Neurocognitive and Emotional Outcomes in Childhood Cancer: A Developmental Perspective
Simple Summary
Abstract
1. Introduction
2. Methodological Approach
- PubMed:
- Scopus:
- PsycINFO (via APA PsycNet):
- Web of Science (Core Collection):
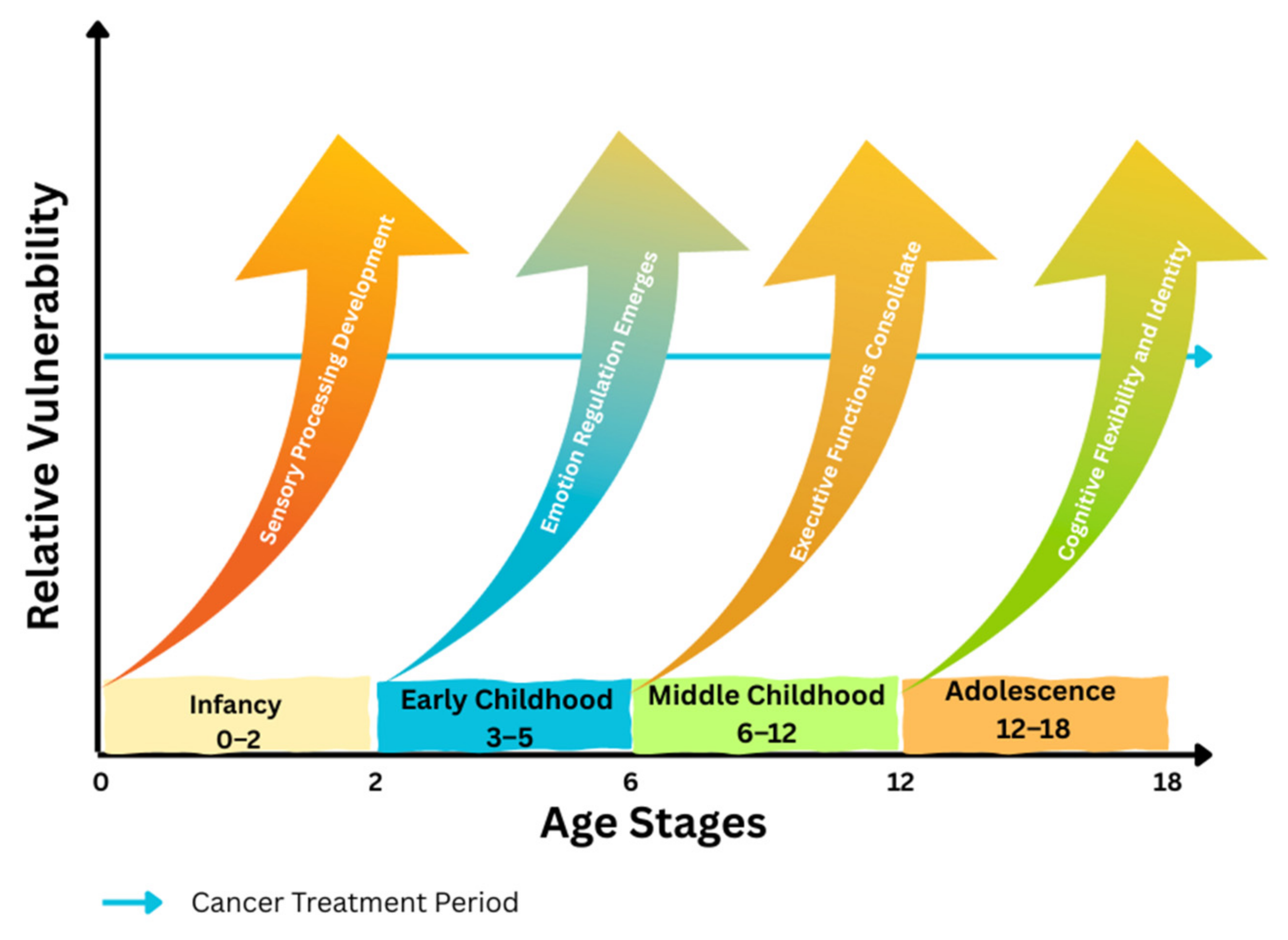
3. Developmental Overview
4. Neurocognitive Outcomes in Childhood Cancer Survivors
4.1. Infancy and Toddlerhood
4.2. Early Childhood
4.3. Middle Childhood
4.4. Adolescence
5. Emotional Outcomes in Childhood Cancer Survivors
5.1. Infancy and Toddlerhood
5.2. Early Childhood
5.3. Middle Childhood
5.4. Adolescence
5.5. Family Dynamics and Environmental Influences
5.6. Methodological Considerations in Survivor Research
6. Neuropsychological Assessment in Survivorship
6.1. Assessment Tools and Practices
6.2. Factors Affecting Assessment Outcomes
7. Interventions and Support Strategies
7.1. Cognitive Rehabilitation
7.2. Psychosocial and Emotional Support
7.3. Educational and School Reintegration
7.4. Innovative Therapies and Recreational Programs
8. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EF | Executive Functions |
| ALL | Acute Lymphoblastic Leukemia |
| CNS | Central Nervous System |
| SPS | Sensory Processing Sensitivity |
| CBT | Cognitive–Behavioral Therapy |
| RCT | Randomized Controlled Trial |
References
- Armstrong, G.T.; Chen, Y.; Yasui, Y.; Leisenring, W.; Gibson, T.M.; Mertens, A.C.; Stovall, M.; Oeffinger, K.C.; Bhatia, S.; Krull, K.R.; et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N. Engl. J. Med. 2016, 374, 833–842. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Recklitis, C.J.; Michel, G.; Grootenhuis, M.A.; Klosky, J.L. Psychological Symptoms, Social Outcomes, Socioeconomic Attainment, and Health Behaviors among Survivors of Childhood Cancer: Current State of the Literature. J. Clin. Oncol. 2018, 36, 2190–2197. [Google Scholar] [CrossRef]
- Krull, K.R.; Hardy, K.K.; Kahalley, L.S.; Schuitema, I.; Kesler, S.R. Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer. J. Clin. Oncol. 2018, 36, 2181–2189. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Krull, K.R. Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia Treated on Contemporary Protocols: A Systematic Review. Neurosci. Biobehav. Rev. 2015, 53, 108–120. [Google Scholar] [CrossRef]
- Alemany, M.; Velasco, R.; Simó, M.; Bruna, J. Late effects of cancer treatment: Consequences for long-term brain cancer survivors. Neurooncol. Pract. 2021, 8, 18–30. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Ness, K.K.; Li, Z.; Huang, I.-C.; Krull, K.R.; Gajjar, A.; Merchant, T.E.; Klosky, J.L.; Partin, R.E.; Tonning Olsson, I.; et al. Attainment of functional and social independence in adult survivors of pediatric CNS tumors: A report from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2018, 36, 2762–2769. [Google Scholar] [CrossRef]
- Robinson, K.E.; Kuttesch, J.F.; Champion, J.E.; Andreotti, C.F.; Hipp, D.W.; Bettis, A.; Barnwell, A.; Compas, B.E. A Quantitative Meta-Analysis of Neurocognitive Sequelae in Survivors of Pediatric Brain Tumors. Pediatr. Blood Cancer 2010, 55, 525–531. [Google Scholar] [CrossRef]
- Mabbott, D.J.; Penkman, L.; Witol, A.; Strother, D.; Bouffet, E. Core Neurocognitive Functions in Children Treated for Posterior Fossa Tumors. Neuropsychology 2008, 22, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, R.K.; Palmer, S.L.; Merchant, T.E.; Wallace, D.; Kocak, M.; Brouwers, P.; Krull, K.R.; Chintagumpala, M.; Woo, S.Y.; Douglas Ris, M. Neurocognitive Consequences of Risk-Adapted Therapy for Childhood Medulloblastoma. J. Clin. Oncol. 2005, 23, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Godber, T.; Smibert, E.; Weiskop, S.; Ekert, H. Cognitive and academic outcome following cranial irradiation and chemotherapy in children: A longitudinal study. Br. J. Cancer 2000, 82, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wengenroth, L.; Rueegg, C.S.; Michel, G.; Essig, S.; Ammann, R.A.; Bergstraesser, E.; Kuehni, C.E.; Swiss Paediatric Oncology Group (SPOG). Life partnerships in childhood cancer survivors, their siblings, and the general population. Pediatr. Blood Cancer 2014, 61, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.L.; Conklin, H.M.; Tyc, V.L.; Stancel, H.; Hinds, P.S.; Hudson, M.M.; Kahalley, L.S. Executive function late effects in survivors of pediatric brain tumors and acute lymphoblastic leukemia. J. Clin. Exp. Neuropsychol. 2014, 36, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Hocking, M.C.; Hobbie, W.L.; Deatrick, J.A.; Lucas, M.S.; Szabo, M.M.; Volpe, E.M.; Barakat, L.P. Neurocognitive and family functioning and quality of life among young adult survivors of childhood brain tumors. Clin. Neuropsychol. 2011, 25, 942–962. [Google Scholar] [CrossRef]
- Steinhausen, H.C.; Helenius, D. The association between medication for attention-deficit/hyperactivity disorder and cancer. J. Child Adolesc. Psychopharmacol. 2013, 23, 208–213. [Google Scholar] [CrossRef]
- Kahalley, L.S.; Conklin, H.M.; Tyc, V.L.; Hudson, M.M.; Wilson, S.J.; Wu, S.; Xiong, X.; Stancel, H.H.; Hinds, P.S. Slower Processing Speed after Treatment for Pediatric Brain Tumor and Acute Lymphoblastic Leukemia. Psychooncology 2013, 22, 1979–1986. [Google Scholar] [CrossRef]
- Michel, G.; Rebholz, C.E.; von der Weid, N.X.; Bergstraesser, E.; Kuehni, C.E. Psychological distress in adult survivors of childhood cancer: The Swiss Childhood Cancer Survivor Study. J. Clin. Oncol. 2010, 28, 1740–1748. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Li, C.; Vannatta, K.; Marchak, J.G.; Muriel, A.C.; Banerjee, P.; Srivastava, D.K.; Robison, L.L.; Armstrong, G.T.; Krull, K.R. Behavioral, Social, and Emotional Symptom Comorbidity and Profiles in Adolescent Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2016, 34, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.Y.B.; Low, C.E.; Yau, C.E.; Li, J.; Ho, R.; Ho, C.S.H. Lifetime Burden of Psychological Symptoms, Disorders, and Suicide Due to Cancer in Childhood, Adolescent, and Young Adult Years: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2023, 177, 790–799. [Google Scholar] [CrossRef]
- Klosky, J.L.; Krull, K.R.; Kawashima, T.; Leisenring, W.; Zebrack, B.; Stuber, M.L.; Robison, L.L.; Mertens, A.C. Relations between Posttraumatic Stress and Posttraumatic Growth in Long-Term Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Health Psychol. 2014, 33, 878–882. [Google Scholar] [CrossRef]
- Kenney, L.B.; Cohen, L.E.; Shnorhavorian, M.; Metzger, M.L.; Lockart, B.; Hijiya, N.; Duffey-Lind, E.; Constine, L.; Green, D.; Meacham, L. Male reproductive health after childhood, adolescent, and young adult cancers: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 3408–3416. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Hyun, G.; Ness, K.K.; Ehrhardt, M.J.; Yasui, Y.; Duprez, D.; Howell, R.M.; Leisenring, W.M.; Constine, L.S.; Tonorezos, E.; et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: Report from the Childhood Cancer Survivor Study cohort. BMJ 2020, 368, l6794. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Ullrich, N.J.; Zhang, N.; Green, D.M.; Zeltzer, L.K.; Lommel, K.M.; Brouwers, P.; Srivastava, D.K.; Jain, N.; Robison, L.L.; et al. Prevalence and predictors of prescription psychoactive medication use in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Cancer Surviv. 2013, 7, 104–114. [Google Scholar] [CrossRef]
- Hjern, A.; Lindblad, F.; Boman, K.K. Disability in adult survivors of childhood cancer: A Swedish national cohort study. J. Clin. Oncol. 2007, 25, 5262–5266. [Google Scholar] [CrossRef]
- Rueegg, C.S.; Gianinazzi, M.E.; Rischewski, J.; Beck Popovic, M.; von der Weid, N.X.; Michel, G.; Kuehni, C.E. Health-related quality of life in survivors of childhood cancer: The role of chronic health problems. J. Cancer Surviv. 2013, 7, 511–522. [Google Scholar] [CrossRef]
- Zeltzer, L.K.; Recklitis, C.; Buchbinder, D.; Zebrack, B.; Casillas, J.; Tsao, J.C.I.; Lu, Q.; Krull, K. Psychological Status in Childhood Cancer Survivors: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2396–2404. [Google Scholar] [CrossRef]
- Kunin-Batson, A.; Kadan-Lottick, N.; Zhu, L.; Cox, C.; Bordes-Edgar, V.; Srivastava, D.K.; Zeltzer, L.; Robison, L.L.; Krull, K.R. Predictors of independent living status in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2011, 57, 1197–1203. [Google Scholar] [CrossRef]
- Hardy, K.K.; Embry, L.; Kairalla, J.A.; Helian, S.; Devidas, M.; Armstrong, D.; Hunger, S.; Carroll, W.L.; Larsen, E.; Raetz, E.A.; et al. Neurocognitive functioning of children treated for high-risk B-acute lymphoblastic leukemia randomly assigned to different methotrexate and corticosteroid treatment strategies: A report from the Children’s Oncology Group. J. Clin. Oncol. 2017, 35, 2700–2707. [Google Scholar] [CrossRef]
- Alias, H.; Mohd Ranai, N.; Lau, S.C.D.; de Sonneville, L.M. Neuropsychological task outcomes among survivors of childhood acute lymphoblastic leukemia in Malaysia. Sci. Rep. 2024, 14, 7915. [Google Scholar] [CrossRef]
- Phillips, N.S.; Baedke, J.L.; Williams, A.; Ji, Q.; Zhang, S.; Scoggins, M.; Sabin, N.; Merchant, T.E.; Gajjar, A.J.; Ness, K.K.; et al. Accelerated brain age and associated neurocognitive impairments in adult survivors of childhood cancer. J. Clin. Oncol. 2023, 41, 10028. [Google Scholar] [CrossRef]
- Kesler, S.R.; Sleurs, C.; McDonald, B.C.; Deprez, S.; van der Plas, E.; Nieman, B.J. Brain imaging in pediatric cancer survivors: Correlates of cognitive impairment. J. Clin. Oncol. 2021, 39, 1775–1785. [Google Scholar] [CrossRef]
- Kahalley, L.S.; Douglas Ris, M.; Grosshans, D.R.; Okcu, M.F.; Paulino, A.C.; Chintagumpala, M.; Moore, B.D. Comparing Intelligence Quotient Change after Treatment with Proton versus Photon Radiation Therapy for Pediatric Brain Tumors. J. Clin. Oncol. 2016, 34, 1043–1049. [Google Scholar] [CrossRef]
- Seghatol-Eslami, V.C.; Cook, E.W., III; Sharafeldin, N.; Wolfson, J.; Murdaugh, D.L. Adaptive functioning and academic achievement in pediatric survivors of acute lymphoblastic leukemia: Associations with executive functioning, socioeconomic status, and academic support. Eur. J. Haematol. 2024, 112, 266–275. [Google Scholar] [CrossRef]
- van der Plas, E.; Schachar, R.J.; Hitzler, J.; Crosbie, J.; Guger, S.L.; Spiegler, B.J.; Ito, S.; Nieman, B.J. Brain structure, working memory and response inhibition in childhood leukemia survivors. Brain Behav. 2016, 7, e00621. [Google Scholar] [CrossRef]
- Phillips, S.M.; Padgett, L.S.; Leisenring, W.M.; Stratton, K.K.; Bishop, K.; Krull, K.R.; Alfano, C.M.; Gibson, T.M.; de Moor, J.S.; Hartigan, D.B.; et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol. Biomark. Prev. 2015, 24, 653–663. [Google Scholar] [CrossRef]
- Escherich, G.; Bielack, S.; Maier, S.; Braungart, R.; Brümmendorf, T.H.; Freund, M.; Grosse, R.; Hoferer, A.; Kampschulte, R.; Koch, B.; et al. Building a national framework for adolescent and young adult hematology and oncology and transition from pediatric to adult care: Report of the inaugural meeting of the “AjET” working group of the German Society for Pediatric Oncology and Hematology. J. Adolesc. Young Adult Oncol. 2017, 6, 194–199. [Google Scholar] [CrossRef]
- Prasad, P.K.; Hardy, K.K.; Zhang, N.; Edelstein, K.; Srivastava, D.; Zeltzer, L.; Stovall, M.; Seibel, N.L.; Leisenring, W.; Armstrong, G.T.; et al. Psychosocial and neurocognitive outcomes in adult survivors of adolescent and early young adult cancer: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2015, 33, 2545–2552. [Google Scholar] [CrossRef]
- Kunin-Batson, A.; Kadan-Lottick, N.; Neglia, J.P. The contribution of neurocognitive functioning to quality of life after childhood acute lymphoblastic leukemia. Psychooncology 2014, 23, 692–699. [Google Scholar] [CrossRef]
- Ford, J.S.; Kawashima, T.; Whitton, J.; Leisenring, W.; Laverdière, C.; Stovall, M.; Zeltzer, L.; Robison, L.L.; Sklar, C.A. Psychosexual functioning among adult female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2014, 32, 3126–3136. [Google Scholar] [CrossRef]
- Wengenroth, L.; Gianinazzi, M.E.; Rueegg, C.S.; Lüer, S.; Bergstraesser, E.; Kuehni, C.E.; Michel, G. Health-related quality of life in young survivors of childhood cancer. Qual. Life Res. 2015, 24, 2151–2161. [Google Scholar] [CrossRef][Green Version]
- Zebrack, B.J.; Chesler, M.A. Quality of life in childhood cancer survivors. Psychooncology 2002, 11, 132–141. [Google Scholar] [CrossRef]
- Norsker, F.N.; Pedersen, C.; Armstrong, G.T.; Robison, L.L.; McBride, M.L.; Hawkins, M.; Kuehni, C.E.; de Vathaire, F.; Berbis, J.; Kremer, L.C.; et al. Late Effects in Childhood Cancer Survivors: Early Studies, Survivor Cohorts, and Significant Contributions to the Field of Late Effects. Pediatr. Clin. N. Am. 2020, 67, 1033–1049. [Google Scholar] [CrossRef]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical Ascertainment of Health Outcomes among Adults Treated for Childhood Cancer. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Ness, K.K.; Kirkland, J.L.; Gramatges, M.M.; Wang, Z.; Kundu, M.; McCastlain, K.; Li-Harms, X.; Zhang, J.; Tchkonia, T.; Pluijm, S.M.F.; et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J. Clin. Oncol. 2018, 36, 2206–2215. [Google Scholar] [CrossRef]
- Armenian, S.H.; Armstrong, G.T.; Aune, G.; Chow, E.J.; Ehrhardt, M.J.; Ky, B.; Moslehi, J.; Mulrooney, D.A.; Nathan, P.C.; Ryan, T.D.; et al. Cardiovascular Disease in Survivors of Childhood Cancer: Insights into Epidemiology, Pathophysiology, and Prevention. J. Clin. Oncol. 2018, 36, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Alberts, N.M.; Leisenring, W.; Whitton, J.; Stratton, K.; Jibb, L.; Flynn, J.; Pizzo, A.; Brinkman, T.M.; Birnie, K.; Gibson, T.M.; et al. Characterization of Chronic Pain, Pain Interference, and Daily Pain Experiences in Adult Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Pain 2024, 165, 2530–2543. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Sabin, N.D.; Reddick, W.E.; Bhojwani, D.; Liu, W.; Brinkman, T.M.; Glass, J.O.; Hwang, S.N.; Srivastava, D.; Pui, C.H.; et al. Leukoencephalopathy and Long-Term Neurobehavioural, Neurocognitive, and Brain Imaging Outcomes in Survivors of Childhood Acute Lymphoblastic Leukaemia Treated with Chemotherapy: A Longitudinal Analysis. Lancet Haematol. 2016, 3, e456–e466. [Google Scholar] [CrossRef]
- Janssen, S.H.M.; van der Graaf, W.T.A.; van der Meer, D.J.; Manten-Horst, E.; Husson, O. Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview. Cancers 2021, 13, 4847. [Google Scholar] [CrossRef]
- Kahalley, L.S.; Peterson, R.; Ris, M.D.; Janzen, L.; Okcu, M.F.; Grosshans, D.; Ramaswamy, V.; Paulino, A.C.; Conklin, H.M. Superior Intellectual Outcomes after Proton Radiotherapy Compared with Photon Radiotherapy for Pediatric Medulloblastoma. J. Clin. Oncol. 2020, 38, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Jacola, L.M.; Ashford, J.M.; Reddick, W.E.; Glass, J.O.; Ogg, R.J.; Merchant, T.E.; Conklin, H.M. The Relationship between Working Memory and Cerebral White Matter Volume in Survivors of Childhood Brain Tumors Treated with Conformal Radiation Therapy. J. Neurooncol. 2014, 119, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Liu, W.; Leisenring, W.; Yasui, Y.; Hammond, S.; Bhatia, S.; Neglia, J.P.; Stovall, M.; Srivastava, D.; Robison, L.L. Occurrence of Multiple Subsequent Neoplasms in Long-Term Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2011, 29, 3056–3064. [Google Scholar] [CrossRef]
- Huang, T.T.; Hudson, M.M.; Stokes, D.C.; Krasin, M.J.; Spunt, S.L.; Ness, K.K. Pulmonary Outcomes in Survivors of Childhood Cancer: A Systematic Review. Chest 2011, 140, 881–901. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, T.M.; Zhang, N.; Recklitis, C.J.; Kimberg, C.; Zeltzer, L.K.; Muriel, A.C.; Stovall, M.; Srivastava, D.K.; Sklar, C.A.; Robison, L.L.; et al. Suicide Ideation and Associated Mortality in Adult Survivors of Childhood Cancer. Cancer 2014, 120, 271–277. [Google Scholar] [CrossRef]
- Klosky, J.L.; Howell, C.R.; Li, Z.; Foster, R.H.; Mertens, A.C.; Robison, L.L.; Hudson, M.M. Risky Health Behavior among Adolescents in the Childhood Cancer Survivor Study Cohort. J. Pediatr. Psychol. 2012, 37, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Zebrack, B.; Isaacson, S. Psychosocial Care of Adolescent and Young Adult Patients with Cancer and Survivors. J. Clin. Oncol. 2012, 30, 1221–1226. [Google Scholar] [CrossRef]
- Sansom-Daly, U.M.; Wakefield, C.E.; Bryant, R.A.; Butow, P.; Sawyer, S.; Patterson, P.; Anazodo, A.; Thompson, K.; Cohn, R.J. Online Group-Based Cognitive-Behavioural Therapy for Adolescents and Young Adults after Cancer Treatment: A Multicenter Randomised Controlled Trial of Recapture Life-AYA. BMC Cancer 2012, 12, 339. [Google Scholar] [CrossRef]
- Michel, G.; Brinkman, T.M.; Wakefield, C.E.; Grootenhuis, M. Psychological Outcomes, Health-Related Quality of Life, and Neurocognitive Functioning in Survivors of Childhood Cancer and Their Parents. Pediatr. Clin. N. Am. 2020, 67, 1103–1134. [Google Scholar] [CrossRef]
- Schwartz, L.F.; Dhaduk, R.; Howell, C.R.; Brinkman, T.M.; Ehrhardt, M.J.; Delaney, A.; Srivastava, D.K.; Lanctot, J.Q.; Armstrong, G.T.; Robison, L.L.; et al. The Association of Neighborhood Characteristics and Frailty in Childhood Cancer Survivors: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol. Biomark. Prev. 2023, 32, 1021–1029. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Hudson, M.M. Long-Term Complications Following Childhood and Adolescent Cancer: Foundations for Providing Risk-Based Health Care for Survivors. CA Cancer J. Clin. 2004, 54, 208–236. [Google Scholar] [CrossRef]
- van Kalsbeek, R.J.; Hudson, M.M.; Mulder, R.L.; Ehrhardt, M.; Green, D.M.; Mulrooney, D.A.; Hakkert, J.; den Hartogh, J.; Nijenhuis, A.; van Santen, H.M.; et al. A Joint International Consensus Statement for Measuring Quality of Survival for Patients with Childhood Cancer. Nat. Med. 2023, 29, 1340–1348. [Google Scholar] [CrossRef]
- Robison, L.L.; Hudson, M.M. Survivors of Childhood and Adolescent Cancer: Life-Long Risks and Responsibilities. Nat. Rev. Cancer 2014, 14, 61–70. [Google Scholar] [CrossRef]
- Sørensen, G.V.; Belmonte, F.; Erdmann, F.; Mogensen, H.; Albieri, V.; Holmqvist, A.S.; Madanat-Harjuoja, L.; Talbäck, M.; Heyman, M.M.; Malila, N.; et al. Late mortality among survivors of childhood acute lymphoblastic leukemia diagnosed during 1971–2008 in Denmark, Finland, and Sweden: A population-based cohort study. Pediatr. Blood Cancer 2022, 69, e29356. [Google Scholar] [CrossRef]
- Kadan-Lottick, N.S.; Zeltzer, L.K.; Liu, Q.; Yasui, Y.; Ellenberg, L.; Gioia, G.; Robison, L.L.; Ness, K.K. Neurocognitive Functioning in Adult Survivors of Childhood Non-CNS Cancers. J. Natl. Cancer Inst. 2010, 102, 881–893. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Brinkman, T.M.; Li, C.; Mzayek, Y.; Srivastava, D.; Ness, K.K.; Patel, S.K.; Howell, R.M.; Oeffinger, K.C.; Robison, L.L.; et al. Chronic Health Conditions and Neurocognitive Function in Aging Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2018, 110, 411–419. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Kawashima, T.; Leisenring, W.; Stratton, K.; Stovall, M.; Hudson, M.M.; Sklar, C.A.; Robison, L.L.; Oeffinger, K.C. Aging and Risk of Severe, Disabling, Life-Threatening, and Fatal Events in the Childhood Cancer Survivor Study. J. Clin. Oncol. 2014, 32, 1218–1227. [Google Scholar] [CrossRef]
- Hayashi, H.; Makimoto, A.; Yuza, Y. Treatment of Pediatric Acute Lymphoblastic Leukemia: A Historical Perspective. Cancers 2024, 16, 723. [Google Scholar] [CrossRef]
- Huang, I.C.; Brinkman, T.M.; Kenzik, K.; Gurney, J.G.; Ness, K.K.; Lanctot, J.; Shenkman, E.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Association between the Prevalence of Symptoms and Health-Related Quality of Life in Adult Survivors of Childhood Cancer: A Report from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2013, 31, 4242–4251. [Google Scholar] [CrossRef]
- Lassaletta, Á.; Morales, J.S.; Valenzuela, P.L.; Esteso, B.; Kahalley, L.S.; Mabbott, D.J.; Unnikrishnan, S.; Panizo, E.; Calvo, F. Neurocognitive Outcomes in Pediatric Brain Tumors after Treatment with Proton versus Photon Radiation: A Systematic Review and Meta-Analysis. World J. Pediatr. 2023, 19, 727–740. [Google Scholar] [CrossRef]
- King, T.Z.; Wang, L.; Mao, H. Disruption of White Matter Integrity in Adult Survivors of Childhood Brain Tumors: Correlates with Long-Term Intellectual Outcomes. PLoS ONE 2015, 10, e0131744. [Google Scholar] [CrossRef]
- Edelmann, M.N.; Krull, K.R.; Liu, W.; Glass, J.O.; Ji, Q.; Ogg, R.J.; Sabin, N.D.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; et al. Diffusion Tensor Imaging and Neurocognition in Survivors of Childhood Acute Lymphoblastic Leukaemia. Brain 2014, 137, 2973–2983. [Google Scholar] [CrossRef]
- Zeller, B.; Tamnes, C.K.; Kanellopoulos, A.; Amlien, I.K.; Andersson, S.; Due-Tønnessen, P.; Fjell, A.M.; Walhovd, K.B.; Westlye, L.T.; Ruud, E. Reduced Neuroanatomic Volumes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2013, 31, 2078–2085. [Google Scholar] [CrossRef]
- Palmer, S.L.; Glass, J.O.; Li, Y.; Ogg, R.; Qaddoumi, I.; Armstrong, G.T.; Wright, K.; Wetmore, C.; Broniscer, A.; Gajjar, A.; et al. White Matter Integrity Is Associated with Cognitive Processing in Patients Treated for a Posterior Fossa Brain Tumor. Neuro-Oncology 2012, 14, 1185–1193. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild Cognitive Impairment: A Concept in Evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Otth, M.; Michel, G.; Gerber, N.U.; Guerreiro Stücklin, A.S.; von Bueren, A.O.; Scheinemann, K.; On Behalf of the Swiss Pediatric Oncology Group (SPOG). Educational Attainment and Employment Outcome of Survivors of Pediatric CNS Tumors in Switzerland—A Report from the Swiss Childhood Cancer Survivor Study. Children 2022, 9, 411. [Google Scholar] [CrossRef]
- Kirchhoff, A.C.; Krull, K.R.; Ness, K.K.; Armstrong, G.T.; Park, E.R.; Stovall, M.; Robison, L.L.; Leisenring, W. Physical, Mental, and Neurocognitive Status and Employment Outcomes in the Childhood Cancer Survivor Study Cohort. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1838–1849. [Google Scholar] [CrossRef]
- Askins, M.A.; Moore, B.D., III. Preventing Neurocognitive Late Effects in Childhood Cancer Survivors. J. Child Neurol. 2008, 23, 1160–1171. [Google Scholar] [CrossRef]
- Di Giuseppe, G.; Pagalan, L.; Jetha, A.; Pechlivanoglou, P.; Pole, J.D. Financial toxicity among adolescent and young adult cancer survivors: A systematic review of educational attainment, employment, and income. Crit. Rev. Oncol./Hematol. 2023, 183, 103914. [Google Scholar] [CrossRef]
- Buizer, A.I.; de Sonneville, L.M.J.; Veerman, A.J.P. Effects of Chemotherapy on Neurocognitive Function in Children with Acute Lymphoblastic Leukemia: A Critical Review of the Literature. Pediatr. Blood Cancer 2009, 52, 447–454. [Google Scholar] [CrossRef]
- Edelmann, M.N.; Krull, K.R.; Jones, M.M.; Ogg, R.J.; Liu, W.; Sabin, N.D.; Glass, J.O.; Wu, S.; Srivastava, D.; Reddick, W.E. Cognitive Processing Speed, White Matter Integrity, and Medical Risk Factors in Adult Survivors of Childhood Acute Lymphoblastic Leukemia. J. Natl. Cancer Inst. 2014, 106, dju273. [Google Scholar] [CrossRef]
- Fidler, M.M.; Reulen, R.C.; Winter, D.L.; Kelly, J.; Jenkinson, H.C.; Skinner, R.; Frobisher, C.; Hawkins, M.M. Long-Term Cause-Specific Mortality among 34,489 Five-Year Survivors of Childhood Cancer in Great Britain: Population Based Cohort Study. BMJ 2016, 354, i4351. [Google Scholar] [CrossRef]
- Moore, B.D., III. Neurocognitive Outcomes in Survivors of Childhood Cancer. J. Pediatr. Psychol. 2005, 30, 51–63. [Google Scholar] [CrossRef]
- Schuitema, I.; Deprez, S.; Van Hecke, W.; Daams, M.; Uyttebroeck, A.; Sunaert, S.; Barkhof, F.; van Dulmen-den Broeder, E.; van der Pal, H.J.; van den Bos, C.; et al. Accelerated Aging, Decreased White Matter Integrity, and Associated Neuropsychological Dysfunction 25 Years after Pediatric Lymphoid Malignancies. J. Clin. Oncol. 2013, 31, 3378–3388. [Google Scholar] [CrossRef]
- Edelmann, M.N.; Krull, K.R. Brain Volume and Cognitive Function in Adult Survivors of Childhood Acute Lymphoblastic Leukemia. Transl Pediatr. 2013, 2, 143–147. [Google Scholar] [CrossRef]
- Stavinoha, P.L.; Askins, M.A.; Powell, S.K.; Smiley, N.P.; Robert, R.S. Neurocognitive and Psychosocial Outcomes in Pediatric Brain Tumor Survivors. Bioengineering 2018, 5, 73. [Google Scholar] [CrossRef]
- Pui, C.H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood Acute Lymphoblastic Leukemia: Progress through Collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef]
- Tonning Olsson, I.; Perrin, S.; Lundgren, J.; Hjorth, L.; Johanson, A. Long-Term Cognitive Sequelae after Pediatric Brain Tumor Related to Medical Risk Factors, Age, and Sex. Pediatr. Neurol. 2014, 51, 515–521. [Google Scholar] [CrossRef]
- Devine, K.A.; Christen, S.; Mulder, R.L.; Brown, M.C.; Ingerski, L.M.; Mader, L.; Potter, E.J.; Sleurs, C.; Viola, A.S.; Waern, S.; et al. Recommendations for the Surveillance of Education and Employment Outcomes in Survivors of Childhood, Adolescent, and Young Adult Cancer: A Report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Cancer 2022, 128, 2405–2419. [Google Scholar] [CrossRef]
- Rosenberg, A.R.; Zhou, C.; Bradford, M.C.; Salsman, J.M.; Sexton, K.; O’Daffer, A.; Yi-Frazier, J.P. Assessment of the promoting resilience in stress management intervention for adolescent and young adult survivors of cancer at 2 years: Secondary analysis of a randomized clinical trial. JAMA Netw. Open 2021, 4, e2136039. [Google Scholar] [CrossRef]
- Kasteler, R.; Fuchs, P.; Otth, M.; Scheinemann, K. Interventions to Improve Neurocognitive Late-Effects in Pediatric and Adolescent CNS Tumor Patients and Survivors—A Systematic Review. Front. Oncol. 2023, 13, 1150166. [Google Scholar] [CrossRef]
- Reddick, W.E.; Glass, J.O.; Palmer, S.L.; Wu, S.; Gajjar, A.; Langston, J.W.; Kun, L.E.; Xiong, X.; Mulhern, R.K. Atypical White Matter Volume Development in Children Following Craniospinal Irradiation. Neuro Oncol. 2005, 7, 12–19. [Google Scholar] [CrossRef]
- Deegan, A.; Brennan, C.; Gallagher, P.; Lambert, V.; Dunne, S. Social Support and Childhood Cancer Survivors: A Systematic Review (2006–2022). Psychooncology 2023, 32, 819–833. [Google Scholar] [CrossRef]
- Clanton, N.R.; Klosky, J.L.; Li, C.; Jain, N.; Srivastava, D.K.; Mulrooney, D.; Zeltzer, L.; Robison, L.L.; Krull, K.R. Fatigue, Vitality, Sleep, and Neurocognitive Function in Adult Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Cancer 2011, 117, 2559–2568. [Google Scholar] [CrossRef]
- Wengenroth, L.; Rueegg, C.S.; Michel, G.; Gianinazzi, M.E.; Essig, S.; von der Weid, N.X.; Grotzer, M.; Kuehni, C.E.; Swiss Paediatric Oncology Group SPOG. Concentration, Working Speed and Memory: Cognitive Problems in Young Childhood Cancer Survivors and Their Siblings. Pediatr. Blood Cancer 2015, 62, 875–882. [Google Scholar] [CrossRef]
- Willard, V.W. Social Skills Interventions for Survivors of Pediatric Brain Tumors: A Review and Reformulation. Pediatr. Blood Cancer 2018, 65, e27434. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, D.J.; Monsalves, E.; Spiegler, B.J.; Bartels, U.; Janzen, L.; Guger, S.; Laperriere, N.; Andrews, N.; Bouffet, E. Longitudinal Evaluation of Neurocognitive Function after Treatment for Central Nervous System Germ Cell Tumors in Childhood. Cancer 2011, 117, 5402–5411. [Google Scholar] [CrossRef] [PubMed]
- Kadan-Lottick, N.S.; Robison, L.L.; Gurney, J.G.; Neglia, J.P.; Yasui, Y.; Hayashi, R.; Hudson, M.; Greenberg, M.; Mertens, A.C. Childhood Cancer Survivors’ Knowledge about Their Past Diagnosis and Treatment: Childhood Cancer Survivor Study. JAMA 2002, 287, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Lubas, M.M.; Mirzaei Salehabadi, S.; Lavecchia, J.; Alberts, N.M.; Krull, K.R.; Ehrhardt, M.J.; Srivastava, D.; Robison, L.L.; Hudson, M.M.; Brinkman, T.M. Suicidality among Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer 2020, 126, 5347–5355. [Google Scholar] [CrossRef]
- Oyefiade, A.; Paltin, I.; De Luca, C.R.; Hardy, K.K.; Grosshans, D.R.; Chintagumpala, M.; Mabbott, D.J.; Kahalley, L.S. Cognitive Risk in Survivors of Pediatric Brain Tumors. J. Clin. Oncol. 2021, 39, 1718–1726. [Google Scholar] [CrossRef]
- Krull, K.R.; Brinkman, T.M.; Li, C.; Armstrong, G.T.; Ness, K.K.; Srivastava, D.K.; Gurney, J.G.; Kimberg, C.; Krasin, M.J.; Pui, C.H.; et al. Neurocognitive Outcomes Decades after Treatment for Childhood Acute Lymphoblastic Leukemia: A Report from the St. Jude Lifetime Cohort Study. J. Clin. Oncol. 2013, 31, 4407–4415. [Google Scholar] [CrossRef]
- Bhatia, S.; Pappo, A.S.; Acquazzino, M.; Allen-Rhoades, W.A.; Barnett, M.; Borinstein, S.C.; Casey, R.; Choo, S.; Chugh, R.; Dinner, S.; et al. Adolescent and Young Adult (AYA) Oncology, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 851–880. [Google Scholar] [CrossRef]
- Bhatia, S.; Tonorezos, E.S.; Landier, W. Clinical Care for People Who Survive Childhood Cancer: A Review. JAMA 2023, 330, 1175–1186. [Google Scholar] [CrossRef]
- Wu, J.; Heidelberg, R.E.; Gajjar, A. Adolescents and Young Adults with Cancer: CNS Tumors. J. Clin. Oncol. 2024, 42, 686–695. [Google Scholar] [CrossRef]
- Semendric, I.; Pollock, D.; Haller, O.J.; George, R.P.; Collins-Praino, L.E.; Whittaker, A.L. “Chemobrain” in Childhood Cancer Survivors—The Impact on Social, Academic, and Daily Living Skills: A Qualitative Systematic Review. Support. Care Cancer 2023, 31, 532. [Google Scholar] [CrossRef] [PubMed]
- Gandy, K.; Scoggins, M.A.; Jacola, L.M.; Litten, M.; Reddick, W.E.; Krull, K.R. Structural and Functional Brain Imaging in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia Treated with Chemotherapy: A Systematic Review. JNCI Cancer Spectr. 2021, 5, pkab069. [Google Scholar] [CrossRef]
- van der Plas, E.; Modi, A.J.; Li, C.K.; Krull, K.R.; Cheung, Y.T. Cognitive Impairment in Survivors of Pediatric Acute Lymphoblastic Leukemia Treated with Chemotherapy Only. J. Clin. Oncol. 2021, 39, 1705–1717. [Google Scholar] [CrossRef]
- Castellino, S.M.; Ullrich, N.J.; Whelen, M.J.; Lange, B.J. Developing Interventions for Cancer-Related Cognitive Dysfunction in Childhood Cancer Survivors. J. Natl. Cancer Inst. 2014, 106, dju186. [Google Scholar] [CrossRef] [PubMed]
- Horan, M.R.; Srivastava, D.K.; Bhakta, N.; Ehrhardt, M.J.; Brinkman, T.M.; Baker, J.N.; Yasui, Y.; Krull, K.R.; Ness, K.K.; Robison, L.L.; et al. Determinants of Health-Related Quality-of-Life in Adult Survivors of Childhood Cancer: Integrating Personal and Societal Values through a Health Utility Approach. EClinicalMedicine 2023, 58, 101921. [Google Scholar] [CrossRef]
- Mash, L.E.; Kahalley, L.S.; Raghubar, K.P.; Goodrich-Hunsaker, N.J.; Abildskov, T.J.; De Leon, L.A.; MacLeod, M.; Stancel, H.; Parsons, K.; Biekman, B.; et al. Cognitive Sparing in Proton versus Photon Radiotherapy for Pediatric Brain Tumor Is Associated with White Matter Integrity: An Exploratory Study. Cancers 2023, 15, 1844. [Google Scholar] [CrossRef]
- King, T.Z.; Na, S.; Mao, H. White Matter Integrity in Adult Survivors of Childhood Brain Tumors and Its Relation to Neurocognitive Outcomes. Neuropsychology 2015, 29, 743–753. [Google Scholar] [CrossRef]
- Child, A.E.; Warren, E.A.; Grosshans, D.R.; Paulino, A.C.; Okcu, M.F.; Ris, M.D.; Mahajan, A.; Orobio, J.; Cirino, P.T.; Minard, C.G.; et al. Long-Term Cognitive and Academic Outcomes among Pediatric Brain Tumor Survivors Treated with Proton versus Photon Radiotherapy. Pediatr. Blood Cancer 2021, 68, e29125. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; White, H.A.; Glass, J.O.; Wheeler, G.C.; Thompson, S.J.; Gajjar, A.; Leigh, L.; Mulhern, R.K. Developmental Model Relating White Matter Volume to Neurocognitive Deficits in Pediatric Brain Tumor Survivors. Cancer 2003, 97, 2512–2519. [Google Scholar] [CrossRef]
- Major, N.; Patel, N.A.; Bennett, J.; Novakovic, E.; Poloni, D.; Abraham, M.; Brown, N.J.; Gendreau, J.L.; Sahyouni, R.; Loya, J. The Current State of Radiotherapy for Pediatric Brain Tumors: An Overview of Post-Radiotherapy Neurocognitive Decline and Outcomes. J. Pers. Med. 2022, 12, 1050. [Google Scholar] [CrossRef]
- Martinez-Santos, A.E.; Fernandez-De-La-Iglesia, J.D.C.; Sheaf, G.; Coyne, I. A Systematic Review of the Educational Experiences and Needs of Children with Cancer Returning to School. J. Adv. Nurs. 2021, 77, 2971–2994. [Google Scholar] [CrossRef]
- Inaba, H.; Pui, C.H. Advances in the Diagnosis and Treatment of Pediatric Acute Lymphoblastic Leukemia. J. Clin. Med. 2021, 10, 1926. [Google Scholar] [CrossRef] [PubMed]
- Jacola, L.M.; Edelstein, K.; Liu, W.; Pui, C.H.; Hayashi, R.; Kadan-Lottick, N.S.; Srivastava, D.; Henderson, T.; Leisenring, W.; Robison, L.L.; et al. Cognitive, behaviour, and academic functioning in adolescent and young adult survivors of childhood acute lymphoblastic leukaemia: A report from the Childhood Cancer Survivor Study. Lancet Psychiatry 2016, 3, 965–972. [Google Scholar] [CrossRef] [PubMed]
- McLoone, J.K.; Sansom-Daly, U.M.; Paglia, A.; Chia, J.; Larsen, H.B.; Fern, L.A.; Cohn, R.J.; Signorelli, C. A Scoping Review Exploring Access to Survivorship Care for Childhood, Adolescent, and Young Adult Cancer Survivors: How Can We Optimize Care Pathways? Adolesc. Health Med. Ther. 2023, 14, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Robison, L.L.; Look, A.T. Acute Lymphoblastic Leukaemia. Lancet 2008, 371, 1030–1043. [Google Scholar] [CrossRef]
- Peterson, C.C.; Johnson, C.E.; Ramirez, L.Y.; Huestis, S.; Pai, A.L.; Demaree, H.A.; Drotar, D. A Meta-Analysis of the Neuropsychological Sequelae of Chemotherapy-Only Treatment for Pediatric Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2008, 51, 99–104. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Reddick, W.E.; Luxton, J.; Glass, J.O.; Sabin, N.D.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Cerebral White Matter Integrity and Executive Function in Adult Survivors of Childhood Medulloblastoma. Neuro Oncol. 2012, 14 (Suppl. 4), iv25–iv36. [Google Scholar] [CrossRef]
- Schultz, K.A.P.; Chen, L.; Chen, Z.; Kawashima, T.; Oeffinger, K.C.; Woods, W.G.; Neglia, J.P.; Mertens, A.C.; Yasui, Y.; Leisenring, W.; et al. Health Conditions and Mortality in Adult Survivors of Childhood Germ Cell Tumors: A Report from the Childhood Cancer Survivor Study. Cancer 2010, 116, 4950–4960. [Google Scholar] [CrossRef]
- Campbell, L.K.; Scaduto, M.; Sharp, W.; Dufton, L.; Van Slyke, D.; Whitlock, J.A.; Compas, B.E. A Meta-Analysis of the Cognitive Effects of Chemotherapy in Children Treated for Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2007, 49, 65–73. [Google Scholar] [CrossRef]
- Ullrich, N.J.; Embry, L. Neurocognitive Dysfunction in Survivors of Childhood Brain Tumors. Semin. Pediatr. Neurol. 2012, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zeltzer, L.K.; Lu, Q.; Leisenring, W.; Tsao, J.C.; Recklitis, C.; Armstrong, G.; Mertens, A.C.; Robison, L.L.; Ness, K.K. Psychosocial Outcomes and Health-Related Quality of Life in Adult Childhood Cancer Survivors: A Report from the Childhood Cancer Survivor Study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Morales, S.; Salehabadi, S.M.; Srivastava, D.; Gibson, T.M.; Leisenring, W.M.; Alderfer, M.A.; Lown, E.A.; Zeltzer, L.K.; Armstrong, G.T.; Krull, K.R.; et al. Health-Related and Cancer Risk Concerns among Siblings of Childhood Cancer Survivors: A Report from the Childhood Cancer Survivor Study (CCSS). J. Cancer Surviv. 2022, 16, 624–637. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Treatment Modalities | Cognitive Sequelae | Emotional Sequelae |
|---|---|---|---|
| ALL (Acute Lymphoblastic Leukemia) | Chemotherapy, intrathecal methotrexate, occasional cranial irradiation (historic) | Processing speed, attention, executive dysfunction, memory deficits; higher vulnerability when treated at younger ages [23,69,78] | Anxiety, depression, social withdrawal, PTSD symptoms [20,62,80] |
| CNS Tumors | Surgery, cranial/spinal irradiation, chemotherapy | Global IQ decline, impaired processing speed, executive dysfunction, working memory deficits; radiation dose–response effects [67,70,71,75] | Emotional dysregulation, chronic anxiety/depression, social functioning difficulties [63,73,80] |
| Neuroblastoma | Intensive chemotherapy, surgery, stem cell transplant, immunotherapy | Visual-motor deficits, attention and executive impairments, lower academic outcomes, especially in younger survivors [65,68,72] | Anxiety, sleep disturbances, social difficulties, treatment-related trauma responses [64,79,83] |
| Bone Tumors (e.g., Osteosarcoma, Ewing Sarcoma) | Surgery (amputation/limb salvage), chemotherapy, sometimes radiotherapy | Mostly preserved cognition, though chemotherapy-related attention/processing vulnerabilities possible [74,89,94] | Body image concerns, depression, anxiety, adjustment difficulties [41,73,85] |
| Renal Tumors (e.g., Wilms’ Tumor) | Nephrectomy, chemotherapy, occasional radiotherapy | Generally minimal late cognitive effects; subtle learning/attention difficulties in subgroups exposed to radiation or anthracyclines [62,86,90] | Anxiety about health/recurrence, internalizing symptoms; parental stress impacting adjustment [40,80,88] |
| Developmental Stage | Primary Affected Cognitive Domains | Emotional/Psychosocial Outcomes | Representative Studies | Main Methodological Limitations |
|---|---|---|---|---|
| Infancy/Toddlerhood | Delays in processing speed and emerging executive functions; early white-matter and hippocampal disruption. | Dysregulated affect; attachment insecurity; caregiver stress and anxiety. | [25,26,27,28,29,33,46,48,77,82,116] | Very small single-center samples; frequent absence of healthy control groups; cross-sectional designs; retrospective reporting; survivors treated under older protocols. |
| Early Childhood | Working-memory, attention, and language inefficiencies; reduced processing speed following chemotherapy or RT. | Separation anxiety; social withdrawal; early peer avoidance; fear generalization. | [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,88,118] | Lack of randomized controls; moderate sample sizes (20–80 participants); short follow-up; attrition bias; heterogeneity in imaging and testing protocols. |
| Middle Childhood | Slowed processing speed; deficits in executive planning, working memory, and academic achievement. | Internalizing symptoms, social isolation, reduced self-esteem. | [36,37,38,39,56,57,58,59,60,61,95,96,97,120] | Cognitive outcomes often secondary endpoints; incomplete follow-up; no healthy controls; reliance on self-report in some cohorts; heterogeneity in treatment exposures. |
| Adolescence | Persistent inefficiencies in higher-order executive function, working memory, and metacognition; slowed processing speed. | Depression, anxiety, post-traumatic stress symptoms, and identity difficulties during school and transition to adulthood. | [107,112,122] | Cross-sectional or retrospective designs; selective participation of higher-functioning survivors; reliance on self-report; older treatment eras; limited cultural generalizability. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christou, A.I.; Kalfadeli, G.; Tsermentseli, S.; Bacopoulou, F. Neurocognitive and Emotional Outcomes in Childhood Cancer: A Developmental Perspective. Curr. Oncol. 2025, 32, 611. https://doi.org/10.3390/curroncol32110611
Christou AI, Kalfadeli G, Tsermentseli S, Bacopoulou F. Neurocognitive and Emotional Outcomes in Childhood Cancer: A Developmental Perspective. Current Oncology. 2025; 32(11):611. https://doi.org/10.3390/curroncol32110611
Chicago/Turabian StyleChristou, Antonios I., Georgia Kalfadeli, Stella Tsermentseli, and Flora Bacopoulou. 2025. "Neurocognitive and Emotional Outcomes in Childhood Cancer: A Developmental Perspective" Current Oncology 32, no. 11: 611. https://doi.org/10.3390/curroncol32110611
APA StyleChristou, A. I., Kalfadeli, G., Tsermentseli, S., & Bacopoulou, F. (2025). Neurocognitive and Emotional Outcomes in Childhood Cancer: A Developmental Perspective. Current Oncology, 32(11), 611. https://doi.org/10.3390/curroncol32110611









