Predictive Factors and Clinical Impact of Radioactive Seed Migration After Prostate Brachytherapy: A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Timing, Migration Sites, and Frequency of Seed Migration
3.2. Analysis of Predictive Factors for Seed Migration
3.3. Relationship Between Seed Migration and the Learning Curve
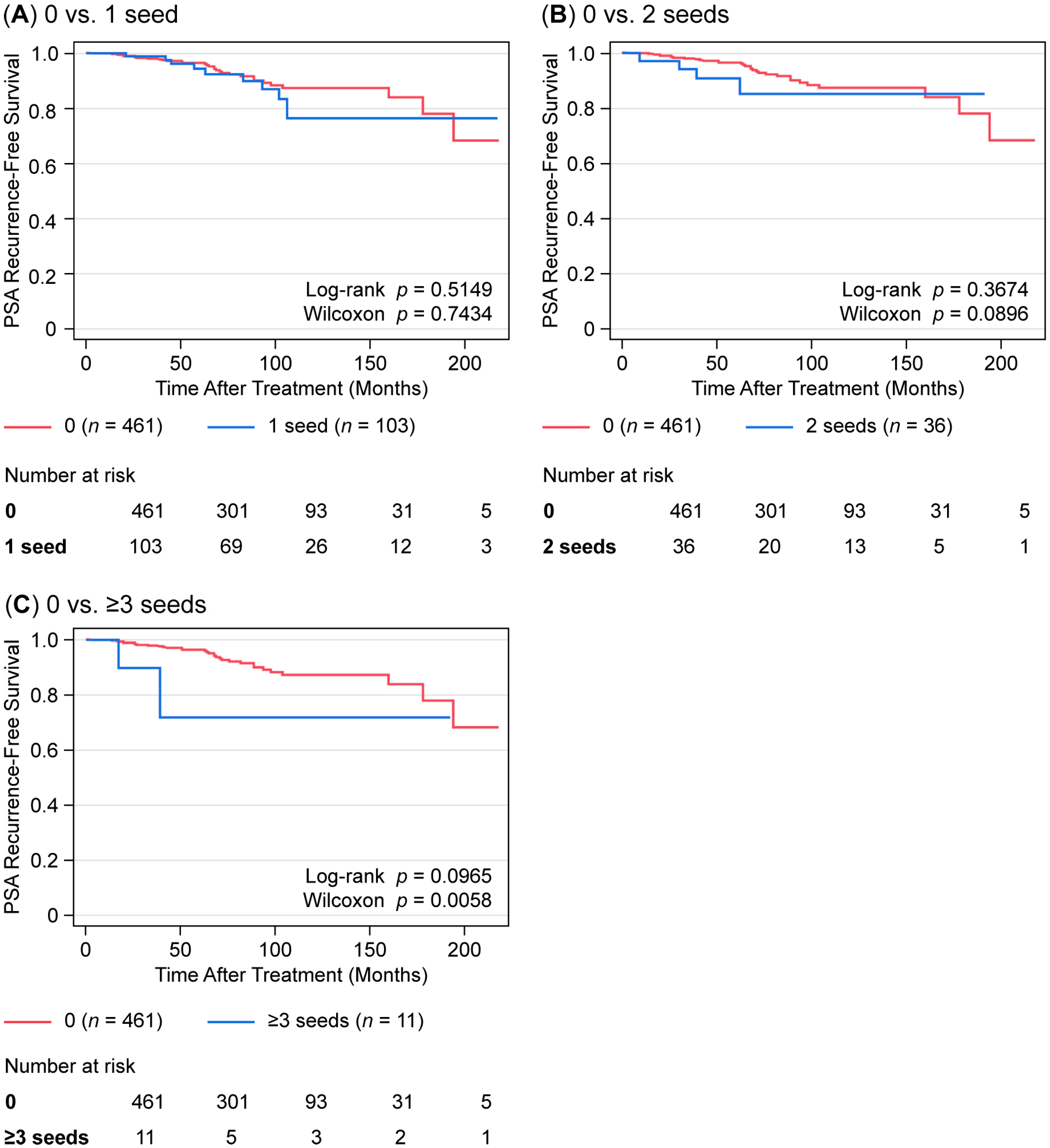
3.4. Impact of Seed Migration on Treatment Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LDR-BT | Low-dose-rate brachytherapy |
| EBRT | External beam radiation therapy |
| CT | Computed tomography |
| NCCN | National Comprehensive Cancer Network |
| PSA | Prostate specific antigen |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
References
- Wyler, S.F.; Engeler, D.S.; Seelentag, W.; Ries, G.; Schmid, H.P. Health-related quality of life after radical prostatectomy and low-dose-rate brachytherapy for localized prostate cancer. Urol. Int. 2009, 82, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.; Billiet, I.; Bostwick, D.; Dicker, A.P.; Frank, S.; Immerzeel, J.; Keyes, M.; Kupelian, P.; Lee, W.R.; Machtens, S.; et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU. Int. 2012, 109 (Suppl. S1), S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Asakawa, I.; Hasegawa, M.; Fujimoto, K. Low-dose-rate brachytherapy for prostate cancer: A 15-year experience in Japan. Int. J. Urol. 2020, 27, 17–23. [Google Scholar] [CrossRef]
- Mäkelä, L.; Pétas, A.; Mikkola, A.; Visapää, H. Long-term outcomes of LDR-brachytherapy for localized prostate cancer. Front. Oncol. 2025, 14, 1326355. [Google Scholar] [CrossRef]
- Stanberry, B.; Webber-Jones, N. Low-dose-rate brachytherapy as a primary treatment for localised and locally advanced prostate cancer: A systematic review of economic evaluations. Prostate. Cancer. Prostatic. Dis. 2025, 28, 23–36. [Google Scholar] [CrossRef]
- Kono, Y.; Kubota, K.; Aruga, T.; Ishibashi, A.; Morooka, M.; Ito, K.; Itami, J.; Kanemura, M.; Minowada, S.; Tanaka, T. Swelling of the prostate gland by permanent brachytherapy may affect seed migration. Jpn. J. Clin. Oncol. 2010, 40, 1159–1165. [Google Scholar] [CrossRef]
- Stone, N.N.; Stock, R.G. Reduction of pulmonary migration of permanent interstitial sources in patients undergoing prostate brachytherapy. Urology 2005, 66, 119–123. [Google Scholar] [CrossRef]
- Davis, B.J.; Bresnahan, J.F.; Stafford, S.L.; Karon, B.L.; King, B.F.; Wilson, T.M. Prostate brachytherapy seed migration to a coronary artery found during angiography. J. Urol. 2002, 168, 1103. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.; Pfeifer, E.A.; Wilson, T.M.; King, B.F.; Eshleman, J.S.; Pisansky, T.M. Prostate brachytherapy seed migration to the right ventricle found at autopsy following acute cardiac dysrhythmia. J. Urol. 2000, 164, 1661. [Google Scholar] [CrossRef]
- Nakiri, M.; Ueda, K.; Hoshino, R.; Ito, N.; Kurose, H.; Nohara, S.; Muraki, K.; Hattori, C.; Ogo, E.; Igawa, T. Prostate brachytherapy seed migration to the right renal artery due to right-to-left shunting across a patent foramen ovale. IJU Case. Rep. 2024, 7, 221–224. [Google Scholar] [CrossRef]
- Nguyen, B.D. Cardiac and hepatic seed implant embolization after prostate brachytherapy. Urology 2006, 68, 673.e17–673.e19. [Google Scholar] [CrossRef]
- Stock, R.G.; Stone, N.N.; Wesson, M.F.; DeWyngaert, J.K. A modified technique allowing interactive ultrasound-guided three-dimensional transperineal prostate implantation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 219–225. [Google Scholar] [CrossRef]
- Stock, R.G.; Stone, N.N.; Cesaretti, J.A.; Rosenstein, B.S. Biologically effective dose values for prostate brachytherapy: Effects on PSA failure and posttreatment biopsy results. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Kunos, C.A.; Resnick, M.I.; Kinsella, T.J.; Ellis, R.J. Migration of implanted free radioactive seeds for adenocarcinoma of the prostate using a Mick applicator. Brachytherapy 2004, 3, 71–77. [Google Scholar] [CrossRef]
- Chauveinc, L.; Osseili, A.; Flam, T.; Thiounn, N.; Rosenwald, J.C.; Savignoni, A.; Cosset, J.M. Iodin 125 seed migration after prostate brachytherapy: A study of 170 patients. Cancer. Radiother. 2004, 8, 211–216. [Google Scholar] [CrossRef]
- Eshleman, J.S.; Davis, B.J.; Pisansky, T.M.; Wilson, T.M.; Haddock, M.G.; King, B.F.; Darby, C.H.; Lajoie, W.N.; Oberg, A.L. Radioactive seed migration to the chest after transperineal interstitial prostate brachytherapy: Extraprostatic seed placement correlates with migration. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 419–425. [Google Scholar] [CrossRef]
- Taussky, D.; Moumdjian, C.; Larouche, R.; Béliveau-Nadeau, D.; Boudreau, C.; Hervieux, Y.; Donath, D. Seed migration in prostate brachytherapy depends on experience and technique. Brachytherapy 2012, 11, 452–456. [Google Scholar] [CrossRef]
- Sugawara, A.; Nakashima, J.; Kunieda, E.; Nagata, H.; Mizuno, R.; Seki, S.; Shiraishi, Y.; Kouta, R.; Oya, M.; Shigematsu, N. Incidence of seed migration to the chest, abdomen, and pelvis after transperineal interstitial prostate brachytherapy with loose (125)I seeds. Radiat. Oncol. 2011, 6, 130. [Google Scholar] [CrossRef]
- Hirose, K.; Aoki, M.; Sato, M.; Akimoto, H.; Hashimoto, Y.; Imai, A.; Kamimura, N.; Kawaguchi, H.; Hatayama, Y.; Fujioka, I.; et al. The retrospective analysis of the relationship between prescribed dose and risk factor for seed migration in iodine-125 prostate brachytherapy. Jpn. J. Radiol. 2016, 34, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Yorozu, A.; Saito, S.; Sugawara, A.; Maruo, S.; Kojima, S.; Kikuchi, T.; Fukushima, M.; Dokiya, T.; Yamanaka, H. Seed migration after transperineal interstitial prostate brachytherapy by using loose seeds: Japanese prostate cancer outcome study of permanent iodine-125 seed implantation (J-POPS) multi-institutional cohort study. Radiat. Oncol. 2015, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Matsuo, M.; Mori, T.; Noda, Y.; Makita, C.; Hyodo, F.; Iinuma, K.; Nakano, M.; Koie, T.; Tanaka, H. Seed density as a new predictive index of seed migration in brachytherapy for prostate cancer using iodine-125 loose seed. Curr. Oncol. 2023, 30, 4060–4066. [Google Scholar] [CrossRef]
- Maletzki, P.; Schwab, C.; Markart, P.; Engeler, D.; Schiefer, J.; Plasswilm, L.; Schmid, H.P. Late seed migration after prostate brachytherapy with Iod-125 permanent implants. Prostate. Int. 2018, 6, 66–70. [Google Scholar] [CrossRef]
- Furuya, R.; Hisasue, S.; Furuya, S.; Saitoh, N.; Ogura, H.; Takahashi, S.; Tsukamoto, T. Fate of seminal vesicles and prostate after medical castration: How long is the optimal duration of neoadjuvant treatment for prostate cancer before radiation? Urology. 2008, 72, 417–421. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.Z.; Nag, S.; Gupta, N. Effects of seed migration on post-implant dosimetry of prostate brachytherapy. Med. Phys. 2007, 34, 471–480. [Google Scholar] [CrossRef]
- Wei, G.; Jiang, P.; Li, C.; Wei, S.; Jiang, Y.; Sun, H.; Wang, J. A review on permanent implants for prostate brachytherapy with comparison between stranded and loose seeds. Jpn. J. Radiol. 2022, 40, 135–146. [Google Scholar] [CrossRef]
- Zhu, A.X.; Wallner, K.E.; Frivold, G.P.; Ferry, D.; Jutzy, K.R.; Foster, G.P. Prostate brachytherapy seed migration to the right coronary artery associated with an acute myocardial infarction. Brachytherapy 2006, 5, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Kusuhara, Y.; Numata, K.; Shirato, A.; Hashine, K.; Sumiyoshi, Y.; Kataoka, M.; Takechi, S. Radiation pneumonitis caused by a migrated brachytherapy seed lodged in the lung. Jpn. J. Clin. Oncol. 2008, 38, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Katcher, J.; Nunez, C.; Tirgan, A.M.; Ellis, R.J. Radioactive seed migration after transperineal interstitial prostate brachytherapy and associated development of small-cell lung cancer. Brachytherapy 2012, 11, 354–358. [Google Scholar] [CrossRef] [PubMed]
| Variables | Value (Range or %) |
|---|---|
| Age (years), median (range) | 71 (47–86) |
| Initial PSA (ng/mL), median (range) | 7.46 (0.114–391) |
| Gleason score (ISUP grade) | |
| Gleason ≤ 6 (ISUP 1) | 258 (42.4) |
| Gleason 7 (3 + 4) (ISUP 2) | 140 (23.0) |
| Gleason 7 (4 + 3) (ISUP 3) | 96 (15.8) |
| Gleason 8 (4 + 4, 3 + 5, 5 + 3) (ISUP 4) | 71 (11.7) |
| Gleason 9–10 (ISUP 5) | 43 (7.1) |
| Unknown | 3 |
| Clinical T stage | |
| <T3 | 567 (93.9) |
| ≥T3 | 37 (6.1) |
| Unknown | 7 |
| NCCN risk classification | |
| Low | 203 (33.3) |
| Intermediate | 252 (41.4) |
| High | 154 (25.9) |
| Unknown | 2 |
| Prostate volume (cc), median (range) | 24.64 (8.1–70.5) |
| Number of seeds, median (range) | 68 (25–103) |
| Number of needles, median (range) | 16 (10–31) |
| Treatment method: Hormone therapy | |
| Yes | 370 (60.6) |
| No | 241 (39.4) |
| Treatment method: Treated with EBRT | |
| Yes | 156 (25.5) |
| No | 455 (74.5) |
| V100 (%), median (range) | 95.3 (66.0–99.9) |
| D90 (Gy), median (range) Combination | 130.2 (78.9–193.4) |
| Mono | 176.1 (91–225.3) |
| All Sites (n = 611) | Lung, Vein, and Other Organs (n = 611) | Seminal Vesicle (n = 611) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Migration (n = 150) | No Migration (n = 461) | p Value | Migration (n = 92) | No Migration (n = 519) | p Value | Migration (n = 61) | No Migration (n = 550) | p Value |
| Age (years), median (range) | 71 (51–84) | 71 (47–86) | 0.4825 | 71 (51–84) | 71 (47–86) | 0.5359 | 71 (55–84) | 71 (47–86) | 0.8448 |
| Initial PSA (ng/mL), median (range) | 7.18 (4–75.4) | 7.50 (0.114–391) | 0.1529 | 6.86 (4–75.4) | 7.52 (0.11–391) | 0.2548 | 7.5 (4.3–56.6) | 7.45 (0.11–391) | 0.2548 |
| ISUP grade, n (%) | |||||||||
| 1 | 65 (25.2) | 193 (74.8) | 0.1574 | 43 (16.7) | 215 (83.3) | 0.4410 | 23 (8.9) | 235 (91.1) | 0.1278 |
| 2 | 42 (30.0) | 98 (70.0) | 25 (17.9) | 115 (82.1) | 18 (12.9) | 122 (87.1) | |||
| 3 | 23 (24.0) | 73 (76.0) | 12 (12.5) | 84 (87.5) | 12 (12.5) | 84 (87.5) | |||
| 4 | 15 (21.1) | 56 (78.9) | 7 (9.9) | 64 (90.1) | 8 (11.3) | 63 (88.7) | |||
| 5 | 5 (11.6) | 38 (88.4) | 5 (11.6) | 38 (88.4) | 0 (0) | 43 (100) | |||
| T stage, n (%) | |||||||||
| <T3 | 144 (25.4) | 423 (74.6) | 0.0456 * | 89 (15.7) | 478 (84.3) | 0.090 | 60 (10.6) | 507 (89.4) | 0.1233 |
| ≥T3 | 4 (10.8) | 33 (89.2) | 2 (5.4) | 35 (94.6) | 1 (2.7) | 36 (97.3) | |||
| NCCN risk classification, n (%) | |||||||||
| Low | 54 (26.6) | 149 (73.4) | 0.0599 | 38 (18.7) | 165 (81.3) | 0.060 | 18 (8.9) | 185 (91.1) | 0.1557 |
| Intermediate | 69 (27.4) | 183 (72.6) | 39 (15.5) | 213 (84.5) | 32 (12.7) | 220 (87.3) | |||
| High | 27 (17.5) | 127 (82.5) | 15 (9.7) | 139 (90.3) | 11 (7.1) | 143 (92.9) | |||
| Prostate volume (cc), median (range) | 27.9 (10.5–70.5) | 24.2 (8.1–60.4) | 0.0004 * | 29.35 (10.5–70.5) | 24.2 (8.1–60.4) | 0.0001 * | 24.1 (11.74–49.9) | 24.69 (8.1–70.5) | 0.7428 |
| Number of seeds, median (range) | 70.5 (40–90) | 66 (25–85) | 0.0001 * | 75 (40–103) | 66 (25–100) | 0.0001 * | 69 (42–97) | 67 (25–103) | 0.2426 |
| Number of needles, median (range) | 17 (10–31) | 16 (10–27) | 0.0181 * | 17 (10–31) | 16 (10–27) | 0.0604 | 17 (11–22) | 16 (10–31) | 0.1433 |
| Hormone therapy, n (%) | |||||||||
| Yes | 74 (20.0) | 296 (80.0) | 0.0012 * | 45 (18.7) | 196 (81.3) | 0.0438 * | 28 (7.6) | 348 (92.4) | 0.0136 * |
| No | 76 (31.5) | 165 (68.5) | 47 (12.7) | 323 (87.3) | 33 (13.7) | 208 (86.3) | |||
| Combination with EBRT, n (%) | |||||||||
| Yes | 26 (16.7) | 130 (83.3) | 0.008 * | 13 (8.3) | 143 (91.7) | 0.0065 * | 11 (7.1) | 145 (92.9) | 0.1568 |
| No | 124 (27.3) | 331 (72.7) | 79 (17.4) | 376 (82.6) | 50 (11.0) | 405 (89.0) | |||
| Order of patients treated, n (%) | |||||||||
| 0–204 (204) | 61 (29.9) | 143 (70.1) | 0.0533 | 42 (20.6) | 162 (79.4) | 0.0222 * | 21 (10.3) | 183 (89.7) | 0.1330 |
| 205–408 (204) | 40 (19.6) | 164 (80.4) | 27 (13.2) | 177 (86.8) | 14 (6.9) | 190 (93.1) | |||
| 408–611 (203) | 49 (24.1) | 154 (75.9) | 23 (11.3) | 180 (88.7) | 26 (12.8) | 177 (87.2) | |||
| Variables (Reference/Comparator) | Univariate OR (95% CI) | p Value | Multivariable aOR (95% CI) | p Value |
|---|---|---|---|---|
| A. Predictors of all sites migration (n = 611) | ||||
| Prostate volume (cc) (≥23 vs. <23) | 1.72 (1.16–2.54) | 0.007 * | 0.92 (0.55–1.54) | 0.7403 |
| Number of seeds (≥68 vs. <68) | 2.30 (1.57–3.38) | <0.0001 * | 1.75 (1.02–3.00) | 0.0409 * |
| Number of needles (≥18 vs. <18) | 1.97 (1.34–2.91) | 0.0006 * | 1.56 (1.03–2.37) | 0.0371 * |
| Clinical T stage (≥T3 vs. <T3) | 0.35 (0.12–1.02) | 0.055 | - | - |
| Hormone therapy (Yes vs. No) | 0.54 (0.37–0.79) | 0.0013 * | 0.71 (0.47–1.06) | 0.091 |
| Combination with EBRT (Yes vs. No) | 0.53 (0.33–0.85) | 0.0087 * | 0.80 (0.47–1.35) | 0.3963 |
| B. Predictors of lung/vein/other organ migration (n = 611) | ||||
| Prostate volume (cc) (≥23 vs. <23) | 2.40 (1.44–3.99) | 0.0008 * | 1.38 (0.72–2.62) | 0.332 |
| Number of seeds (≥68 vs. <68) | 2.86 (1.76–4.65) | <0.0001 * | 1.96 (1.01–3.80) | 0.0446 * |
| Number of needles (≥18 vs. <18) | 1.60 (1.00–2.55) | 0.0472 * | 1.13 (0.69–1.85) | 0.6407 |
| Clinical T stage (≥T3 vs. <T3) | 0.31 (0.07–1.29) | 0.1086 | - | - |
| Hormone therapy (Yes vs. No) | 0.63 (0.40–0.98) | 0.0449 * | 0.92 (0.57–1.49) | 0.7422 |
| Combination with EBRT (Yes vs. No) | 0.43 (0.23–0.80) | 0.0078 * | 0.68 (0.34–1.35) | 0.2712 |
| C. Predictors of seminal vesicle migration (n = 611) | ||||
| Prostate volume (cc) (≥23 vs. <23) | 1.12 (0.65–1.93) | 0.6878 | 0.67 (0.32–1.37) | 0.2719 |
| Number of seeds (≥68 vs. <68) | 1.45 (0.85–2.48) | 0.1746 | 1.08 (0.50–2.32) | 0.8402 |
| Number of needles (≥18 vs. <18) | 2.20 (1.28–3.79) | 0.0042 * | 2.15 (1.20–3.87) | 0.0102 * |
| Clinical T stage (≥T3 vs. <T3) | 0.23 (0.03–1.74) | 0.1565 | - | - |
| Hormone therapy (Yes vs. No) | 0.52 (0.30–0.88) | 0.0148 * | 0.56 (0.32–1.00) | 0.052 |
| Combination with EBRT (Yes vs. No) | 0.61 (0.31–1.21) | 0.1603 | 0.74 (0.35–1.58) | 0.4444 |
| Number of Migrated Seeds (n) | V100 (Median (Range)) | p Value (vs. No Migration) | D90 (Median (Range)) | p Value (vs. No Migration) |
|---|---|---|---|---|
| No migration (n = 461) | 95.428 (66.862–99.943) | - | 163.106 (78.856–225.253) | - |
| 1 seed (n = 103) | 95.307 (67.486–99.73) | 0.9323 | 171.998 (89.622–202.625) | 0.055 |
| 2 seeds (n = 36) | 94.82 (65.987–99.043) | 0.4542 | 172.594 (101.441–197.625) | 0.2835 |
| ≥3 seeds (n = 11) | 90.126 (76.402–95.786) | 0.0055 * | 144.206 (110.345–179.231) | 0.1343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, S.; Fukagai, T.; Yamatoya, J.; Oshinomi, K.; Nagata, M.; Morita, M.; Toyofuku, K.; Sekimoto, A.; Kato, M.; Morota, M.; et al. Predictive Factors and Clinical Impact of Radioactive Seed Migration After Prostate Brachytherapy: A Retrospective Study. Curr. Oncol. 2025, 32, 567. https://doi.org/10.3390/curroncol32100567
Kikuchi S, Fukagai T, Yamatoya J, Oshinomi K, Nagata M, Morita M, Toyofuku K, Sekimoto A, Kato M, Morota M, et al. Predictive Factors and Clinical Impact of Radioactive Seed Migration After Prostate Brachytherapy: A Retrospective Study. Current Oncology. 2025; 32(10):567. https://doi.org/10.3390/curroncol32100567
Chicago/Turabian StyleKikuchi, Shota, Takashi Fukagai, Jin Yamatoya, Kazuhiko Oshinomi, Masakazu Nagata, Masashi Morita, Kosuke Toyofuku, Atsuhito Sekimoto, Masako Kato, Madoka Morota, and et al. 2025. "Predictive Factors and Clinical Impact of Radioactive Seed Migration After Prostate Brachytherapy: A Retrospective Study" Current Oncology 32, no. 10: 567. https://doi.org/10.3390/curroncol32100567
APA StyleKikuchi, S., Fukagai, T., Yamatoya, J., Oshinomi, K., Nagata, M., Morita, M., Toyofuku, K., Sekimoto, A., Kato, M., Morota, M., & Ito, Y. (2025). Predictive Factors and Clinical Impact of Radioactive Seed Migration After Prostate Brachytherapy: A Retrospective Study. Current Oncology, 32(10), 567. https://doi.org/10.3390/curroncol32100567






