Serum miRNA Signatures in Cancer Cachexia Depend on Systemic Inflammation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection and Assessment
2.3. Patient Reported Outcome Measures (PROMs)
2.4. Muscle Mass Analyses
2.5. Assessment of Cachexia
- (1)
- Non-cachexia without inflammation
- (2)
- Non-cachexia with inflammation
- (3)
- Cachexia without inflammation
- (4)
- Cachexia with inflammation
2.6. RNA Extraction, Sequencing, and Calibration
2.7. Differential Expression Analysis
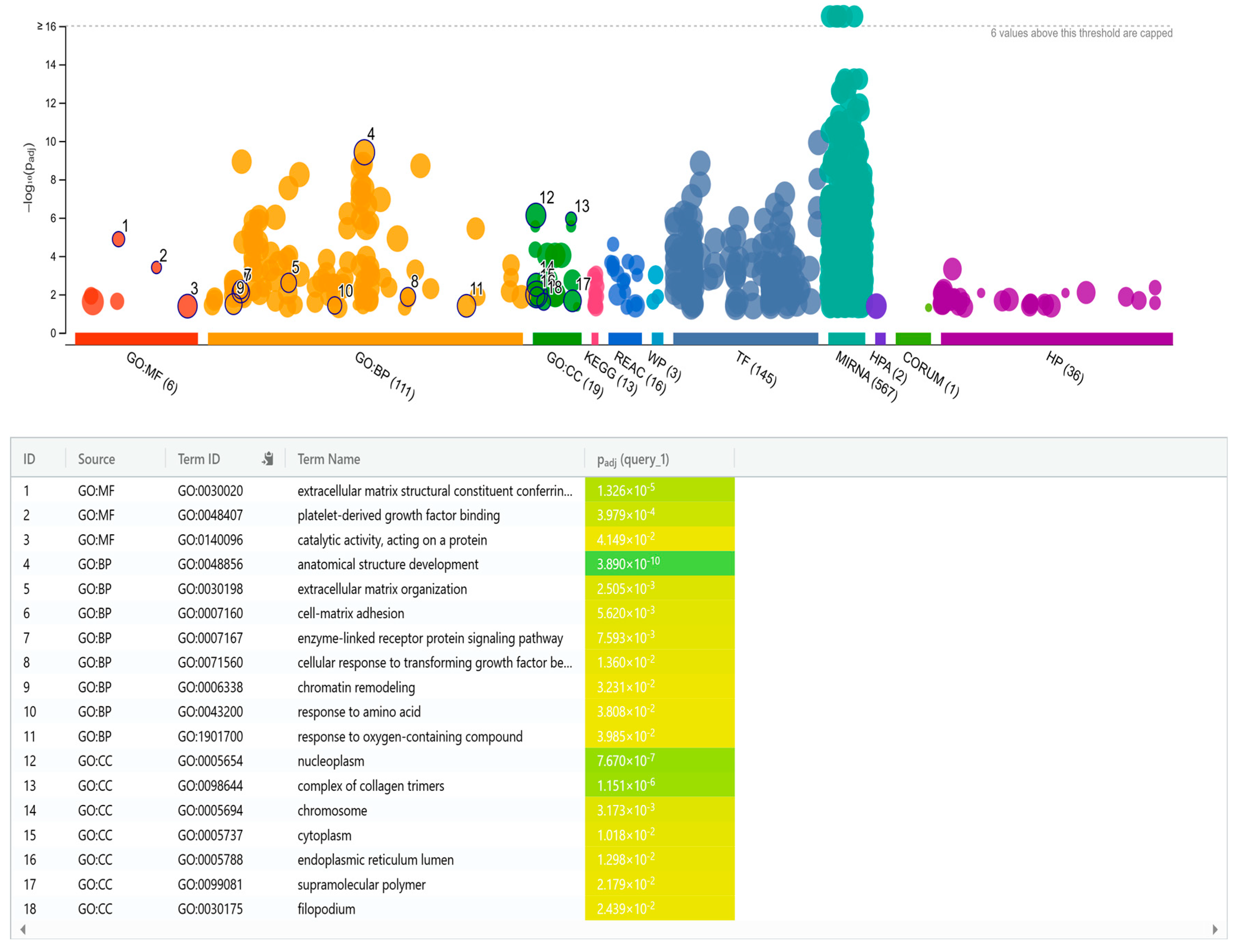
2.8. Predicting Targets for Differentially Expressed miRNAs
2.9. Gene Ontology Analysis for miRNA Targets
2.10. Survival Analysis
2.11. Analysis and Statistical Considerations
3. Results
3.1. Patients
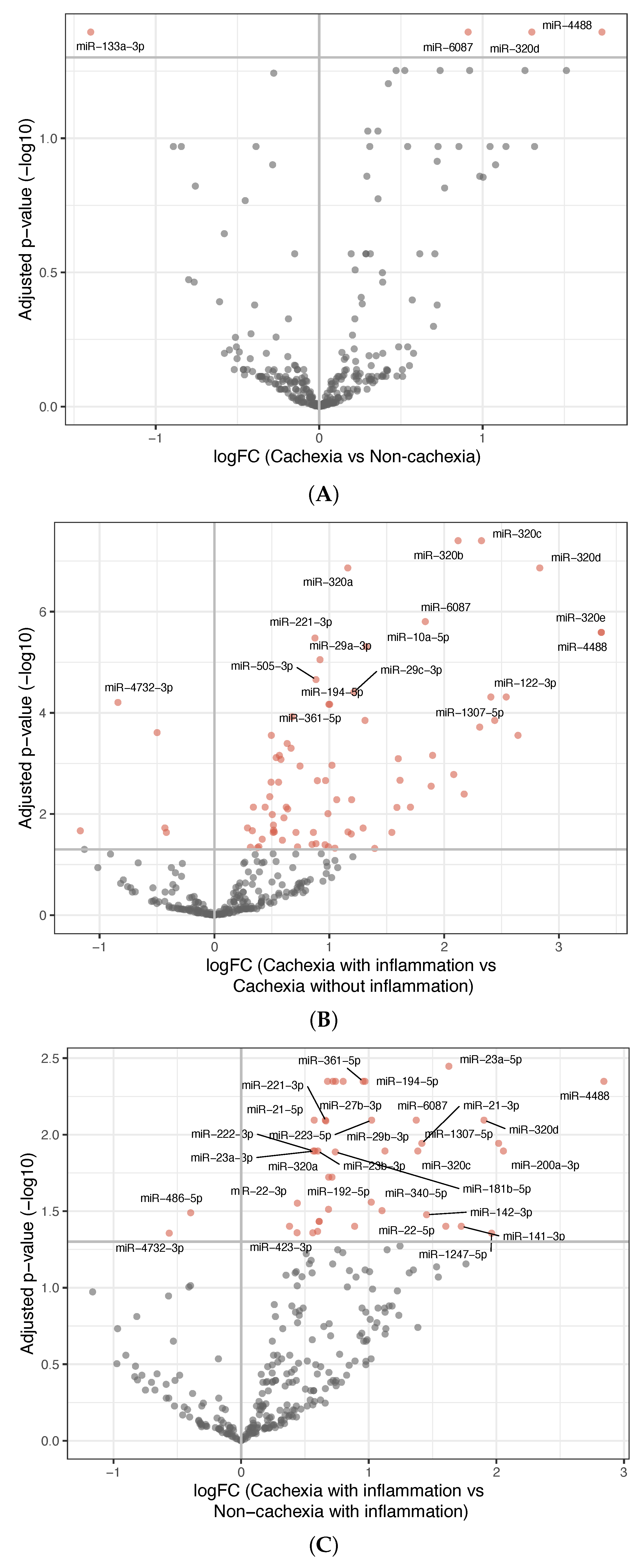
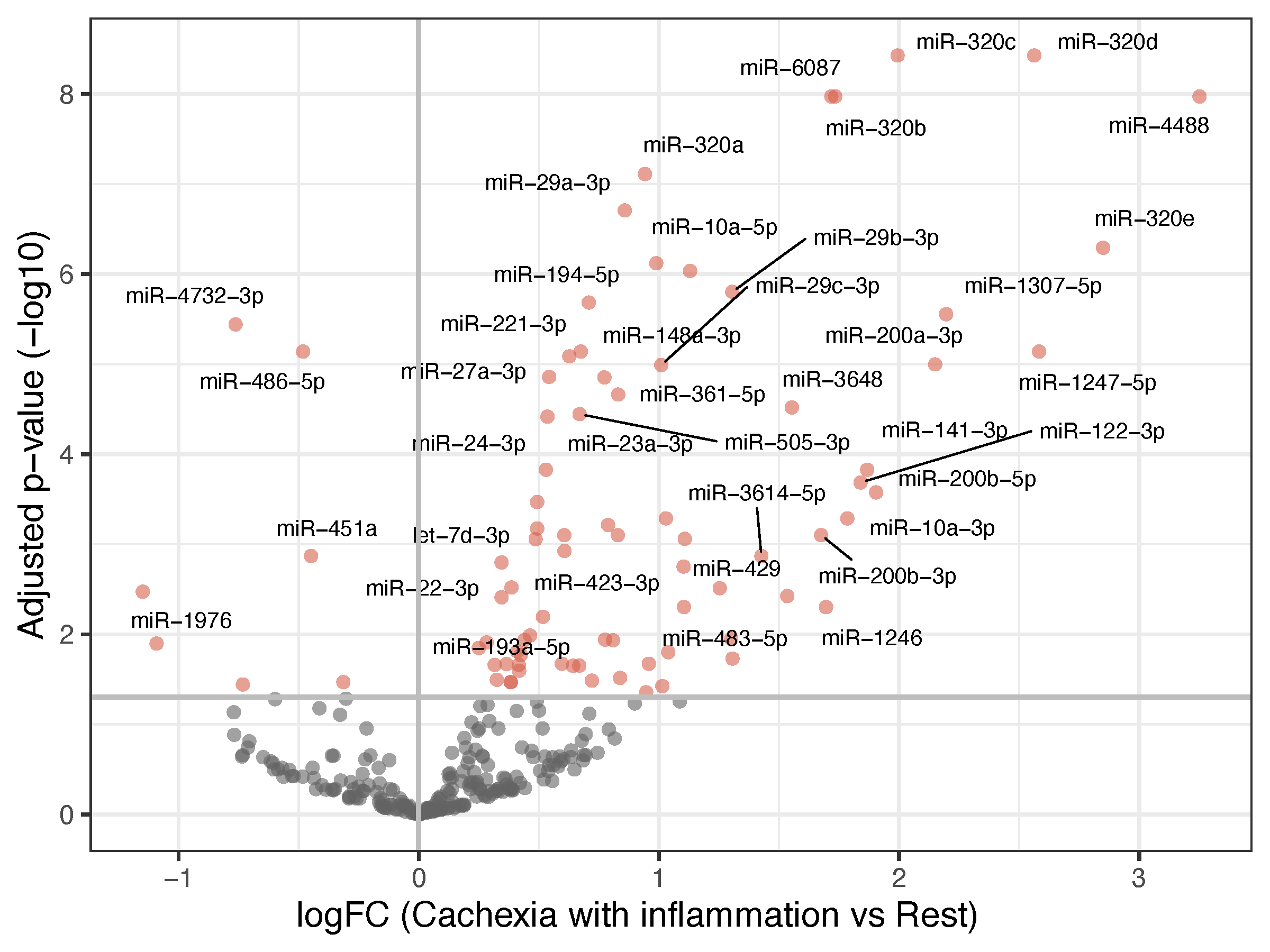
3.2. miRNA Analyses
- Among patients without cachexia, no significant differences in miRNA expression between those with and without inflammation were found (Figure S2A).
- Among patients having inflammation, comparing those with and without cachexia revealed 42 differentially expressed miRNAs; 40 were upregulated and 2 were downregulated in the inflammatory cachexia group (Figure 1C). Although fewer than in the comparison between cachectic patients with and without inflammation, most differentially expressed miRNAs overlapped.
- Among patients without inflammation, no significant differences in miRNA expression were observed between those with and without cachexia (Figure S2B).
3.3. Survival Analyses
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, T.; Clarke, L.; Goldberg, A.; Bishop, K.S. Pancreatic Cancer Cachexia: The Role of Nutritional Interventions. Healthcare 2019, 7, 89. [Google Scholar] [CrossRef]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.G.; Quintas Teixeira Ribeiro, H.; Geraldo, M.V.; Matos-Neto, E.; Neves, R.X.; Carnevali, L.C., Jr.; Donatto, F.F.; Alcântara, P.S.; Ottoch, J.P.; Seelaender, M. Cancer Cachexia and MicroRNAs. Mediat. Inflamm. 2015, 2015, 367561. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Rao, L.V.M. The Role of microRNAs in Inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug. Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- He, W.A.; Calore, F.; Londhe, P.; Canella, A.; Guttridge, D.C.; Croce, C.M. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl. Acad. Sci. USA 2014, 111, 4525–4529. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Chen, X.; Gao, X.; Dong, Y.; Zhao, Y.; Wei, M.; Fan, W.; Yang, G.; Liu, L. Chronic myeloid leukemia-derived exosomes attenuate adipogenesis of adipose derived mesenchymal stem cells via transporting miR-92a-3p. J. Cell. Physiol. 2019, 234, 21274–21283. [Google Scholar] [CrossRef]
- Narasimhan, A.; Ghosh, S.; Stretch, C.; Greiner, R.; Bathe, O.F.; Baracos, V.; Damaraju, S. Small RNAome profiling from human skeletal muscle: Novel miRNAs and their targets associated with cancer cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 405–416. [Google Scholar] [CrossRef] [PubMed]
- van de Worp, W.; Schols, A.; Dingemans, A.C.; den Kamp, C.M.H.O.; Degens, J.; Kelders, M.; Coort, S.; Woodruff, H.C.; Kratassiouk, G.; Harel-Bellan, A.; et al. Identification of microRNAs in skeletal muscle associated with lung cancer cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 452–463. [Google Scholar] [CrossRef]
- Kulyté, A.; Lorente-Cebrián, S.; Gao, H.; Mejhert, N.; Agustsson, T.; Arner, P.; Rydén, M.; Dahlman, I. MicroRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E267–E274. [Google Scholar] [CrossRef]
- Krauss, T.; Heisz, S.; Honecker, J.; Prokopchuk, O.; Martignoni, M.; Janssen, K.P.; Claussnitzer, M.; Hauner, H.; Seeliger, C. Specific miRNAs are associated with human cancer cachexia in an organ-specific manner. J. Cachexia Sarcopenia Muscle 2023, 14, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.O.; da Silva, S.P.; da Costa, R.M.G.; Medeiros, R. The Emerging Role of MicroRNAs and Other Non-Coding RNAs in Cancer Cachexia. Cancers 2020, 12, 1004. [Google Scholar] [CrossRef]
- Powrózek, T.; Mlak, R.; Brzozowska, A.; Mazurek, M.; Gołębiowski, P.; Małecka-Massalska, T. miRNA-130a Significantly Improves Accuracy of SGA Nutritional Assessment Tool in Prediction of Malnutrition and Cachexia in Radiotherapy-Treated Head and Neck Cancer Patients. Cancers 2018, 10, 294. [Google Scholar] [CrossRef]
- Okugawa, Y.; Yao, L.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Yin, C.; Omura, Y.; Ide, S.; Kitajima, T.; Shimura, T.; et al. Prognostic impact of sarcopenia and its correlation with circulating miR-21 in colorectal cancer patients. Oncol. Rep. 2018, 39, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Hur, K.; Yamamoto, A.; Yin, C.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Koike, Y.; et al. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2019, 10, 536–548. [Google Scholar] [CrossRef]
- Yehia, R.; Schaalan, M.; Abdallah, D.M.; Saad, A.S.; Sarhan, N.; Saleh, S. Impact of TNF-α Gene Polymorphisms on Pancreatic and Non-Small Cell Lung Cancer-Induced Cachexia in Adult Egyptian Patients: A Focus on Pathogenic Trajectories. Front. Oncol. 2021, 11, 783231. [Google Scholar] [CrossRef]
- Sun, D.; Ding, Z.; Shen, L.; Yang, F.; Han, J.; Wu, G. miR-410-3P inhibits adipocyte differentiation by targeting IRS-1 in cancer-associated cachexia patients. Lipids Health Dis. 2021, 20, 115. [Google Scholar] [CrossRef]
- Molfino, A.; Beltrà, M.; Amabile, M.I.; Belli, R.; Birolo, G.; Belloni, E.; De Lucia, S.; Garcia-Castillo, L.; Penna, F.; Imbimbo, G.; et al. Small non-coding RNA profiling in patients with gastrointestinal cancer. J. Cachexia Sarcopenia Muscle 2023, 14, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Tambaro, F.; Imbimbo, G.; Pace, V.; Amabile, M.I.; Rizzo, V.; Orlando, S.; Lauteri, G.; Ramaccini, C.; Catalano, C.; Nigri, G.; et al. Circulating adipose-tissue miRNAs in gastrointestinal cancer patients and their association with the level and type of adiposity at body composition analysis. Front. Mol. Biosci. 2024, 11, 1449197. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, Y.; Zhang, S.; Guo, Y.; Wang, F.; Du, Y.; Wang, L.; Li, P.; Xu, Y.; Zhang, H.; et al. Tumor-derived miR-203a-3p potentiates muscle wasting by inducing muscle ferroptosis in pancreatic cancer. Cancer Lett. 2025, 614, 217523. [Google Scholar] [CrossRef]
- A Longitudinal Study of Colorectal Cancer Patients with Metastatic Disease in Middle-Norway, ClinicalTrials.gov. 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02395224?term=A+longitudinal+study+of+colorectal+cancer+patients+with+metastatic+disease+in+Middle-Norway&draw=2&rank=1 (accessed on 28 October 2025).
- Huo, Z.; Chong, F.; Yin, L.; Li, N.; Liu, J.; Zhang, M.; Guo, J.; Fan, Y.; Zhang, L.; Lin, X.; et al. Comparison of the performance of the GLIM criteria, PG-SGA and mPG-SGA in diagnosing malnutrition and predicting survival among lung cancer patients: A multicenter study. Clin. Nutr. 2023, 42, 1048–1058. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Mjelle, R.; Kristensen, A.K.; Tora, S.S.; Westvik, G.S.; Elvebakken, H.; Hofsli, E. Serum small RNAs in metastatic colorectal cancer predict response to chemotherapy and characterize high-risk patients. Mol. Cancer 2024, 23, 133. [Google Scholar] [CrossRef]
- Mjelle, R.; Sellæg, K.; Sætrom, P.; Thommesen, L.; Sjursen, W.; Hofsli, E. Identification of metastasis-associated microRNAs in serum from rectal cancer patients. Oncotarget 2017, 8, 90077–90089. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g:Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]
- Huot, J.R.; Novinger, L.J.; Pin, F.; Narasimhan, A.; Zimmers, T.A.; O’Connell, T.M.; Bonetto, A. Formation of colorectal liver metastases induces musculoskeletal and metabolic abnormalities consistent with exacerbated cachexia. JCI Insight 2020, 5, 136687. [Google Scholar] [CrossRef]
- Cardaci, T.D.; Bastian, A.V.; Roark, K.; Bullard, B.M.; NeSmith, M.M.; Unger, C.A.; Price, R.L.; Kubinak, J.L.; Murphy, E.A.; VanderVeen, B.N. Profibrotic immune cells and fibro-adipogenic progenitors contribute to skeletal muscle extracellular matrix remodeling and fibrosis in cancer cachexia. Am. J. Physiol. Cell. Physiol. 2025, 329, C1283–C1299. [Google Scholar] [CrossRef]
- Cui, Q.; Li, S.; Liu, X.; Liu, J.; Chen, W.; Sheng, Y.; Xie, P.; Jin, L.; Zeng, F.; Lv, F.; et al. MIF-ACKR3 causes irreversible fat loss by impairing adipogenesis in cancer cachexia. Cell Metab. 2025, 37, 954–970.e958. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.S.; Tomaz da Silva, M.; Kumar, S.; Kumar, A. Signaling networks governing skeletal muscle growth, atrophy, and cachexia. Skelet. Muscle 2025, 15, 29. [Google Scholar] [CrossRef] [PubMed]

| Non-Cachexia Without Inflammation n = 49 (29%) | Non-Cachexia with Inflammation n = 25 (15%) | Cachexia Without Inflammation n = 41 (24%) | Cachexia with Inflammation n = 53 (32%) | Total n = 168 (100%) | p-Value | |
|---|---|---|---|---|---|---|
| Sex | 0.76 a | |||||
| Male | 36 (73%) | 17 (68%) | 26 (63%) | 35 (66%) | 114 (68%) | |
| Female | 13 (27%) | 8 (32%) | 15 (37%) | 18 (34%) | 54 (32%) | |
| Age, mean (SD) | 66 (11) | 69 (6.6) | 67 (12) | 67 (10) | 67 (10) | 0.53 b |
| Cachexia criterion | ||||||
| Weight loss > 5% last six months | - | - | 33 (80%) | 41 (77%) | 74 (44%) | |
| BMI < 20 and weight loss > 2% | - | - | 1 (2.4%) | 3 (5.7%) | 4 (2.4%) | |
| Sarcopenia and weight loss > 2% | - | - | 27 (87%) | 42 (91%) | 69 (46%) | |
| WHO performance status | 0.01 c | |||||
| 0 | 30 (61%) | 13 (52%) | 18 (45%) | 16 (30%) | 77 (46%) | |
| 1 | 17 (35%) | 7 (28%) | 16 (40%) | 28 (53%) | 68 (41%) | |
| ≥2 | 2 (4.1%) | 5 (20%) | 6 (15%) | 9 (17%) | 22 (13%) | |
| Location of primary tumor | 0.54 a | |||||
| Right-sided tumor | 15 (31%) | 9 (36%) | 15 (38%) | 22 (45%) | 61 (37%) | |
| Left-sided tumor | 34 (69%) | 16 (64%) | 25 (63%) | 27 (55%) | 102 (63%) | |
| Metastases | ||||||
| Liver | 30 (61%) | 18 (72%) | 26 (63%) | 46 (87%) | 120 (71%) | 0.02 a |
| Lung | 19 (39%) | 14 (56%) | 10 (24%) | 17 (32%) | 60 (36%) | 0.06 a |
| Peritoneal | 10 (20%) | 3 (12%) | 13 (32%) | 13 (25%) | 39 (23%) | 0.30 a |
| Non-regional lymph nodes | 11 (22%) | 3 (12%) | 14 (34%) | 15 (28%) | 43 (26%) | 0.22 a |
| Histological grade | 0.15 a | |||||
| Highly or moderately diff | 31 (86%) | 13 (65%) | 24 (80%) | 21 (66%) | 89 (75%) | |
| Poorly or undiff | 5 (14%) | 7 (35%) | 6 (20%) | 11 (34%) | 29 (25%) | |
| RAS status | 0.46 a | |||||
| Mutation KRAS or NRAS | 17 (37%) | 14 (56%) | 18 (45%) | 24 (48%) | 73 (45%) | |
| WT KRAS and NRAS | 29 (63%) | 11 (44%) | 22 (55%) | 26 (52%) | 88 (55%) | |
| BRAF status | 0.56 a | |||||
| Mutation BRAF | 6 (13%) | 4 (16%) | 10 (25%) | 10 (20%) | 30 (19%) | |
| WT BRAF | 39 (87%) | 21 (84%) | 30 (75%) | 40 (80%) | 130 (81%) | |
| Groups Compared | Differentially Expressed miRNAs |
|---|---|
| Cachexia vs. non-cachexia | 4 in total; 3 upregulated, 1 downregulated in the cachexia group (Table S2A) |
| Cachexia with inflammation vs. cachexia without inflammation | 80 in total; 75 upregulated, 5 downregulated in the inflammatory cachexia group (Table S2B) |
| Non-cachexia with inflammation vs. non-cachexia without inflammation | None |
| Cachexia with inflammation vs. non-cachexia with inflammation | 42 in total; 40 upregulated, 2 downregulated in the inflammatory cachexia group (Table S2C) |
| Cachexia without inflammation vs. non-cachexia without inflammation | None |
| Cachexia with inflammation vs. non-cachexia without inflammation | 46 in total; 37 upregulated, 9 downregulated in the inflammatory cachexia group (Table S2D) |
| Cachexia without inflammation vs. non-cachexia with inflammation | None |
| Cachexia with inflammation vs. all other patients | 82 in total; 75 upregulated, 7 downregulated in the inflammatory cachexia group (Table S3) |
| Hazard Ratio | p-Value | 95% Confidence Interval | |
|---|---|---|---|
| Reference group: Non-cachexia without inflammation | |||
| Non-cachexia with inflammation | 1.37 | 0.22 | 0.83–2.27 |
| Cachexia without inflammation | 1.12 | 0.60 | 0.73–1.73 |
| Cachexia with inflammation | 2.10 | <0.001 | 1.40–3.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsen, T.L.S.; Mjelle, R.; Vagnildhaug, O.M.; Balstad, T.R.; Kristensen, A.K.; Slagsvold, J.E.; Westwik, G.S.; Elvebakken, H.; Hofsli, E.; Hatlevoll, I.; et al. Serum miRNA Signatures in Cancer Cachexia Depend on Systemic Inflammation. Curr. Oncol. 2025, 32, 620. https://doi.org/10.3390/curroncol32110620
Karlsen TLS, Mjelle R, Vagnildhaug OM, Balstad TR, Kristensen AK, Slagsvold JE, Westwik GS, Elvebakken H, Hofsli E, Hatlevoll I, et al. Serum miRNA Signatures in Cancer Cachexia Depend on Systemic Inflammation. Current Oncology. 2025; 32(11):620. https://doi.org/10.3390/curroncol32110620
Chicago/Turabian StyleKarlsen, Terese Louise Schmidberger, Robin Mjelle, Ola Magne Vagnildhaug, Trude Rakel Balstad, Are Korsnes Kristensen, Jens Erik Slagsvold, Ganna S. Westwik, Hege Elvebakken, Eva Hofsli, Ingunn Hatlevoll, and et al. 2025. "Serum miRNA Signatures in Cancer Cachexia Depend on Systemic Inflammation" Current Oncology 32, no. 11: 620. https://doi.org/10.3390/curroncol32110620
APA StyleKarlsen, T. L. S., Mjelle, R., Vagnildhaug, O. M., Balstad, T. R., Kristensen, A. K., Slagsvold, J. E., Westwik, G. S., Elvebakken, H., Hofsli, E., Hatlevoll, I., & Solheim, T. S. (2025). Serum miRNA Signatures in Cancer Cachexia Depend on Systemic Inflammation. Current Oncology, 32(11), 620. https://doi.org/10.3390/curroncol32110620





