Incidence of Hypothyroidism and Thyroid Function Monitoring After Immune Checkpoint Inhibitor Therapy Completion for Lung Cancer: A Nationwide Analysis of a Japanese Claims Database
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
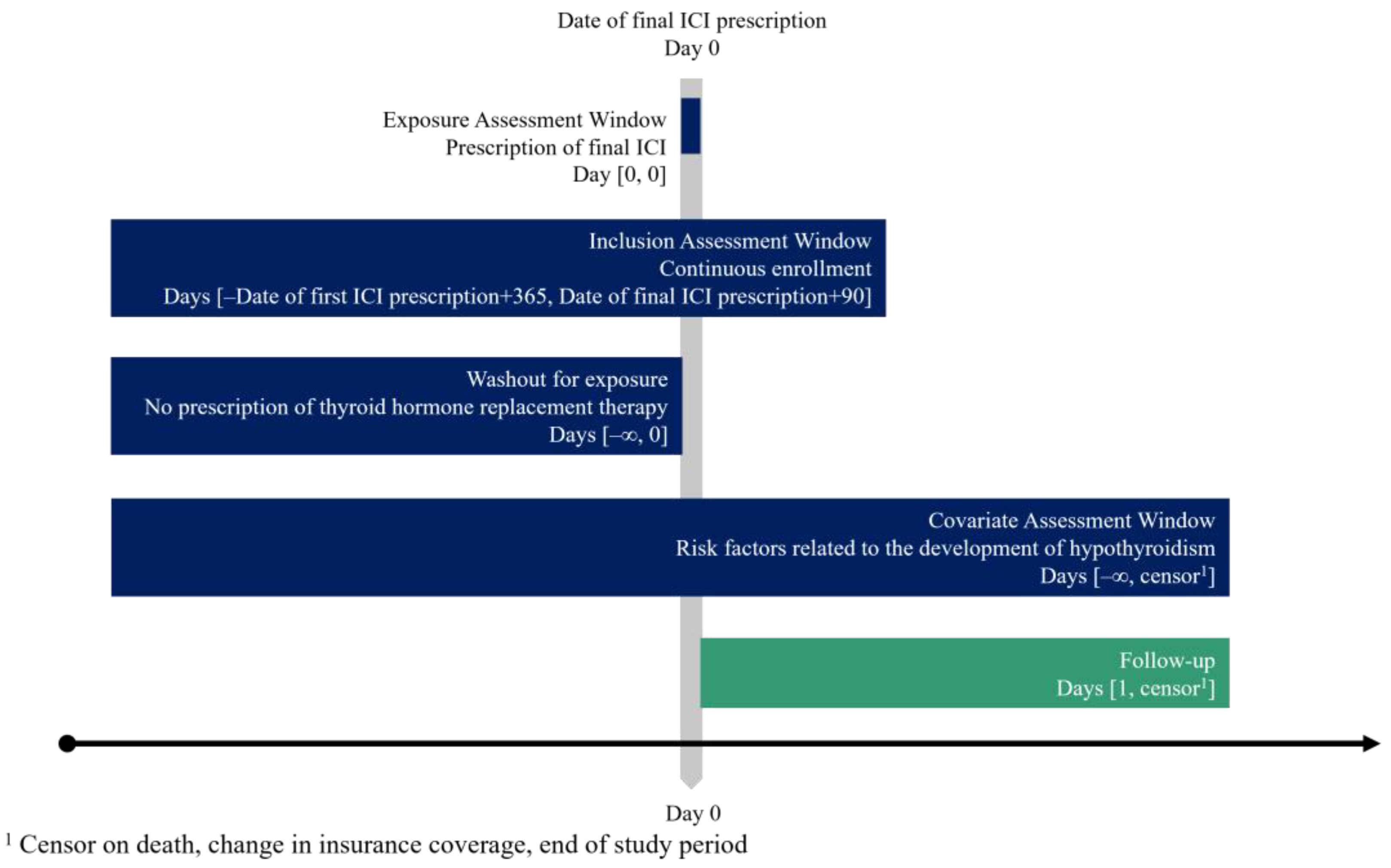
2.1. Study Design and Data Source
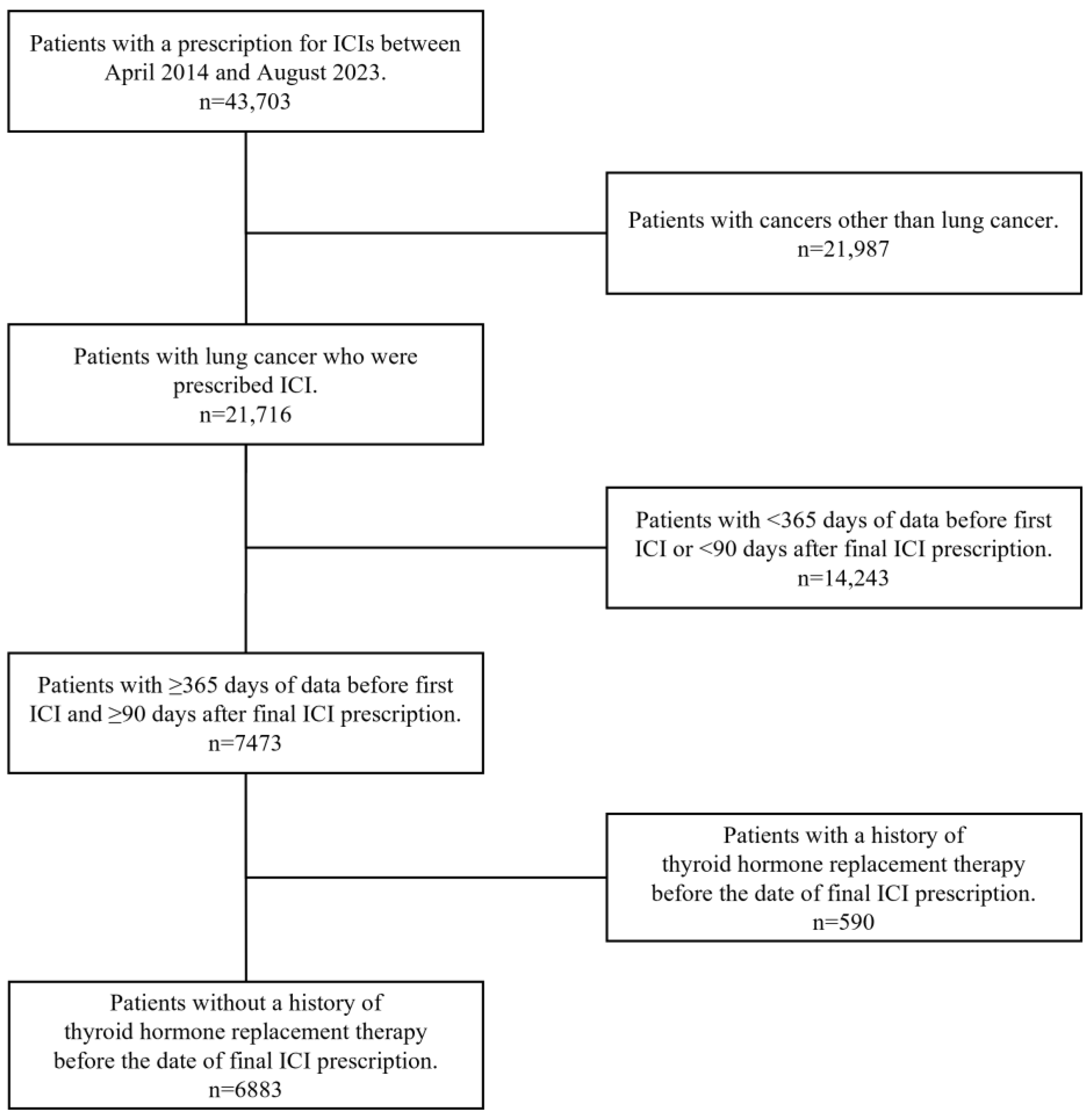
2.2. Study Population
2.3. Outcomes
2.3.1. Hypothyroidism After ICI Therapy Completion
2.3.2. Thyroid Function Monitoring After ICI Therapy Completion
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Incidence of Hypothyroidism After ICI Therapy Completion
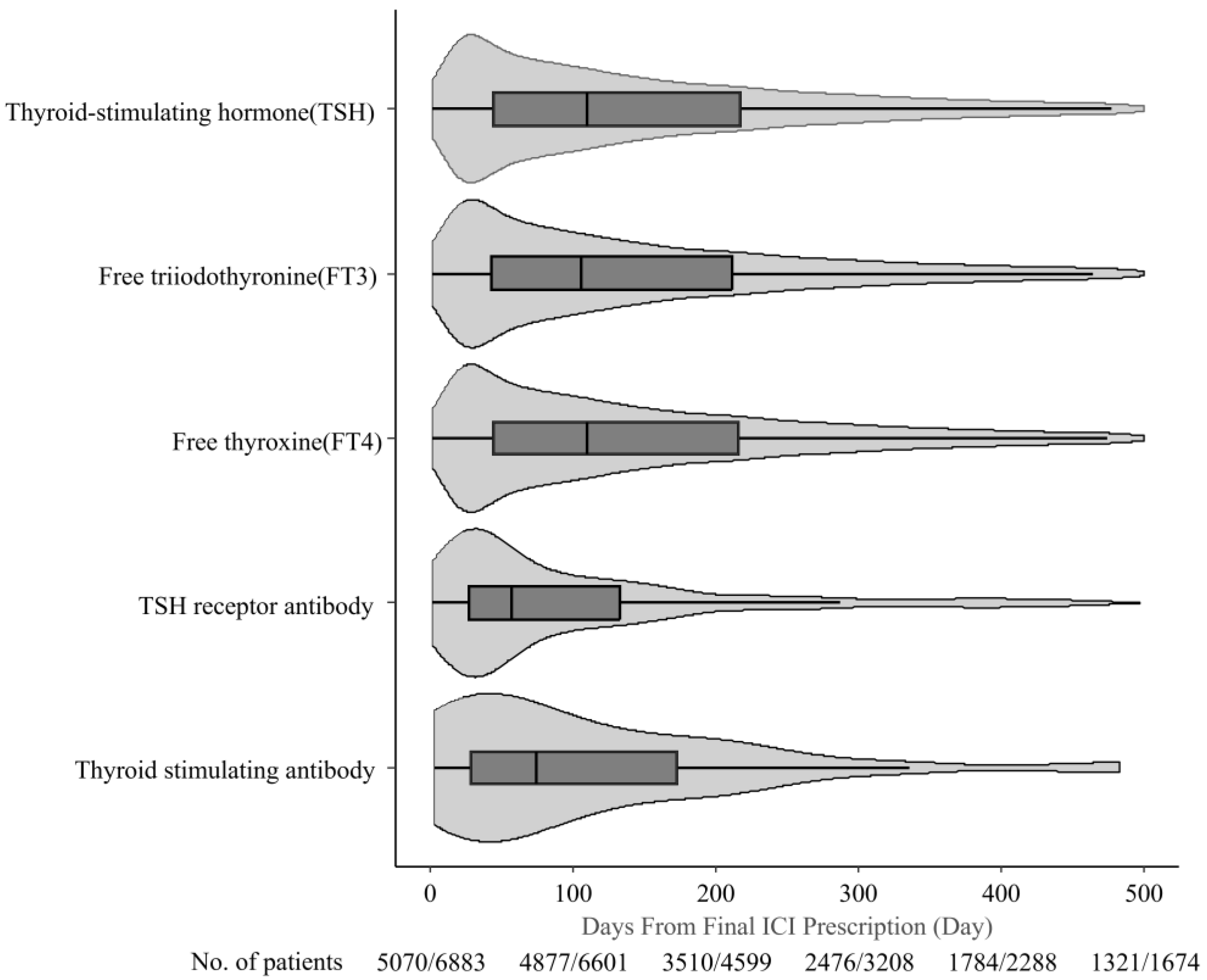
3.3. Thyroid Function Monitoring After ICI Therapy Completion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| FT3 | free triiodothyronine |
| FT4 | free thyroxine |
| ICI | immune checkpoint inhibitor |
| IQR | interquartile range |
| irAE | immune-related adverse events |
| OR | odds ratio |
| PD-1 | programmed death receptor 1 |
| TSH | thyroid-stimulating hormone |
| VEGF | vascular endothelial growth factor |
References
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in Advanced nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Sacchi de Camargo Correia, G.; Pai, T.; Li, S.; Connor, D.; Zhao, Y.; Lou, Y.; Manochakian, R. Immune-related adverse events in patients with lung cancer. Curr. Oncol. Rep. 2023, 25, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Zaid, M.A.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bermas, B.; Braaten, T.; Budde, L.E.; et al. NCCN guidelines® insights: Management of immunotherapy-related toxicities, version 2.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 582–592. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Stelmachowska-Banaś, M.; Czajka-Oraniec, I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: An updated review. Endocr. Connect. 2020, 9, R207–R228. [Google Scholar] [CrossRef]
- Ghisoni, E.; Wicky, A.; Bouchaab, H.; Imbimbo, M.; Delyon, J.; Gautron Moura, B.; Gérard, C.L.; Latifyan, S.; Özdemir, B.C.; Caikovski, M.; et al. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: An overlooked aspect in immunotherapy. Eur. J. Cancer 2021, 149, 153–164. [Google Scholar] [CrossRef]
- Husebye, E.S.; Castinetti, F.; Criseno, S.; Curigliano, G.; Decallonne, B.; Fleseriu, M.; Higham, C.E.; Lupi, I.; Paschou, S.A.; Toth, M.; et al. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: An ESE clinical practice guideline. Eur. J. Endocrinol. 2022, 187, G1–G21. [Google Scholar] [CrossRef] [PubMed]
- Girotra, M.; Hansen, A.; Farooki, A.; Byun, D.J.; Min, L.; Creelan, B.C.; Callahan, M.K.; Atkins, M.B.; Sharon, E.; Antonia, S.J.; et al. The current understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for management. JNCI Cancer Spectr. 2018, 2, pky021. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 25 August 2025).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Rong, Y.; Bentley, J.P.; Bhattacharya, K.; Yang, Y.; Chang, Y.; Earl, S.; Ramachandran, S. Incidence and risk factors of immune-related adverse events induced by immune checkpoint inhibitors among older adults with non-small cell lung cancer. Cancer Med. 2024, 13, e6879. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Velez, M.A.; Bachrach, B.; Gukasyan, J.; Fares, C.M.; Cummings, A.L.; Lind-Lebuffe, J.P.; Akingbemi, W.O.; Li, D.Y.; Brodrick, P.M.; et al. Immune checkpoint inhibitor induced thyroid dysfunction is a frequent event post-treatment in NSCLC. Lung Cancer 2021, 161, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Boucher, Y.; Kumar, A.S.; Posada, J.M.; Gjini, E.; Pfaff, K.; Lipschitz, M.; Lako, A.; Duda, D.G.; Rodig, S.J.; Hodi, F.S.; et al. Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer patients. NPJ Precis. Oncol. 2021, 5, 62. [Google Scholar] [CrossRef]
- Danlos, F.X.; Voisin, A.L.; Dyevre, V.; Michot, J.M.; Routier, E.; Taillade, L.; Champiat, S.; Aspeslagh, S.; Haroche, J.; Albiges, L.; et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur. J. Cancer 2018, 91, 21–29. [Google Scholar] [CrossRef]
- Gulati, N.; Celen, A.; Johannet, P.; Mehnert, J.M.; Weber, J.; Krogsgaard, M.; Osman, I.; Zhong, J. Preexisting immune-mediated inflammatory disease is associated with improved survival and increased toxicity in melanoma patients who receive immune checkpoint inhibitors. Cancer Med. 2021, 10, 7457–7465. [Google Scholar] [CrossRef]
- van der Kooij, M.K.; Suijkerbuijk, K.P.M.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; Blank, C.U.; Boers-Sonderen, M.J.; van Breeschoten, J.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Haanen, J.B.A.G.; et al. Safety and efficacy of checkpoint inhibition in patients with melanoma and preexisting autoimmune disease: A cohort study. Ann. Intern. Med. 2021, 174, 641–648. [Google Scholar] [CrossRef]
- Strehl, C.; Buttgereit, F. Optimized glucocorticoid therapy: Teaching old drugs new tricks. Mol. Cell Endocrinol. 2013, 380, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.F.L.; Mana, D.L.; Serra, H.A. Drug-induced hypothyroidism. Medicina 2017, 77, 394–404. [Google Scholar] [PubMed]
| Total, n (%) | |
|---|---|
| 6883 | |
| Male sex | 5290 (76.9) |
| Age: median (IQR) | 74.0 (69.0–79.0) |
| Type of ICI regimen | |
| Monotherapy | 5181 (75.3) |
| Combination | 1702 (24.3) |
| PD-1 + CTLA-4 | 160 (2.3) |
| ICI + Cytotoxic anticancer agents | 1365 (19.8) |
| ICI + Bevacizumab | 116 (1.7) |
| ICI + Cytotoxic anticancer agents + Bevacizumab | 60 (0.9) |
| ICI + Lenvatinib | 1 (0.01) |
| ICI-Type | |
| Pembrolizumab | 3118 (45.3) |
| Nivolumab | 1328 (19.3) |
| Atezolizumab | 1416 (20.6) |
| Durvalumab | 1018 (14.8) |
| Ipilimumab | 189 (2.7) |
| Number of ICI cycles | 5.0 (3.0–10.0) |
| Duration of ICI therapy | 105.0 (42.0–226.0) |
| Thyroid monitoring during ICI therapy | 5354 (77.8) |
| Thyroid monitoring after ICI therapy completion | 5070 (73.7) |
| Thyroid monitoring ≤90 d post-ICI therapy | 4481 (65.1) |
| Autoimmune disease (at the initiation of ICI therapy) | |
| Systemic lupus erythematosus | 23 (0.3) |
| Crohn’s disease | 2 (0.03) |
| Ulcerative colitis | 12 (0.2) |
| Myasthenia gravis | 6 (0.1) |
| Rheumatoid arthritis | 265 (3.9) |
| Type 1 diabetes | 20 (0.3) |
| Steroid use ≥ 28 d during ICI treatment | 761 (11.1) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Male sex | 0.77 (0.59–1.01) | 0.062 | 0.79 (0.60–1.03) | 0.079 |
| Age ≥ 75 years | 0.96 (0.76–1.23) | 0.781 | ||
| Type of ICI regimen | ||||
| Monotherapy | 0.75 (0.57–0.97) | 0.028 | 0.94 (0.69–1.27) | 0.665 |
| Combination | ||||
| PD-1 + CTLA-4 | 1.98 (1.08–3.60) | 0.026 | 1.12 (0.24–5.30) | 0.889 |
| ICI + Cytotoxic anticancer agents | 1.05 (0.78–1.41) | 0.751 | ||
| ICI + Bevacizumab | 2.30 (1.19–4.44) | 0.014 | 2.17 (1.07–4.39) | 0.031 |
| ICI + Cytotoxic anticancer agents + Bevacizumab | 2.19 (0.87–5.51) | 0.096 | 2.11 (0.81–5.50) | 0.126 |
| ICI + Lenvatinib | NA | NA | ||
| ICI-Type | ||||
| Pembrolizumab | 1.12 (0.88–1.43) | 0.354 | ||
| Nivolumab | 1.09 (0.81–1.46) | 0.581 | ||
| Atezolizumab | 0.87 (0.64–1.18) | 0.364 | ||
| Durvalumab | 0.86 (0.60–1.22) | 0.391 | ||
| Ipilimumab | 1.96 (1.12–3.42) | 0.019 | 1.79 (0.42–7.75) | 0.433 |
| Autoimmune disease (at the initiation of ICI therapy) | ||||
| Systemic lupus erythematosus | NA | NA | ||
| Crohn’s disease | NA | NA | ||
| Ulcerative colitis | 2.17 (0.28–16.90) | 0.458 | ||
| Myasthenia gravis | 12.0 (2.19–65.80) | 0.004 | 12.70 (2.31–70.00) | 0.003 |
| Rheumatoid arthritis | 1.03 (0.60–1.91) | 0.915 | ||
| Type 1 diabetes | NA | NA | ||
| Steroid use ≥28 d during ICI treatment | 0.48 (0.29–0.80) | 0.005 | 0.52 (0.31–0.87) | 0.013 |
| Thyroid-Stimulating Hormone (TSH) | Free Triiodothyronine (FT3) | Free Thyroxine (FT4) | TSH Receptor Antibody | Thyroid Stimulating Antibody | |
|---|---|---|---|---|---|
| Median (IQR), d | 126.0 (50.0–273.0) | 126.0 (49.0–273.0) | 126.0 (49.0–273.0) | 60.0 (27.0–154.0) | 75.0 (28.8–183.5) |
| Mode, d | 21.0 | 21.0 | 21.0 | 21.0 | 42.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, H.; Tsugane, H.; Yasu, T. Incidence of Hypothyroidism and Thyroid Function Monitoring After Immune Checkpoint Inhibitor Therapy Completion for Lung Cancer: A Nationwide Analysis of a Japanese Claims Database. Curr. Oncol. 2025, 32, 558. https://doi.org/10.3390/curroncol32100558
Ohta H, Tsugane H, Yasu T. Incidence of Hypothyroidism and Thyroid Function Monitoring After Immune Checkpoint Inhibitor Therapy Completion for Lung Cancer: A Nationwide Analysis of a Japanese Claims Database. Current Oncology. 2025; 32(10):558. https://doi.org/10.3390/curroncol32100558
Chicago/Turabian StyleOhta, Hiroaki, Hinako Tsugane, and Takeo Yasu. 2025. "Incidence of Hypothyroidism and Thyroid Function Monitoring After Immune Checkpoint Inhibitor Therapy Completion for Lung Cancer: A Nationwide Analysis of a Japanese Claims Database" Current Oncology 32, no. 10: 558. https://doi.org/10.3390/curroncol32100558
APA StyleOhta, H., Tsugane, H., & Yasu, T. (2025). Incidence of Hypothyroidism and Thyroid Function Monitoring After Immune Checkpoint Inhibitor Therapy Completion for Lung Cancer: A Nationwide Analysis of a Japanese Claims Database. Current Oncology, 32(10), 558. https://doi.org/10.3390/curroncol32100558





