Small Extracellular Vesicles Derived from NF2-Associated Schwannoma Cells Modulate Tumor Progression and Immunity via HSP90
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Primary Schwannoma Cells Isolation and Culture
2.3. SEVs Isolation
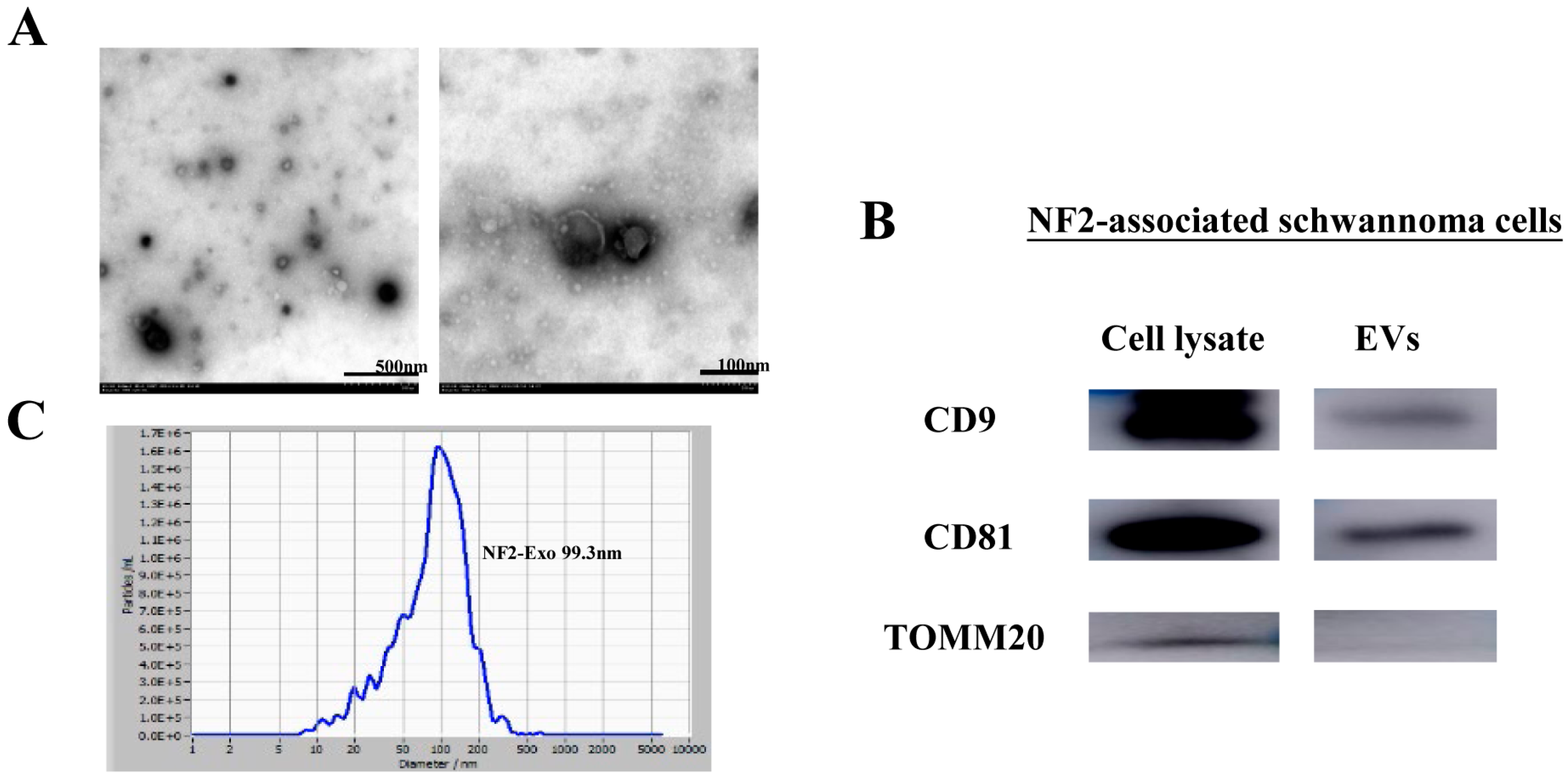
2.4. Transmission Electron Microscopy
2.5. Nanoparticle Tracking Analysis (NTA)
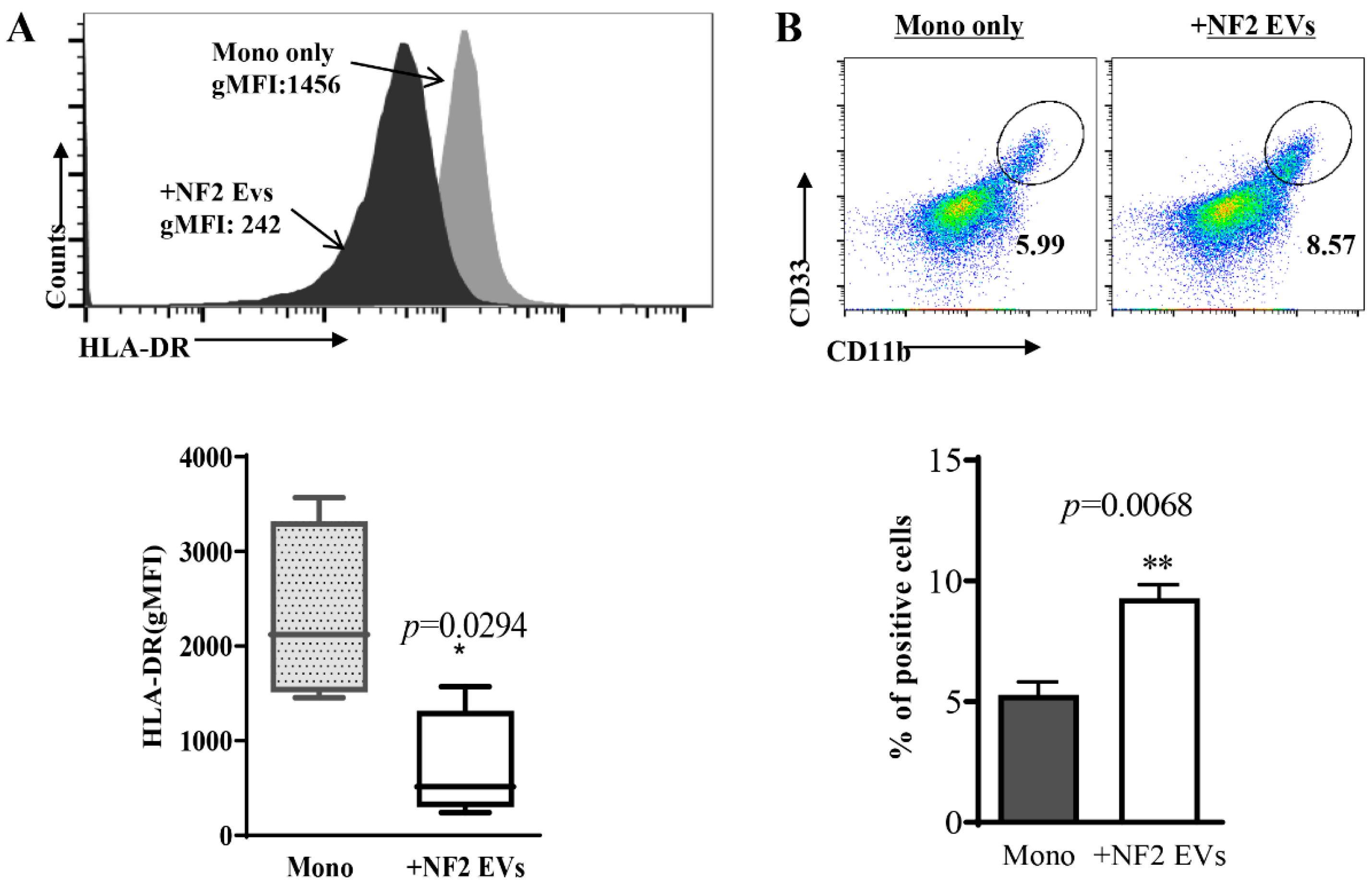
2.6. Analysis of MDSC Percentages in Co-Culture
2.7. Detection of Intracellular ROS Generation
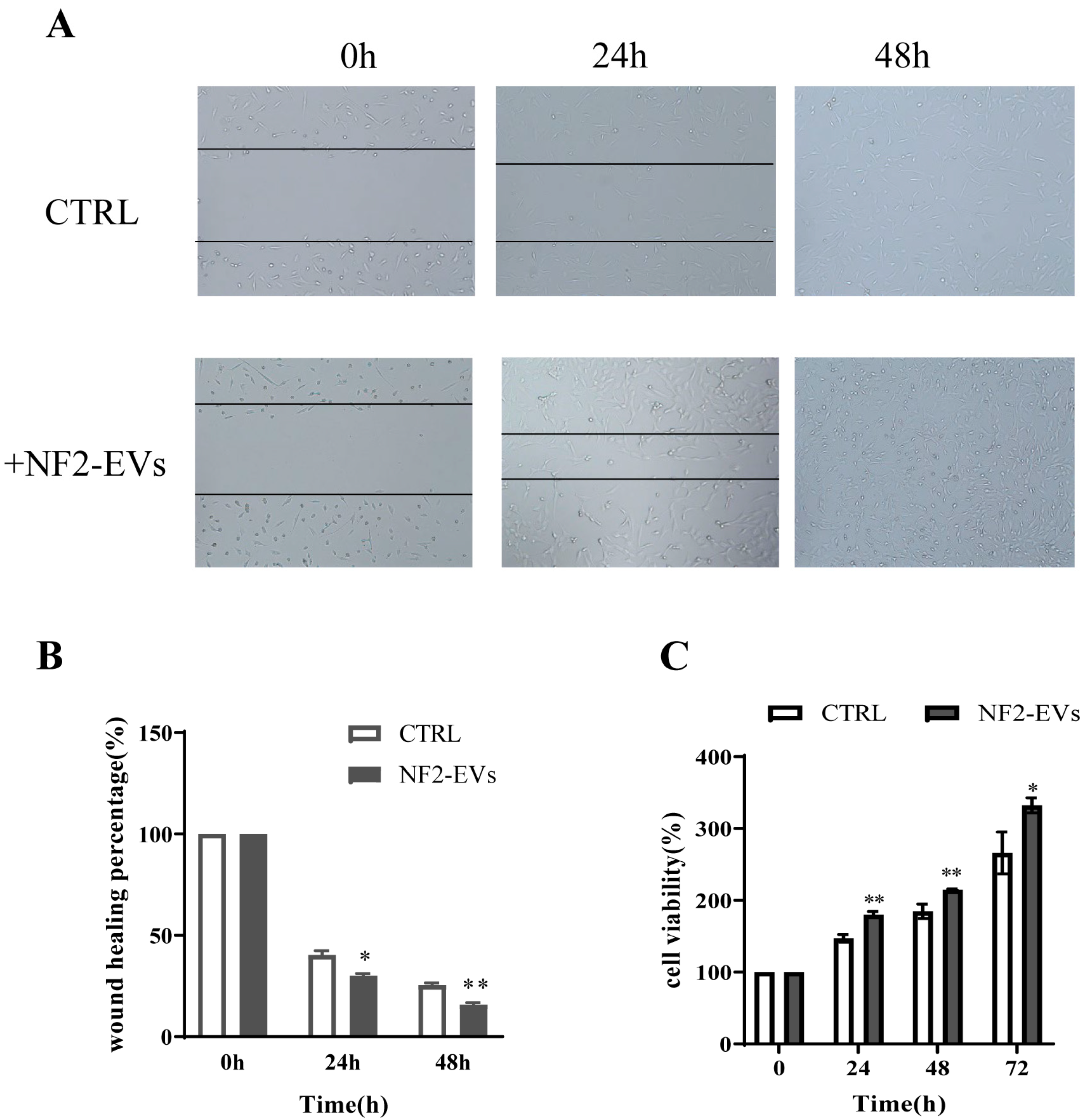
2.8. Cell Viability Assay
2.9. Luminex Liquid Suspension Chip Detection
2.10. Mass Spectrum Analysis
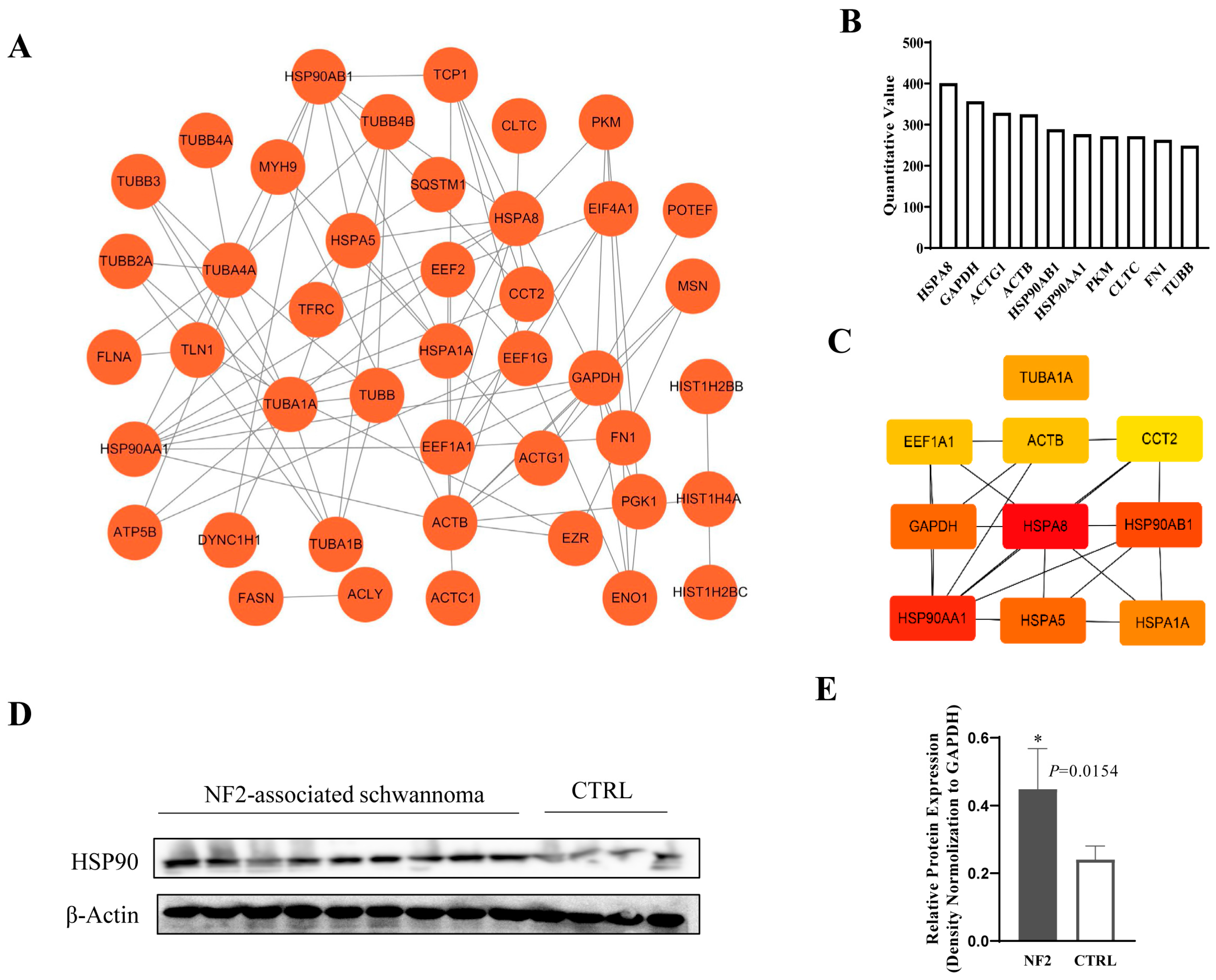
2.11. Western Blot
2.12. PPI Regulatory Network Analysis
2.13. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Characterization of NF2-EVs
3.3. Monocytes Co-Cultured with NF2-EVs Resemble MDSC Cells in Phenotype and Function
3.4. NF2-EVs Enhance NF2-Associated Schwannoma Cells Proliferation
3.5. Exosomal HSP90 Is a Key Modulator in NF2-Associated Tumor Progression and Immunity
3.6. HSP90 Modulates NF2-Associated Tumor Progression and Immunity by Regulating AKT
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamura, R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int. J. Mol. Sci. 2021, 22, 5850. [Google Scholar] [CrossRef]
- Garcia, M.R.; Hagiwara, M.; Yaffe, A.; Mitchell, C.; Akshintala, S.; Nicolaides, T.; Phadnis, S.S.; Yohay, K.; Feng, Y.; Goldberg, J.D.; et al. Phase II study of axitinib in patients with NF2-related schwannomatosis and progressive vestibular schwannomas. Neurooncol. Adv. 2025, 7, vdaf083. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.; Nassar, S.; Jagger, D.J. NF2-related schwannomatosis: A view from within the inner ear. Hear. Res. 2025, 460, 109226. [Google Scholar] [CrossRef] [PubMed]
- Coy, S.; Rashid, R.; Stemmer-Rachamimov, A.; Santagata, S. An update on the CNS manifestations of neurofibromatosis type 2. Acta Neuropathol. 2020, 139, 643–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Chung, Y.H.; Woo, T.G.; Kang, S.M.; Park, S.; Kim, M.; Park, B.J. NF2-Related Schwannomatosis (NF2): Molecular Insights and Therapeutic Avenues. Int. J. Mol. Sci. 2024, 25, 6558. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, J.; Chen, G.; Chen, L.; Lu, X. Advances in Tumor Microenvironment and Immunotherapeutic Strategies for Hepatocellular Carcinoma. Oncol. Res. 2025, 33, 2309–2329. [Google Scholar] [CrossRef]
- Mayasin, Y.; Osinnikova, M.; Osadchaya, D.; Dmitrienko, V.; Gorodilova, A.; Kharisova, C.; Kitaeva, K.; Filin, I.; Solovyeva, V.; Rizvanov, A. Targeting TAMs & CAFs in melanoma: New approaches to tumor microenvironment therapy. Oncol. Res. 2025, 33, 2221–2242. [Google Scholar]
- Wang, Y.; Li, P.; Wang, B.; Wang, S.; Liu, P. Identification of myeloid-derived suppressor cells that have an immunosuppressive function in NF2 patients. J. Cancer Res. Clin. Oncol. 2019, 145, 523–533. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Ortiz, A. Extracellular vesicles in cancer progression. Semin. Cancer Biol. 2021, 76, 139–142. [Google Scholar] [CrossRef]
- Nowak, M.; Górczyńska, J.; Kołodzińska, K.; Rubin, J.; Choromańska, A. Extracellular Vesicles as Drug Transporters. Int. J. Mol. Sci. 2023, 24, 10267. [Google Scholar] [CrossRef]
- Javdani-Mallak, A.; Mowla, S.J.; Alibolandi, M. Tumor-derived exosomes and their application in cancer treatment. J. Transl. Med. 2025, 23, 751. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef] [PubMed]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, C.; Kluwe, L.; Mautner, V.F.; Friedrich, R.E.; Müller, H.W.; Hanemann, C.O. Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiol. Dis. 1998, 5, 55–64. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Clancy, J.; D’Souza-Schorey, C. Extracellular Vesicles in Cancer: Purpose and Promise. Cancer J. 2018, 24, 65–69. [Google Scholar] [CrossRef]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Jackson, S.E. Hsp90: Structure and function. Top. Curr. Chem. 2013, 328, 155–240. [Google Scholar]
- Prodromou, C. Mechanisms of Hsp90 regulation. Biochem. J. 2016, 473, 2439–2452. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, F.; Li, H.; Li, L.; Yang, Y.; Liu, F. HSP90 mediates the connection of multiple programmed cell death in diseases. Cell Death Dis. 2022, 13, 929. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef]
- Mielczarek-Lewandowska, A.; Hartman, M.L.; Czyz, M. Inhibitors of HSP90 in melanoma. Apoptosis 2020, 25, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Eskin, A.; Chareyre, F.; Jessen, W.J.; Manent, J.; Niwa-Kawakita, M.; Chen, R.; White, C.H.; Vitte, J.; Jaffer, Z.M.; et al. Therapeutic potential of HSP90 inhibition for neurofibromatosis type 2. Clin. Cancer Res. 2013, 19, 3856–3870. [Google Scholar] [CrossRef]
- Arkhypov, I.; Özbay Kurt, F.G.; Bitsch, R.; Novak, D.; Petrova, V.; Lasser, S.; Hielscher, T.; Groth, C.; Lepper, A.; Hu, X.; et al. HSP90α induces immunosuppressive myeloid cells in melanoma via TLR4 signaling. J. Immunother. Cancer 2022, 10, e005551. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.; Speigl, L.; Pawelec, G.; Niessner, H.; Shipp, C. Inhibiting HSP90 prevents the induction of myeloid-derived suppressor cells by melanoma cells. Cell. Immunol. 2018, 327, 68–76. [Google Scholar] [CrossRef] [PubMed]



| Details of NF2-SWN Patients | Numbers (%) |
|---|---|
| All patients (n = 11) | |
| Median age (±SD) | 28 ± 14.02 |
| Gender | |
| Male | 5 (45.45%) |
| Female | 6 (54.55%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ren, Y.; Zhang, Q.; Zhang, C.; Yan, M.; Ma, X.; Wang, B.; Li, P.; Liu, P. Small Extracellular Vesicles Derived from NF2-Associated Schwannoma Cells Modulate Tumor Progression and Immunity via HSP90. Curr. Oncol. 2025, 32, 569. https://doi.org/10.3390/curroncol32100569
Wang Y, Ren Y, Zhang Q, Zhang C, Yan M, Ma X, Wang B, Li P, Liu P. Small Extracellular Vesicles Derived from NF2-Associated Schwannoma Cells Modulate Tumor Progression and Immunity via HSP90. Current Oncology. 2025; 32(10):569. https://doi.org/10.3390/curroncol32100569
Chicago/Turabian StyleWang, Ying, Yuan Ren, Qi Zhang, Chao Zhang, Minjun Yan, Xin Ma, Bo Wang, Peng Li, and Pinan Liu. 2025. "Small Extracellular Vesicles Derived from NF2-Associated Schwannoma Cells Modulate Tumor Progression and Immunity via HSP90" Current Oncology 32, no. 10: 569. https://doi.org/10.3390/curroncol32100569
APA StyleWang, Y., Ren, Y., Zhang, Q., Zhang, C., Yan, M., Ma, X., Wang, B., Li, P., & Liu, P. (2025). Small Extracellular Vesicles Derived from NF2-Associated Schwannoma Cells Modulate Tumor Progression and Immunity via HSP90. Current Oncology, 32(10), 569. https://doi.org/10.3390/curroncol32100569





