing of follow-up remains unspecified | Lodi et al. [15] |
| 3 | M | 27 | R | N c.485G>T | Otorrhea, FN paralysis, Tinnitus, HL | Cranial, Retinal | Hepatic Haemangioma, Pancreatic Cyst, Parotid Nodule, Left Renal cyst | 6 months after ELST resection: No evidence of ELST recurrence | Our research |
| F | 17 | L | P c.499C>T | Tinnitus, Otorrhea, FN paralysis, HL | Spinal | Gallbladder Polyp | 1 year after ELST resection: Showed no progression of residual tumour 14 years after ELST resection: Lost to follow-up | Our research | |
| F | 32 | L | P c.639-2C>A | Headache, dizziness, HL | Spinal | Pancreatic Cyst, Pheochromocytoma | 2 years after ELST resection: No evidence of ELST recurrence | Jensen et al. [16] | |
| F | 30 | R | P Missense Mutation (specific unknown) | vertigo | Cranial, retinal | renal cystic tumours | 6 months after ELST resection: No evidence of ELST recurrence | Codreanu et al. [17] | |
| M | 16 | L | P (His Father carries same gene mutation, had RCC and bilateral pheochromocytomas) c.712C>T | Tinnitus HL disequilibrium | - | Pheochromocytoma | No evidence of ELST recurrence was noted, although the exact timing of follow-up was not specified | Priesemann et al. [18] | |
| M | 21 | L | P c.930delG + D3S1259 LOH | truncal sensory disturbance, left FN paralysis and hypalgia at the level of T6-12, hearing loss | Cranial, spinal and retinal | Bilateral Renal Cysts, Left Clear Cell Renal Carcinoma | No evidence of ELST recurrence was noted, although the exact timing of follow-up was not specified | Kawahara et al. [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Huang, J.; Zhao, Z.; Su, Y.; Wu, N.; Yang, S.; Shen, W.; Sai, N.; Han, W. Von Hippel–Lindau Disease-Associated Endolymphatic Sac Tumours: Seven Cases and Genotype–Phenotype Features. Curr. Oncol. 2025, 32, 633. https://doi.org/10.3390/curroncol32110633
Wang Q, Huang J, Zhao Z, Su Y, Wu N, Yang S, Shen W, Sai N, Han W. Von Hippel–Lindau Disease-Associated Endolymphatic Sac Tumours: Seven Cases and Genotype–Phenotype Features. Current Oncology. 2025; 32(11):633. https://doi.org/10.3390/curroncol32110633
Chicago/Turabian StyleWang, Qin, Junhui Huang, Zhikai Zhao, Yu Su, Nan Wu, Shiming Yang, Weidong Shen, Na Sai, and Weiju Han. 2025. "Von Hippel–Lindau Disease-Associated Endolymphatic Sac Tumours: Seven Cases and Genotype–Phenotype Features" Current Oncology 32, no. 11: 633. https://doi.org/10.3390/curroncol32110633
APA StyleWang, Q., Huang, J., Zhao, Z., Su, Y., Wu, N., Yang, S., Shen, W., Sai, N., & Han, W. (2025). Von Hippel–Lindau Disease-Associated Endolymphatic Sac Tumours: Seven Cases and Genotype–Phenotype Features. Current Oncology, 32(11), 633. https://doi.org/10.3390/curroncol32110633





