SUVmax-IPI as a New Prognostic Index in Metastatic Non-Small Cell Lung Cancer Patients Receiving Nivolumab
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Univariate and Multivariate Analysis of Progression-Free Survival and Overall Survival
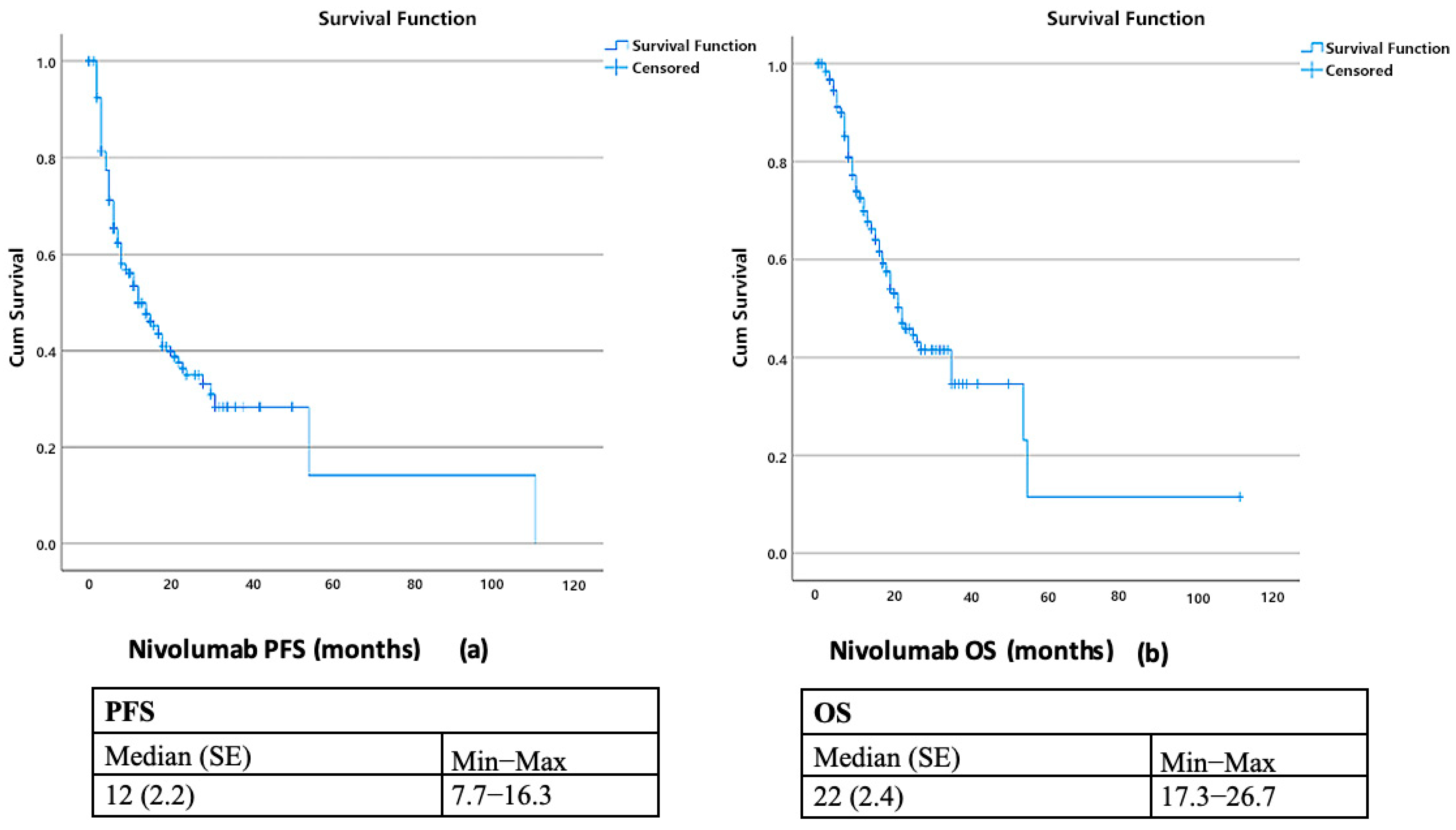
3.3. Survival Outcomes
3.4. Time-Dependent ROC/C-Index and Calibration
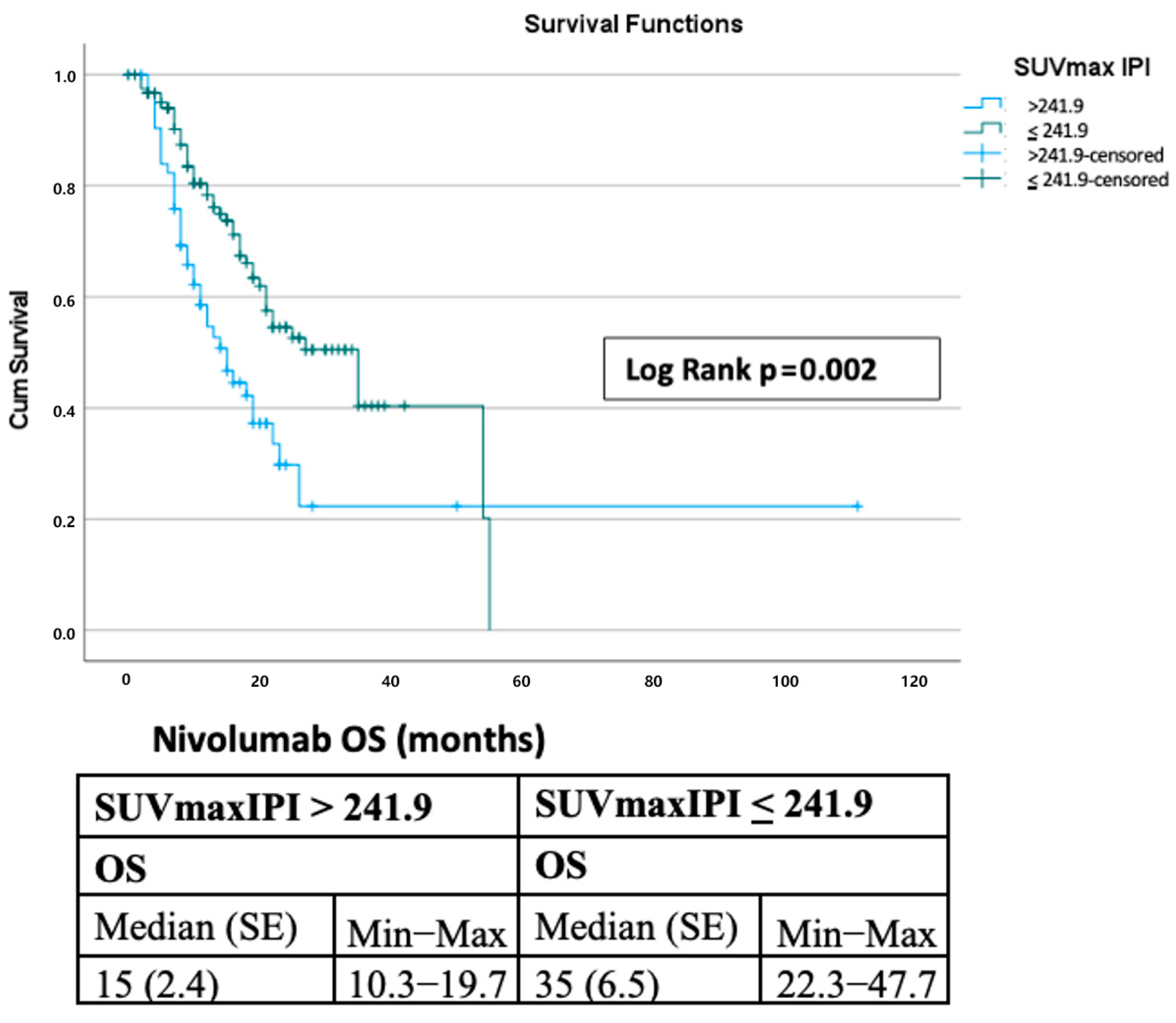
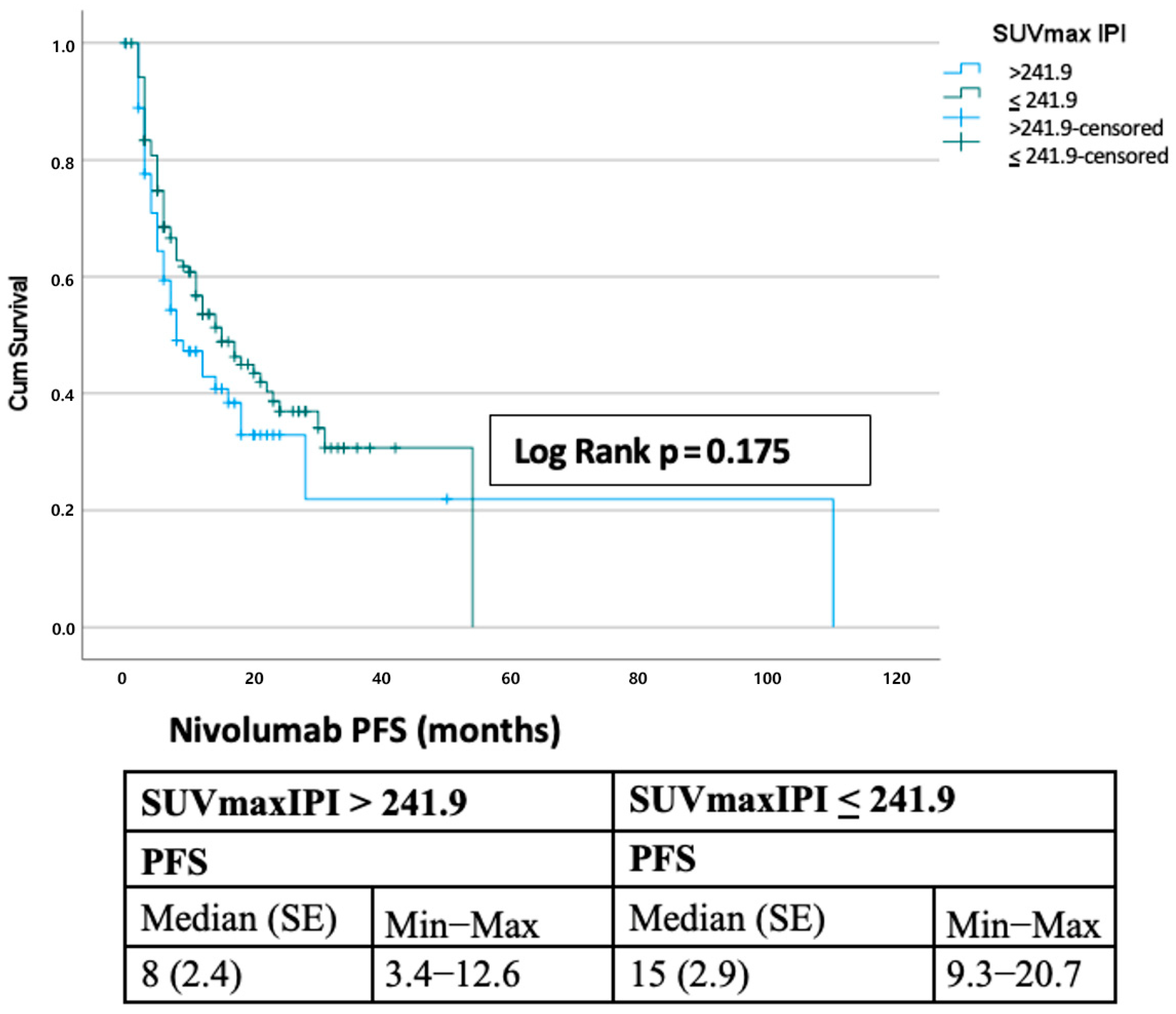
3.5. Comparison with Other Prognostic Scores
3.6. Comparative Performance of SUVmax-IPI Against IPI and SUVmax Alone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SUVmax | maximum standardized uptake value |
| IPI | inflammatory prognostic index |
| FDG-PET | 18F-fluorodeoxyglucose positron emission tomography |
| CRP | C-reactive protein |
| NLR | neutrophil-to-lymphocyte ratio |
| ROC | receiver operating characteristic |
| IQR | interquartile range |
| CI | confidence interval |
| SE | standard error |
| aHR | adjusted hazard ratio |
| SPSS | Statistical Package for the Social Sciences |
| C-index | concordance index |
| DCA | decision curve analysis |
| CR | complete response |
| PR | partial response |
| SD | stable disease |
| PD | progressive disease |
| irAEs | immune-related adverse events |
| HR | hazard ratio |
| LIPI | Lung Immune Prognostic Index |
| dNLR | derived neutrophil-to-lymphocyte ratio |
| SII | systemic immune-inflammation index |
| PLR | platelet-to-lymphocyte ratio |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The Evolving Landscape of Biomarkers for Checkpoint Inhibitor Immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Grootjans, W.; de Geus-Oei, L.F.; Troost, E.G.C.; Visser, E.P.; Oyen, W.J.G.; Bussink, J. PET in the Management of Locally Advanced and Metastatic NSCLC. Nat. Rev. Clin. Oncol. 2015, 12, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Higuchi, T.; Naruse, I.; Arisaka, Y.; Tokue, A.; Altan, B.; Takise, A.; Yanagitani, N.; Sunaga, N.; Hisada, T.; et al. Metabolic Activity by 18F-FDG-PET/CT Is Predictive of Early Response after Nivolumab in Previously Treated NSCLC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, X.; Zhang, A.; Zhang, J.; Zhang, Y.; Guo, F.; Zhang, S.; Li, X.; Wang, Z. The Role of PET Molecular Imaging in Immune Checkpoint Inhibitor Therapy in Lung Cancer: Precision Medicine and Visual Monitoring. Eur. J. Radiol. 2022, 149, 110200. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce-Aix, S.; Mazieres, J.; Caramella, C.; Couraud, S.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Advanced NSCLC. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, S.; Sun, W.; Zhang, C.; Yu, S.; Zhang, H.; Zhang, S.; Wang, Y. Systemic Immune-Inflammation Index Predicts Outcomes in Metastatic NSCLC Treated with Nivolumab. J. Clin. Lab. Anal. 2019, 33, e22964. [Google Scholar] [CrossRef]
- Tomita, M.; Shimizu, T.; Ayabe, T.; Nakamura, K.; Onitsuka, T. Inflammatory Prognostic Index in Resected NSCLC. Asian Pac. J. Cancer Prev. 2018, 19, 2867–2870. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Bryant, A.S.; Ohja, B.; Bartolucci, A.A. The Maximum Standardized Uptake Values on Positron Emission Tomography of a Non–Small Cell Lung Cancer Predict Stage, Recurrence, and Survival. J. Thorac. Cardiovasc. Surg. 2005, 130, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Zhu, H.; Fu, Z.; Liu, J.; Shi, D.; He, X.; Dong, C. Prognostic Value of the Standardized Uptake Value Maximum Change Calculated by Dual-Time-Point (18F)-Fluorodeoxyglucose PET Imaging in Patients with Advanced NSCLC. OncoTargets Ther. 2016, 9, 2993–2999. [Google Scholar] [CrossRef]
- Machtay, M.; Duan, F.; Siegel, B.A.; Snyder, B.S.; Gorelick, J.J.; Reddin, J.S.; Munden, R.F.; Johnson, D.W.; Wilf, L.H.; DeNittis, A.S.; et al. Prediction of Survival by [18F] Fluorodeoxyglucose Positron Emission Tomography in Patients with Locally Advanced NSCLC Undergoing Definitive Chemoradiation Therapy: Results of the ACRIN 6668/RTOG 0235 Trial. J. Clin. Oncol. 2013, 31, 3823–3830. [Google Scholar] [CrossRef]
- Guo, D.; Jin, F.; Jing, W.; Liu, J.; Fu, Z.; Zhu, H. Incorporation of the SUVmax Measured from FDG PET and Neutrophil-to-Lymphocyte Ratio Improves Prediction of Clinical Outcomes in Patients with Locally Advanced NSCLC. Clin. Lung Cancer 2019, 20, 412–419. [Google Scholar] [CrossRef]
- Chen, J.; Wei, S.; Zhao, T.; Xiang, J.; Zhou, J.; Chen, J.; Guo, F.; Zhang, J.; Liang, H.; Chen, X. Clinical Significance of Serum Biomarkers in Stage IV NSCLC Treated with PD-1 Inhibitors: LIPI Score, NLR, dNLR, LMR, and PAB. Dis. Markers 2022, 2022, 7137357. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Alfieri, S.; Signori, A.; Granata, R.; Galli, G.; Cortellini, A.; Buti, S.; Bersanelli, M.; De Toma, A.; Pagani, F.; et al. Prognostic Scores Including Peripheral Blood-Derived Inflammatory Indices in Patients with Advanced NSCLC Treated with Immune Checkpoint Inhibitors. Crit. Rev. Oncol. Hematol. 2022, 179, 103806. [Google Scholar] [CrossRef]
- Rizzo, A.; Cantale, O.; Mogavero, A.; Bortolotti, L.; Mollica, V.; Massari, F. Assessing the Role of Colonic and Other Anatomical Sites Uptake by [18F] FDG-PET/CT and Immune-Inflammatory Peripheral Blood Indexes in Patients with Advanced NSCLC Treated with First-Line Immune Checkpoint Inhibitors. Thorac. Cancer 2023, 14, 2473–2483. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, M.; Jiang, X.; Liu, Y.; Xu, C.; Wang, Y.; Sun, H.; Yang, J. Research Progress and Value of Albumin-Related Inflammatory Markers in the Prognosis of NSCLC: A Review of Clinical Evidence. Ann. Med. 2023, 55, 1294–1307. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, C.; Shi, F.; Liu, H.; Wu, S.; Ding, H.; Liang, Z. Combined Prognostic Value of the SUVmax Derived from FDG-PET and the Lymphocyte-Monocyte Ratio in Patients with Stage IIIB–IV NSCLC Receiving Chemotherapy. BMC Cancer 2021, 21, 66. [Google Scholar] [CrossRef]
- Ke, L.; Lu, S.; Li, X.; Fan, Y.; Zhou, C.; Cheng, Y.; Wu, L.; Yang, Y.; Hu, C.; Zhang, L. Prognostic Significance of SUVmax Combined with Lactate Dehydrogenase in Advanced Lung Cancer Patients Treated with Immune Checkpoint Inhibitors Plus Chemotherapy. Front. Oncol. 2021, 11, 652312. [Google Scholar] [CrossRef]
| SUVmax IPI | ||||||||
|---|---|---|---|---|---|---|---|---|
| n = 187 | Total | >241.9 | ≤241.9 | |||||
| n | % | n | % | n | % | p | ||
| Gender | Male | 159 | 85.0 | 61 | 93.8 | 98 | 80.3 | 0.014 |
| Female | 28 | 15.0 | 4 | 6.2 | 24 | 19.7 | ||
| Age at Diagnosis | Median (IQR) | 66 | (60–70) | 66 (60–70) | 65.5 (58.75–70.25) | 0.764 | ||
| Smoking Status | Smoker | 63 | 33.7 | 24 | 36.9 | 39 | 32.0 | 0.495 |
| Ex-smoker | 124 | 66.3 | 41 | 63.1 | 83 | 68.0 | ||
| Histological Type | Adenocarcinoma | 92 | 49.2 | 21 | 32.3 | 71 | 58.2 | 0.001 |
| Squamous cell | 90 | 48.1 | 43 | 66.2 | 47 | 38.5 | ||
| Other | 5 | 2.7 | 1 | 1.5 | 4 | 3.3 | ||
| Mutational Status | Unknown | 11 | 5.9 | 4 | 6.2 | 7 | 5.7 | 0.938 |
| Positive | 11 | 5.9 | 3 | 4.6 | 8 | 6.6 | ||
| Negative | 165 | 88.2 | 58 | 89.2 | 107 | 87.7 | ||
| PD-L1 Status | Unknown | 53 | 28.3 | 20 | 30.8 | 33 | 27.0 | 0.863 |
| Positive | 50 | 26.7 | 17 | 26.2 | 33 | 27.0 | ||
| Negative | 84 | 44.9 | 28 | 43.1 | 56 | 45.9 | ||
| Stage at Initial Diagnosis | 1 | 4 | 2.2 | 1 | 1.5 | 3 | 2.5 | 0.564 |
| 2 | 11 | 5.9 | 6 | 9.2 | 5 | 4.1 | ||
| 3 | 61 | 32.8 | 20 | 30.8 | 41 | 33.9 | ||
| 4 | 110 | 59.1 | 38 | 58.5 | 72 | 59.5 | ||
| Metastatic Pattern | de novo | 116 | 62.0 | 41 | 63.1 | 75 | 61.5 | 0.830 |
| Recurrence | 71 | 38.0 | 24 | 36.9 | 47 | 38.5 | ||
| SUVmax | Median (IQR) | 11.6 | (7.5–16.2) | 15.1 (11.2–20.7) | 9.65 (7.5–13.5) | ˂0.001 | ||
| Site of Metastasis | Lung | 103 | 55.1 | 36 | 55.4 | 67 | 54.9 | 0.951 |
| Liver | 19 | 10.2 | 9 | 13.8 | 10 | 8.2 | 0.223 | |
| Brain | 38 | 20.3 | 11 | 16.9 | 27 | 22.1 | 0.399 | |
| Bone | 78 | 41.7 | 31 | 47.7 | 47 | 38.5 | 0.226 | |
| Distant lymph node | 80 | 42.8 | 25 | 38.5 | 55 | 45.1 | 0.384 | |
| Other | 55 | 29.4 | 55 | 29.4 | 19 | 29.2 | 0.968 | |
| Prior Therapy Before Nivolumab | Pemetrexed combination | 27 | 14.6 | 6 | 9.4 | 21 | 17.4 | 0.300 |
| Taxane combination | 84 | 45.4 | 33 | 51.6 | 51 | 42.1 | ||
| Gemcitabine combination | 67 | 36.2 | 24 | 37.5 | 43 | 35.5 | ||
| Other | 7 | 3.8 | 1 | 1.6 | 6 | 5.0 | ||
| Treatment Line of Nivolumab | 1 | 3 | 1.6 | 1 | 1.5 | 2 | 1.6 | 0.0717 |
| 2 | 138 | 73.8 | 49 | 75.4 | 89 | 73.0 | ||
| 3 | 33 | 17.6 | 13 | 20.0 | 20 | 16.4 | ||
| 4 | 9 | 4.8 | 1 | 1.5 | 8 | 6.6 | ||
| 5 | 3 | 1.6 | 1 | 1.5 | 2 | 1.6 | ||
| 6 | 1 | 0.5 | 0 | 0.0 | 1 | 0.8 | ||
| Total number of lines of treatment | Median (IQR) | 3 | (2–3) | 2 (2–3) | 3 (2–3) | 0.214 | ||
| Disease Progression | Yes | 108 | 57.8 | 41 | 63.1 | 67 | 54.9 | 0.282 |
| No | 79 | 42.2 | 24 | 36.9 | 55 | 45.1 | ||
| Reason for Nivolumab Cessation | Progression | 105 | 93.8 | 40 | 93.0 | 65 | 94.2 | 0.536 |
| Hyperprogression | 1 | 0.9 | 0 | 0.0 | 1 | 1.4 | ||
| Adverse effect | 1 | 0.9 | 0 | 0.0 | 1 | 1.4 | ||
| Follow-up after CR | 1 | 0.9 | 0 | 0.0 | 1 | 1.4 | ||
| Other | 4 | 3.6 | 3 | 7.0 | 1 | 1.4 | ||
| Best Response to Nivolumab Treatment | CR | 27 | 14.4 | 11 | 16.4 | 16 | 13.3 | 0.465 |
| PR | 94 | 50.2 | 29 | 43.2 | 65 | 54.2 | ||
| SD | 36 | 19.2 | 13 | 19.4 | 23 | 19.2 | ||
| PD | 30 | 16.0 | 14 | 20.8 | 16 | 13.3 | ||
| Nivolumab Related Adverse Events | No | 146 | 78.07 | 58 | 85.3 | 88 | 73.9 | 0.107 |
| Yes | 41 | 21.92 | 10 | 14.7 | 31 | 26.1 | ||
| Grade of Nivolumab Related Adverse Events | Grade 1 | 18 | 43.9 | 8 | 80.0 | 10 | 32.3 | 0.050 |
| Grade 2 | 19 | 46.3 | 2 | 20.0 | 17 | 54.8 | ||
| Grade 3 | 1 | 2.4 | 0 | 0.0 | 1 | 3.2 | ||
| Grade 4 | 3 | 7.3 | 0 | 0.0 | 3 | 9.7 | ||
| Types of Nivolumab Related Adverse Events | Colitis | 2 | 4.9 | 0 | 0.0 | 2 | 6.5 | 0.493 |
| Hepatitis | 1 | 2.4 | 1 | 10.0 | 0 | 0.0 | ||
| Thyroiditis | 15 | 34.1 | 3 | 30.0 | 11 | 35.5 | ||
| Skin-related | 10 | 24.4 | 2 | 20.0 | 8 | 25.8 | ||
| Pneumonitis | 10 | 22.0 | 3 | 30.0 | 6 | 19.4 | ||
| Myocarditis | 1 | 2.4 | 1 | 10.0 | 0 | 0.0 | ||
| Other | 2 | 4.9 | 0 | 0.0 | 2 | 6.5 | ||
| Arthritis | 1 | 2.4 | 0 | 0.0 | 1 | 3.2 | ||
| Treatments for Nivolumab Related Adverse Events | Steroid | 11 | 26.8 | 3 | 30.0 | 8 | 25.8 | 1.000 |
| Steroid and other | 1 | 2.4 | 0 | 0.0 | 1 | 3.2 | ||
| Topical | 9 | 22.0 | 2 | 20.0 | 7 | 22.6 | ||
| Other | 20 | 48.8 | 5 | 50.0 | 15 | 48.4 | ||
| Rechallenge with Nivolumab After Adverse Events | No | 3 | 7.3 | 0 | 0.0 | 3 | 10.3 | 1.000 |
| Yes | 38 | 92.7 | 12 | 100 | 26 | 89.7 | ||
| Death | No | 102 | 54.5 | 27 | 41.5 | 75 | 61.5 | 0.009 |
| Yes | 85 | 45.5 | 38 | 58.5 | 47 | 38.5 | ||
| Follow-up period (months) | Median (IQR) | 28 (18–42) | 25 (16–38.5) | 31.5 (19.75–42.25) | 0.049 | |||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| p | HR (95% Cl Min–Max) | p | HR (95% Cl Min–Max) | |
| Gender Female | 0.227 | 1.360 (0.826–2.238) | ||
| Age at Diagnosis | 0.061 | 1.024 (0.999–1.049) | 0.411 | 1.011 (0.985–1.038) |
| Smoking Status Ex-Smoker | 0.028 | 1.634 (1.054–2.533) | 0.093 | 1.542 (0.931–2.555) |
| Histological Type (Ref: Adenocarcinoma) | 0.737 | |||
| Squamous cell | 0.437 | 1.167 (0.791–1.721) | ||
| Other | 0.955 | 1.034 (0.321–3.331) | ||
| Mutation Status (Ref: Negative) | 0.286 | |||
| Unknown | 0.296 | 1.507 (0.698–3.252) | ||
| Positive | 0.256 | 0.512 (0.161–1.624) | ||
| PD-L1 Status (Ref: Negative) | 0.061 | 0.244 | ||
| Unknown | 0.018 | 0.556 (0.341–0.905) | 0.095 | 0.624 (0.359–1.085) |
| Positive | 0.556 | 0.872 (0.552–1.377) | 0.665 | 0.893 (0.534–1.493) |
| PD-L1 Positivity Rate | 0.241 | 0.993 (0.982–1.005) | ||
| Stage at Initial Diagnosis (Ref: 4) | 0.454 | |||
| Stage 1 | 0.178 | 0.257 (0.035–1.854) | ||
| Stage 2 | 0.534 | 1.264 (0.604–2.644) | ||
| Stage 3 | 0.591 | 0.891 (0.585–1.357) | ||
| Metastatic Pattern Recurrence | 0.213 | 1.288 (0.865–1.916) | 0.019 | |
| SUVmax | 0.201 | 0.983 (0.957–1.009) | 0.623 | 0.990 (0.951–1.031) |
| Lung Metastasis | 0.472 | 1.153 (0.783–1.698) | ||
| Liver Metastasis | 0.805 | 1.083 (0.576–2.034) | ||
| Brain Metastasis | 0.081 | 1.503 (0.951–2.375) | 0.325 | 1.322 (0.759–2.303) |
| Bone Metastasis | 0.146 | 1.330 (0.905–1.953) | 0.389 | 1.200 (0.792–1.817) |
| Distant Lymph Node Metastasis | 0.627 | 1.100 (0.749–1.617) | ||
| Other Metastasis | 0.537 | 0.875 (0.573–1.337) | ||
| Prior Therapy Before Nivolumab (Ref: pemetrexed combination) | 0.450 | |||
| taxan combination | 0.113 | 1.669 (0.887–3.141) | ||
| gemcitabine combination | 0.220 | 1.502 (0.784–2.877) | ||
| other | 0.695 | 1.259 (0.398–3.987) | ||
| Treatment Line of Nivolumab | 0.459 | 0.901 (0.683–1.188) | ||
| Total number of lines of Treatment | ˂0.001 | 1.285 (1.123–1.470) | 0.133 | 1.144 (0.960–1.363) |
| Best Response to Nivolumab Treatment (Ref: CR) | ˂0.001 | ˂ 0.001 | ||
| PR | 0.112 | 1.848 (0.866–3.941) | 0.047 | 2.231 (1.011–4.291) |
| SD | ˂0.001 | 4.363 (1.966–9.683) | 0.001 | 4.416 (1.905–10.237) |
| PD | ˂0.001 | 12.847 (5.628–29.327) | ˂0.001 | 11.018 (4.383–27.696) |
| Nivolumab- Related Adverse Events | 0.004 | 0.451 (0.261–0.781) | ˂0.001 | 0.449 (0.253–0.798) |
| SUVmaxIPI > 241.9 | 0.191 | 1.303 (0.876–1.938) | 0.086 | 1.532 (0.942–2.492) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| p | HR (95%CI Min–Max) | p | HR (95%CI Min–Max) | |
| Gender Female | 0.325 | 1.325 (0.757–2.321) | ||
| Age at Diagnosis | 0.080 | 1.025 (0.997–1.053) | 0.934 | 0.999 (0.968–1.030) |
| Smoking Status Ex-Smoker | 0.033 | 1.763 (1.046–2.971) | 1.030 | 1.866 (0.877–3.791) |
| Histological Type (Ref: Adenocarcinoma) | 0.266 | |||
| Squamous cell | 0.127 | 1.409 (0.907–2.190) | ||
| Other | 0.773 | 0.810 (0.195–3.376) | ||
| Mutation Status (Ref: Negative) | 0.436 | |||
| Unknown | 0.881 | 0.926 (0.339–2.532) | ||
| Positive | 0.199 | 0.274 (0.038–1.977) | ||
| PD-L1 Status (Ref: Negative) | 0.223 | 0.027 | ||
| Unknown | 0.552 | 0.851 (0.501–1.446) | 0.027 | 2.380 (1.105–5.126) |
| Positive | 0.202 | 1.388 (0.839–2.299) | 0.028 | 2.034 (1.081–3.828) |
| PD-L1 Positivity Rate | 0.527 | 0.996 (0.984–1.008) | ||
| Stage at Initial Diagnosis (Ref: 4) | 0.718 | |||
| stage 1 | 0.958 | 0.000 | ||
| stage 2 | 0.733 | 1.159 (0.496–2.705) | ||
| stage 3 | 0.306 | 0.780 (0.484–1.256) | ||
| Metastatic Pattern (Ref: de novo) Recurrence | 0.093 | 0.677 (0.430–1.067) | 0.027 | 2.010 (1.083–3.732) |
| SUVmax | 0.914 | 0.998 (0.970–1.027) | ||
| Lung Metastasis | 0.359 | 1.227 (0.793–1.900) | ||
| Liver Metastasis | 0.883 | 1.054 (0.522–2.127) | ||
| Brain Metastasis | 0.049 | 1.663 (1.002–2.762) | 0.350 | 1.393 (0.695–2.795) |
| Bone Metastasis | 0.312 | 1.250 (0.811–1.925) | ||
| Distant Lymph Node Metastasis | 0.761 | 0.935 (0.604–1.445) | ||
| Other Metastasis | 0.223 | 0.735 (0.448–1.206) | 0.428 | 0.790 (0.441–1.414) |
| Prior Therapy Before Nivolumab (Ref: pemetrexed combination) | 0.154 | 0.842 | ||
| taxan combination | 0.051 | 2.053 (0.998–4.223) | 0.741 | 1.157 (0.488–2.739) |
| gemcitabine combination | 0.252 | 1.556 (0.730–3.319) | 0.727 | 1.175 (0.475–2.902) |
| other | 0.944 | 0.954 (0.252–3.613) | 0.588 | 0.633 (0.121–3.309) |
| Treatment Line of Nivolumab | 0.882 | 0.979 (0.738–1.298) | ||
| Total number of lines of treatment | 0.654 | 1.036 (0.887–1.211) | ||
| Progression | ˂0.001 | 24.422 (7.706–77.394) | ˂0.001 | 28.774 (8.262–100.205) |
| Best Response to Nivolumab Treatment (Ref: CR) | 0.000 | 0.062 | ||
| PR | 0.279 | 1.500 (0.719–3.129) | 0.369 | 0.661 (0.268–1.630) |
| SD | 0.029 | 2.421 (1.094–5.360) | 0.589 | 0.760 (0.280–2.060) |
| PD | ˂0.001 | 4.199 (1.898–9.289) | 0.284 | 1.703 (0.644–4.506) |
| Nivolumab- Related Adverse Events | 0.019 | 0.480 (0.260–0.887) | 0.819 | 1.086 (0.536–2.198) |
| SUVmaxIPI > 241.9 | 0.002 | 1.961 (1.273–3.022) | 0.002 | 2.499 (1.392–4.486) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolkıran, N.; Erdoğan, A.P.; Şahbazlar, M.; Taş, S.; Gököz Doğu, G.; Canaslan, K.; Ünek, İ.T.; Demirkıran, Ö.; Demir, B.; Teküstün, G.N.; et al. SUVmax-IPI as a New Prognostic Index in Metastatic Non-Small Cell Lung Cancer Patients Receiving Nivolumab. Curr. Oncol. 2025, 32, 566. https://doi.org/10.3390/curroncol32100566
Kolkıran N, Erdoğan AP, Şahbazlar M, Taş S, Gököz Doğu G, Canaslan K, Ünek İT, Demirkıran Ö, Demir B, Teküstün GN, et al. SUVmax-IPI as a New Prognostic Index in Metastatic Non-Small Cell Lung Cancer Patients Receiving Nivolumab. Current Oncology. 2025; 32(10):566. https://doi.org/10.3390/curroncol32100566
Chicago/Turabian StyleKolkıran, Nagihan, Atike Pınar Erdoğan, Mustafa Şahbazlar, Semra Taş, Gamze Gököz Doğu, Kübra Canaslan, İlkay Tuğba Ünek, Özge Demirkıran, Bilgin Demir, Güler Nur Teküstün, and et al. 2025. "SUVmax-IPI as a New Prognostic Index in Metastatic Non-Small Cell Lung Cancer Patients Receiving Nivolumab" Current Oncology 32, no. 10: 566. https://doi.org/10.3390/curroncol32100566
APA StyleKolkıran, N., Erdoğan, A. P., Şahbazlar, M., Taş, S., Gököz Doğu, G., Canaslan, K., Ünek, İ. T., Demirkıran, Ö., Demir, B., Teküstün, G. N., Tanrıverdi, Ö., & Ekinci, F. (2025). SUVmax-IPI as a New Prognostic Index in Metastatic Non-Small Cell Lung Cancer Patients Receiving Nivolumab. Current Oncology, 32(10), 566. https://doi.org/10.3390/curroncol32100566





