A Multicenter Retrospective Study of Avelumab First-Line Maintenance and Subsequent Therapies for Locally Advanced and Metastatic Urothelial Carcinoma: Subgroup Analysis of First-Line Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, and Gemcitabine Plus Cisplatin in the Japan AVElumab MAintenance and Continuous Treatment Study (JAVEMACS)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
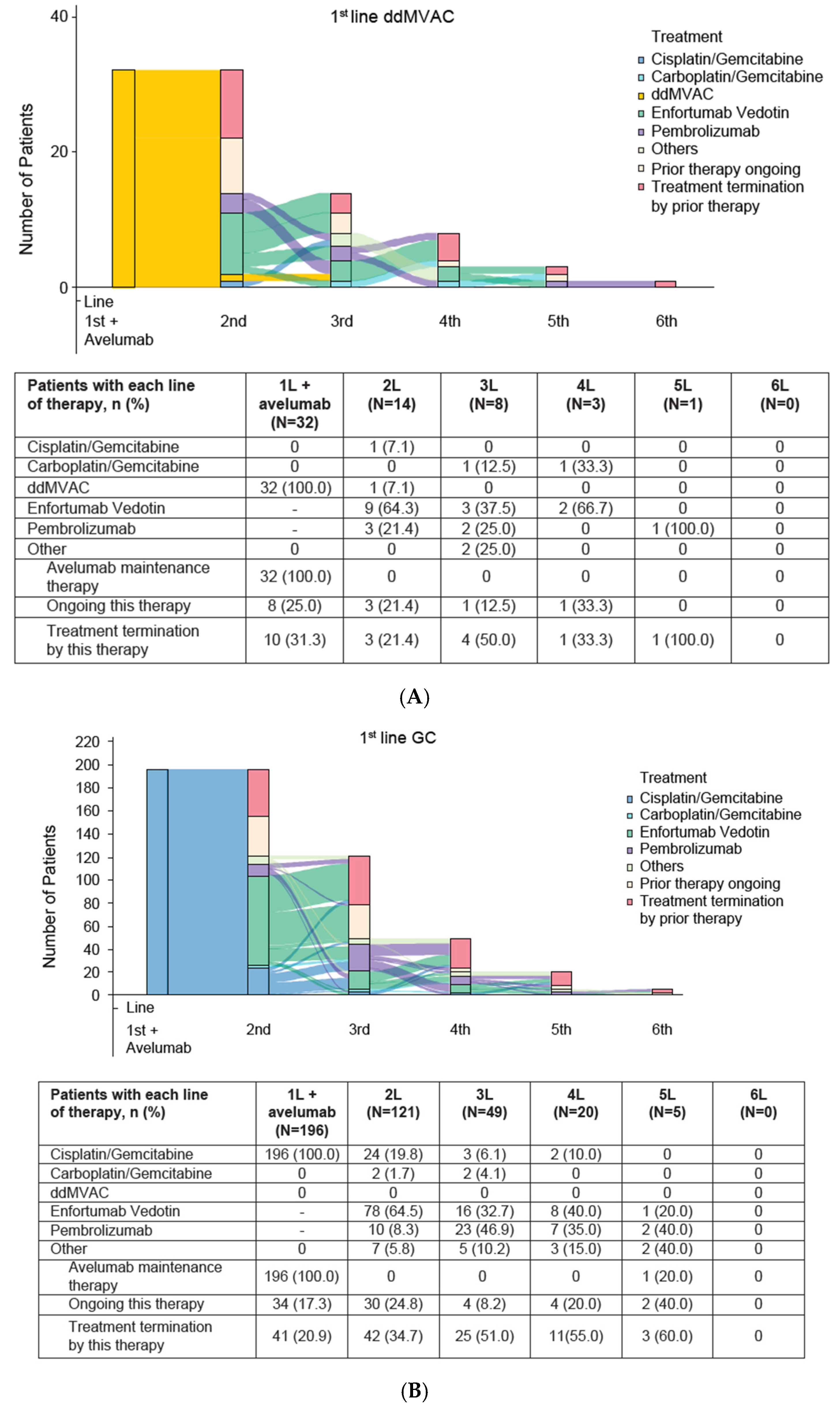
3.2. Subsequent Treatment
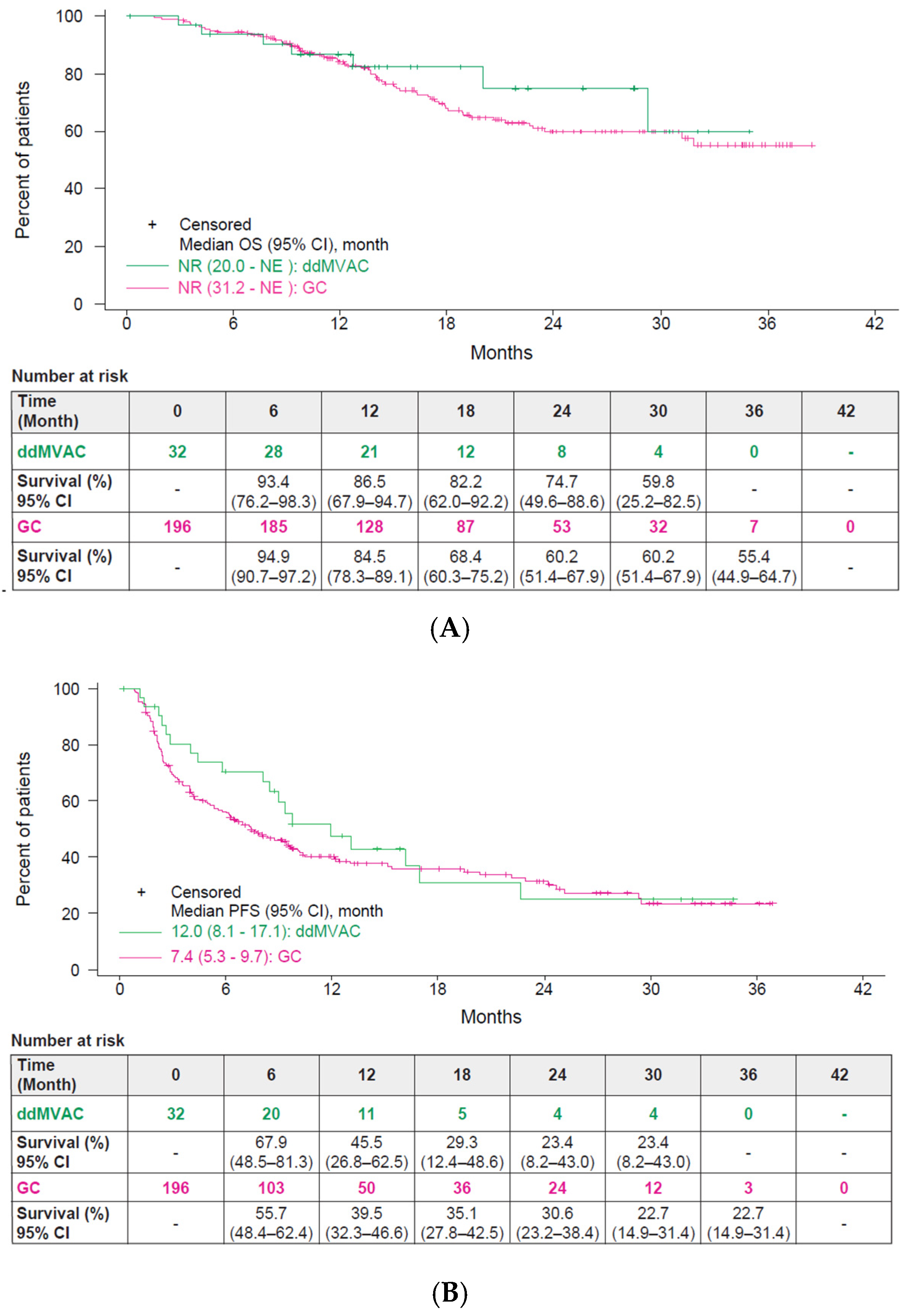
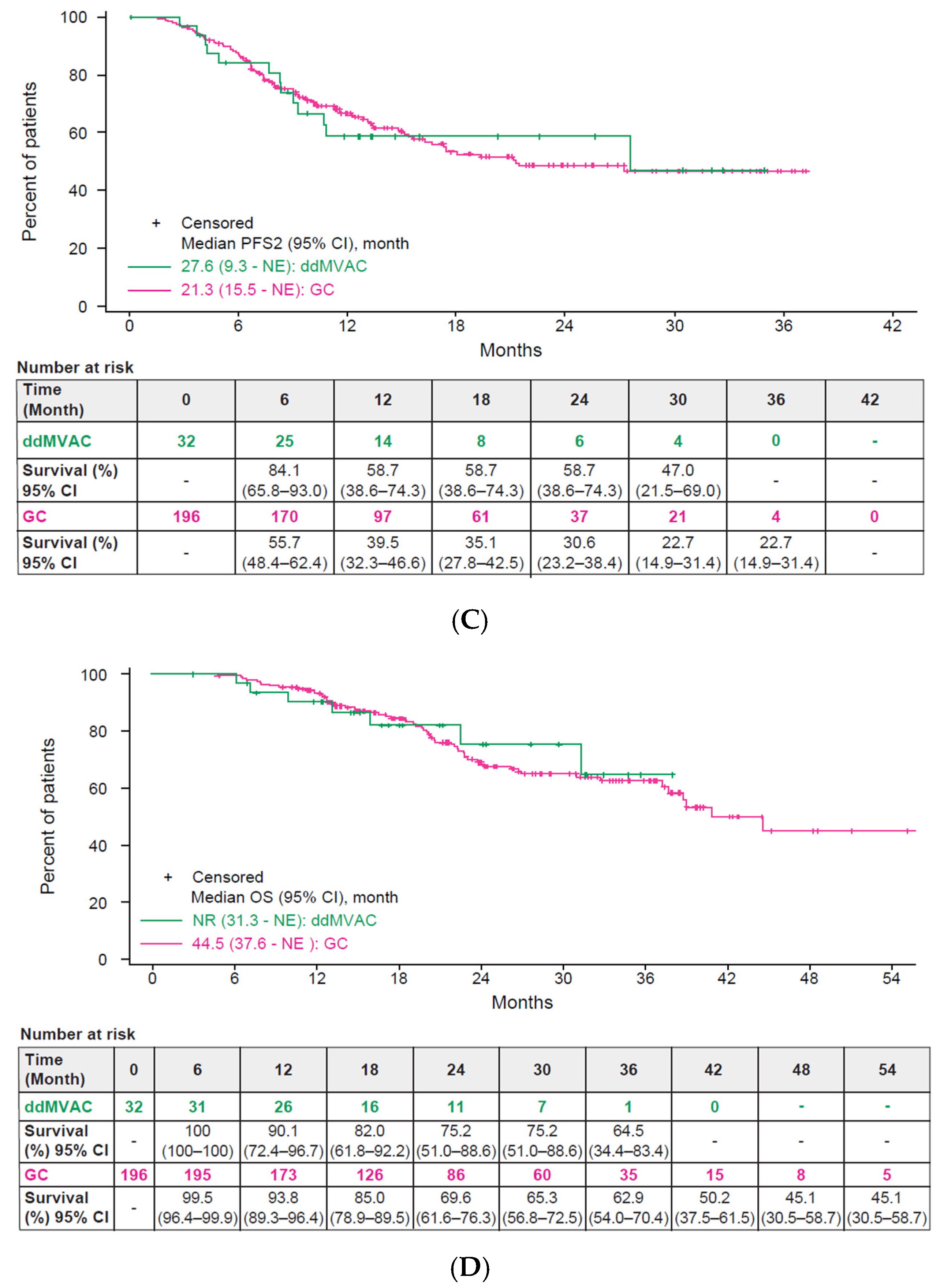
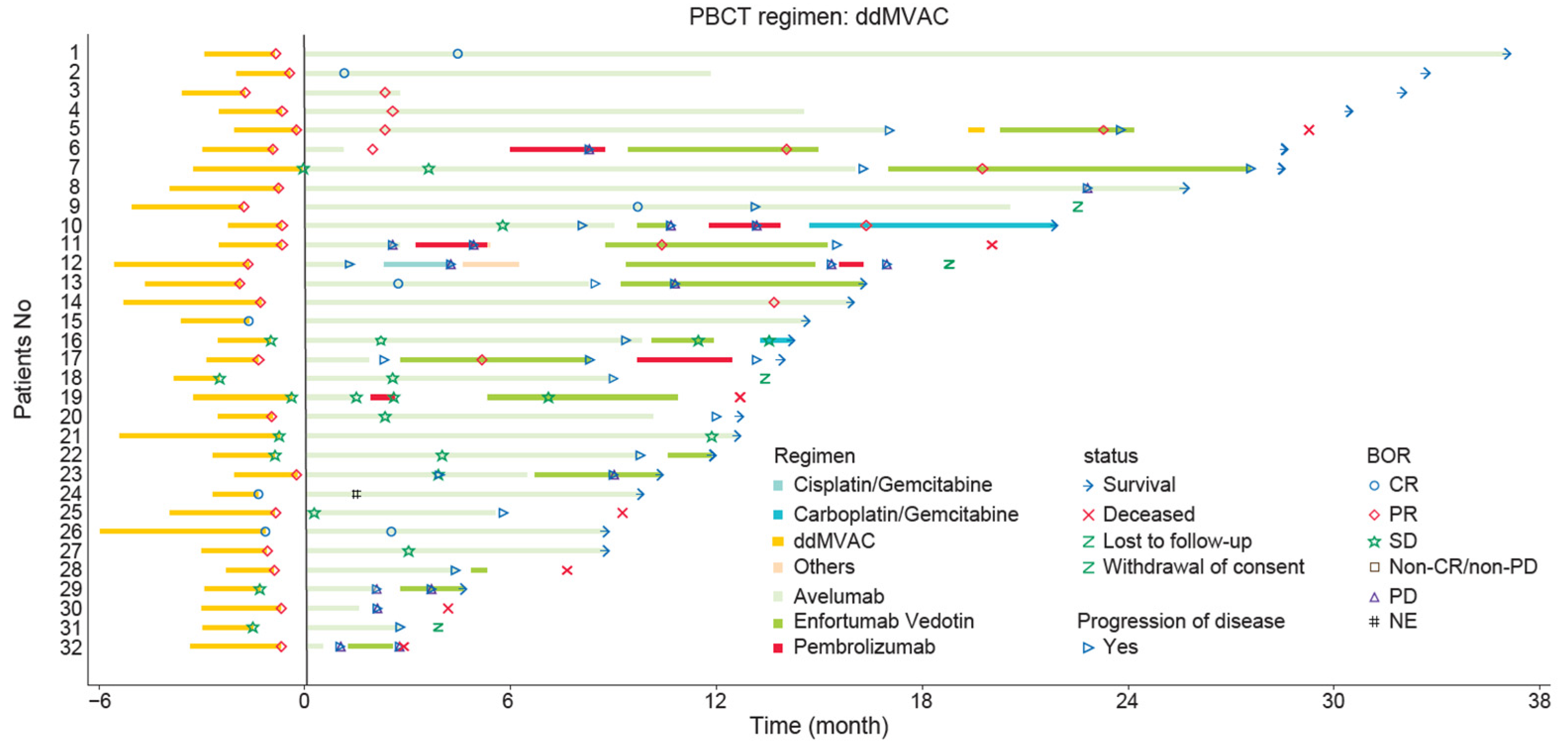
3.3. Effectiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyazaki, J.; Nishiyama, H. Epidemiology of urothelial carcinoma. Int. J. Urol. 2017, 24, 730–734. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Abern, M.; Agarwal, N.; Bangs, R.; Buyyounouski, M.K.; Chan, K.; Chang, S.S.; Chang, P.; Friedlander, T.; et al. NCCN Guidelines® Insights: Bladder Cancer, Version 3.2024. J. Natl. Compr. Canc. Netw. 2024, 22, 216–225. [Google Scholar] [CrossRef]
- World Health Organization. Cancer Factsheets; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- The Cancer Statistical Forecast in 2023 Conducted by the National Cancer Center Japan. Available online: https://ganjoho.jp/reg_stat/statistics/stat/short_pred_en.html (accessed on 4 December 2024).
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Gürses Andersson, I.; Liedberg, F.; Mariappan, P.; Hugh Mostafid, A.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann. Oncol. 2024, 35, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Shiraishi, K.; Azuma, H.; Inoue, K.; Uemura, H.; Eto, M.; Ohyama, C.; Ogawa, O.; Kikuchi, E.; Kitamura, H.; et al. Clinical Practice Guidelines for Bladder Cancer 2019 edition by the Japanese Urological Association: Revision working position paper. Int. J. Urol. 2020, 27, 362–368. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Stecca, C.; Abdeljalil, O.; Sridhar, S.S. Metastatic Urothelial Cancer: A rapidly changing treatment landscape. Ther. Adv. Med. Oncol. 2021, 13, 17588359211047352. [Google Scholar] [CrossRef]
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- Minato, A.; Murooka, K.; Okumura, Y.; Takaba, T.; Higashijima, K.; Nagata, Y.; Tomisaki, I.; Harada, K.; Fujimoto, N. Efficacy of Platinum-based Chemotherapy in Patients With Metastatic Urothelial Carcinoma With Variant Histology. In Vivo 2024, 38, 873–880. [Google Scholar] [CrossRef]
- Sternberg, C.N.; de Mulder, P.; Schornagel, J.H.; Theodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, J.A.; Spina, M.; van Groeningen, C.J.; Duclos, B.; et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur. J. Cancer 2006, 42, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Gravis, G.; Fléchon, A.; Chevreau, C.; Mahammedi, H.; Laguerre, B.; Guillot, A.; Joly, F.; Soulié, M.; Allory, Y.; et al. Perioperative dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin in muscle-invasive bladder cancer (VESPER): Survival endpoints at 5 years in an open-label, randomised, phase 3 study. Lancet Oncol. 2024, 25, 255–264. [Google Scholar] [CrossRef]
- Pfister, C.; Gravis, G.; Fléchon, A.; Soulié, M.; Guy, L.; Laguerre, B.; Mottet, N.; Joly, F.; Allory, Y.; Harter, V.; et al. Randomized Phase III Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients with Muscle-invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur. Urol. 2021, 79, 214–221. [Google Scholar] [CrossRef]
- Dietrich, B.; Siefker-Radtke, A.O.; Srinivas, S.; Yu, E.Y. Systemic Therapy for Advanced Urothelial Carcinoma: Current Standards and Treatment Considerations. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 342–353. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; Sternberg, C.N.; Bellmunt, J.; et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J. Clin. Oncol. 2023, 41, 3486–3492. [Google Scholar] [CrossRef]
- Tomita, Y.; Yamamoto, Y.; Tsuchiya, N.; Kanayama, H.; Eto, M.; Miyake, H.; Powles, T.; Yoshida, M.; Koide, Y.; Umeyama, Y.; et al. Avelumab first-line maintenance plus best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma: JAVELIN Bladder 100 Japanese subgroup analysis. Int. J. Clin. Oncol. 2022, 27, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [CrossRef]
- Gupta, S.; Andreev-Drakhlin, A.; Fajardo, O.; Fassò, M.; Garcia, J.A.; Wee, C.; Schröder, C. Platinum ineligibility and survival outcomes in patients with advanced urothelial carcinoma receiving first-line treatment. J. Natl. Cancer Inst. 2024, 116, 547–554. [Google Scholar] [CrossRef]
- Antonuzzo, L.; Maruzzo, M.; De Giorgi, U.; Santini, D.; Tambaro, R.; Buti, S.; Carrozza, F.; Calabrò, F.; Di Lorenzo, G.; Fornarini, G.; et al. READY: REAl-world Data from an Italian compassionate use program of avelumab first-line maintenance for locallY advanced or metastatic urothelial carcinoma. ESMO Real. World Data Digit. Oncol. 2024, 5, 100068. [Google Scholar] [CrossRef]
- Grivas, P.; Barata, P.C.; Moon, H.H.; Gupta, S.; Hutson, T.E.; Sternberg, C.N.; Brown, J.; Dave, V.; Downey, C.; Shillington, A.C.; et al. Avelumab first-line maintenance therapy for locally advanced/metastatic urothelial carcinoma: Results from the real-world US PATRIOT-II study. J. Clin. Oncol. 2024, 42, 697. [Google Scholar] [CrossRef]
- Barthélémy, P.; Thibault, C.; Fléchon, A.; Gross-Goupil, M.; Voog, E.; Eymard, J.C.; Abraham, C.; Chasseray, M.; Lorgis, V.; Hilgers, W.; et al. Real-world Study of Avelumab First-line Maintenance Treatment in Patients with Advanced Urothelial Carcinoma in France: Overall Results from the Noninterventional AVENANCE Study and Analysis of Outcomes by Second-line Treatment. Eur. Urol. Oncol. 2025, 8, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Harano, T.; Ikeda, M.; Hirano, S.; Shimura, S.; Toyoda, M.; Okuda, S.; Koguchi, D.; Tsumura, H.; Ishii, D.; Matsumoto, K. Safety and efficacy of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim in Japanese patients with advanced or metastatic urothelial carcinoma. Chemotherapy 2025, 70, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Gravis, G.; Fléchon, A.; Chevreau, C.; Mahammedi, H.; Laguerre, B.; Guillot, A.; Joly, F.; Soulié, M.; Allory, Y.; et al. Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Nonmetastatic Muscle-Invasive Bladder Cancer: Results of the GETUG-AFU V05 VESPER Trial. J. Clin. Oncol. 2022, 40, 2013–2022. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Talukder, R.; Lin, G.I.; Makrakis, D.; Diamantopoulos, L.N.; Tripathi, N.; Agarwal, N.; Zakopoulou, R.; Bamias, A.; Brown, J.R.; et al. Response and Outcomes of Maintenance Avelumab After Platinum-Based Chemotherapy (PBC) in Patients With Advanced Urothelial Carcinoma (aUC): “Real World” Experience. Clin. Genitourin. Cancer 2023, 21, 584–593. [Google Scholar] [CrossRef]
- Kikuchi, E.; Hayakawa, N.; Nakayama, M.; Uno, M.; Nakatsu, H.; Kitagawa, C.; Miyake, H.; Yamada, T.; Fujita, K.; Shimoyama, H.; et al. J-AVENUE: A retrospective, real-world study evaluating patient characteristics and outcomes in patients with advanced urothelial carcinoma treated with avelumab first-line maintenance therapy in Japan. Int. J. Urol. 2024, 31, 859–867. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, S.J.; Rha, S.Y.; Beom, S.H.; Seo, H.K.; Keam, B.; Kim, M.; Hong, Y.H.; Yoon, S.; Lee, J.L. Avelumab first-line maintenance treatment in patients with locally advanced or metastatic urothelial carcinoma: Real-world results from a Korean expanded access program. Front. Oncol. 2024, 14, 1403120. [Google Scholar] [CrossRef]
- Chelushkin, M.A.; van Dorp, J.; van Wilpe, S.; Seignette, I.M.; Mellema, J.J.; Alkemade, M.; Gil-Jimenez, A.; Peters, D.; Brugman, W.; Stockem, C.F.; et al. Platinum-Based Chemotherapy Induces Opposing Effects on Immunotherapy Response-Related Spatial and Stromal Biomarkers in the Bladder Cancer Microenvironment. Clin. Cancer Res. 2024, 30, 4227–4239. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Wang, Y.Y.; Wang, J.; Bai, W.J.; Miao, N.J.; Wang, J. Vinblastine resets tumor-associated macrophages toward M1 phenotype and promotes antitumor immune response. J. Immunother. Cancer 2023, 11, e007253. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Guan, X.; Rishipathak, D.; Rapaport, A.S.; Shehata, H.M.; Banchereau, R.; Yuen, K.; Varfolomeev, E.; Hu, R.; Han, C.J.; et al. Immunomodulatory effects and improved soutcomes with cisplatin-versus carboplatin-based chemotherapy plus atezolizumab in urothelial cancer. Cell Rep. Med. 2024, 5, 101393. [Google Scholar] [CrossRef] [PubMed]
| Characteristic, n (%) | 1L PBC | |
|---|---|---|
| ddMVAC (N = 32) | GC (N = 196) | |
| Age, median (range), years | 71 (49–82) | 72 (43–87) |
| <65 years | 10 (31.3) | 44 (22.4) |
| ≥65 years and <75 years | 14 (43.8) | 80 (40.8) |
| ≥75 years and <80 years | 5 (15.6) | 49 (25.0) |
| ≥80 years | 3 (9.4) | 23 (11.7) |
| Sex | ||
| Male | 23 (71.9) | 147 (75.0) |
| Female | 9 (28.1) | 49 (25.0) |
| BMI (kg/m2), median (IQR) | 23.1 (20.3–24.9) | 23.0 (21.0–25.3) |
| <18.5 kg/m2 | 2 (6.3) | 11 (5.6) |
| ≥18.5 kg/m2 and <25 kg/m2 | 22 (68.8) | 120 (61.2) |
| ≥25 kg/m2 | 7 (21.9) | 51 (26.0) |
| Unknown | 1 (3.1) | 14 (7.1) |
| Smoking Status | ||
| No | 10 (31.3) | 51 (26.0) |
| Yes | 21 (65.6) | 142 (72.4) |
| Unknown | 1 (3.1) | 3 (1.5) |
| ECOG PS | ||
| 0 | 22 (68.8) | 171 (87.2) |
| 1 | 8 (25.0) | 20 (10.2) |
| ≥2 | 2 (6.3) | 3 (1.5) |
| Unknown | 0 (0.0) | 2 (1.0) |
| Primary tumor location | ||
| Ureter | 7 (21.9) | 34 (17.3) |
| Renal pelvis | 8 (25.0) | 50 (25.5) |
| Bladder | 15 (46.9) | 110 (56.1) |
| Urethra | 2 (6.3) | 2 (1.0) |
| Metastatic site | ||
| Metastases | 26 (81.3) | 164 (83.7) |
| Regional lymph node | 21 (65.6) | 100 (51.0) |
| Distant lymph node | 14 (43.8) | 47 (24.0) |
| Visceral | 15 (46.9) | 60 (30.6) |
| Lung | 8 (25.0) | 40 (20.4) |
| Liver | 4 (12.5) | 11 (5.6) |
| Peritoneum | 2 (6.3) | 8 (4.1) |
| Other organs | 2 (6.3) | 6 (3.1) |
| Bone | 3 (9.4) | 33 (16.8) |
| Other | 0 (0.0) | 6 (3.1) |
| Variant histology | ||
| Pure UC | 24 (75.0) | 147 (75.0) |
| UC with variant | 2 (6.3) | 32 (16.3) |
| Pure non-UC | 2 (6.3) | 2 (1.0) |
| Unknown | 4 (12.5) | 15 (7.7) |
| Radical surgery history | ||
| No | 21 (65.6) | 115 (58.7) |
| Yes | 11 (34.4) | 81 (41.3) |
| Adjuvant and neoadjuvant history | ||
| No | 29 (90.6) | 161 (82.1) |
| Yes | 3 (9.4) | 32 (16.3) |
| Unknown | 0 (0.0) | 3 (1.5) |
| Hb (g/dL), median (IQR) | 9.6 (9.0–11.5) | 10.7 (9.9–11.7) |
| <10 g/dL | 18 (56.3) | 52 (26.5) |
| ≥10 g/dL | 14 (43.8) | 142 (72.4) |
| Unknown | 0 (0.0) | 2 (1.0) |
| NLR, median (IQR) | 3.00 (2.17–4.31) | 2.31 (1.58–3.27) |
| <3 | 15 (46.9) | 132 (67.3) |
| ≥3 | 16 (50.0) | 61 (31.1) |
| Unknown | 1 (3.1) | 3 (1.5) |
| CRP (mg/dL), median (IQR) | 0.10 (0.03–0.37) | 0.15 (0.07–0.43) |
| ≤0.3 mg/dL | 22 (68.8) | 131 (66.8) |
| >0.3 mg/dL | 9 (28.1) | 60 (30.6) |
| Unknown | 1 (3.1) | 5 (2.6) |
| CCr (mL/min), median (IQR) | 65.0 (43.4–83.0) | 57.2 (45.2–69.3) |
| ≥60 mL/min | 18 (56.3) | 81 (41.3) |
| <60 mL/min | 13 (40.6) | 107 (54.6) |
| Unknown | 1 (3.1) | 8 (4.1) |
| Characteristic, n (%) | 1L PBC Regimen | |
|---|---|---|
| ddMVAC (N = 32) | GC (N = 196) | |
| Platinum eligibility at 1L PBC initiation | ||
| Cisplatin eligible | 16 (50.0) | 78 (39.8) |
| Cisplatin ineligible a/platinum eligible | 14 (43.8) | 91 (46.4) |
| Platinum ineligible b | 1 (3.1) | 2 (1.0) |
| Unknown | 1 (3.1) | 25 (12.8) |
| Number of cycles, median (IQR) | 4.0 (4.0–4.0) | 4.0 (4.0–5.0) |
| 1–3 cycles | 4 (12.5) | 37 (18.9) |
| 4 cycles | 22 (68.8) | 109 (55.6) |
| 5, 6 cycles | 6 (18.8) | 38 (19.4) |
| ≥7 cycles | 0 (0.0) | 12 (6.1) |
| Duration (weeks), median (IQR) | 13.2 (11.2–17.1) | 21.1 (17.4–27.1) |
| Platinum dose reduction, n (%) | 14 (43.8) | 74 (37.8) |
| First cycle when platinum dose reduction occurred, median (IQR) | 1.0 (1.0–2.0) | 1.5 (1.0–2.0) |
| 1 cycle | 8 (25.0) | 37 (18.9) |
| 2 cycles | 3 (9.4) | 20 (10.2) |
| 3 cycles | 2 (6.3) | 8 (4.1) |
| ≥4 cycles | 1 (3.1) | 9 (4.6) |
| TFI (weeks), median (IQR) | 4.2 (3.2–6.1) | 5.1 (3.6–7.4) |
| <4 weeks | 12 (37.5) | 51 (26.0) |
| 4 to 10 weeks | 19 (59.4) | 119 (60.7) |
| >10 weeks | 1 (3.1) | 26 (13.3) |
| BOR | ||
| CR | 3 (9.4) | 21 (10.7) |
| PR | 21 (65.6) | 103 (52.6) |
| SD | 8 (25.0) | 72 (36.7) |
| 1L PBC | ||
|---|---|---|
| ddMVAC (N = 32) | Cisplatin/Gemcitabine (N = 196) | |
| Number of cases with response evaluation, n | 26 | 173 |
| BOR, n (%) | ||
| CR | 5 (19.2) | 20 (10.2) |
| PR | 5 (19.2) | 23 (11.7) |
| SD | 11 (42.3) | 58 (29.6) |
| Non-CR/non-PD | 0 (0.0) | 9 (4.6) |
| PD | 4 (15.4) | 61 (31.1) |
| NE | 1 (3.8) | 2 (1.0) |
| ORR%, (95% CI) a,c | 38.5 (20.2–59.4) | 24.9 (18.6–32.0) |
| DCR% (95% CI) b,c | 80.8 (60.6–93.4) | 63.6 (55.9–70.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, M.; Fujimoto, K.; Miura, N.; Taoka, R.; Nishihara, K.; Ikarashi, D.; Naito, S.; Shimizu, F.; Fujihara, A.; Shono, M.; et al. A Multicenter Retrospective Study of Avelumab First-Line Maintenance and Subsequent Therapies for Locally Advanced and Metastatic Urothelial Carcinoma: Subgroup Analysis of First-Line Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, and Gemcitabine Plus Cisplatin in the Japan AVElumab MAintenance and Continuous Treatment Study (JAVEMACS). Curr. Oncol. 2025, 32, 618. https://doi.org/10.3390/curroncol32110618
Ikeda M, Fujimoto K, Miura N, Taoka R, Nishihara K, Ikarashi D, Naito S, Shimizu F, Fujihara A, Shono M, et al. A Multicenter Retrospective Study of Avelumab First-Line Maintenance and Subsequent Therapies for Locally Advanced and Metastatic Urothelial Carcinoma: Subgroup Analysis of First-Line Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, and Gemcitabine Plus Cisplatin in the Japan AVElumab MAintenance and Continuous Treatment Study (JAVEMACS). Current Oncology. 2025; 32(11):618. https://doi.org/10.3390/curroncol32110618
Chicago/Turabian StyleIkeda, Masaomi, Kiyohide Fujimoto, Noriyoshi Miura, Rikiya Taoka, Kiyoaki Nishihara, Daiki Ikarashi, Sei Naito, Fumitaka Shimizu, Atsuko Fujihara, Michihiro Shono, and et al. 2025. "A Multicenter Retrospective Study of Avelumab First-Line Maintenance and Subsequent Therapies for Locally Advanced and Metastatic Urothelial Carcinoma: Subgroup Analysis of First-Line Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, and Gemcitabine Plus Cisplatin in the Japan AVElumab MAintenance and Continuous Treatment Study (JAVEMACS)" Current Oncology 32, no. 11: 618. https://doi.org/10.3390/curroncol32110618
APA StyleIkeda, M., Fujimoto, K., Miura, N., Taoka, R., Nishihara, K., Ikarashi, D., Naito, S., Shimizu, F., Fujihara, A., Shono, M., Nakagawa, T., & Kikuchi, E. (2025). A Multicenter Retrospective Study of Avelumab First-Line Maintenance and Subsequent Therapies for Locally Advanced and Metastatic Urothelial Carcinoma: Subgroup Analysis of First-Line Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, and Gemcitabine Plus Cisplatin in the Japan AVElumab MAintenance and Continuous Treatment Study (JAVEMACS). Current Oncology, 32(11), 618. https://doi.org/10.3390/curroncol32110618





