Phase Ib/II Study of Pamiparib Plus Radiation Therapy and/or Temozolomide in Adult Patients with Treatment-Naïve or Recurrent/Refractory Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
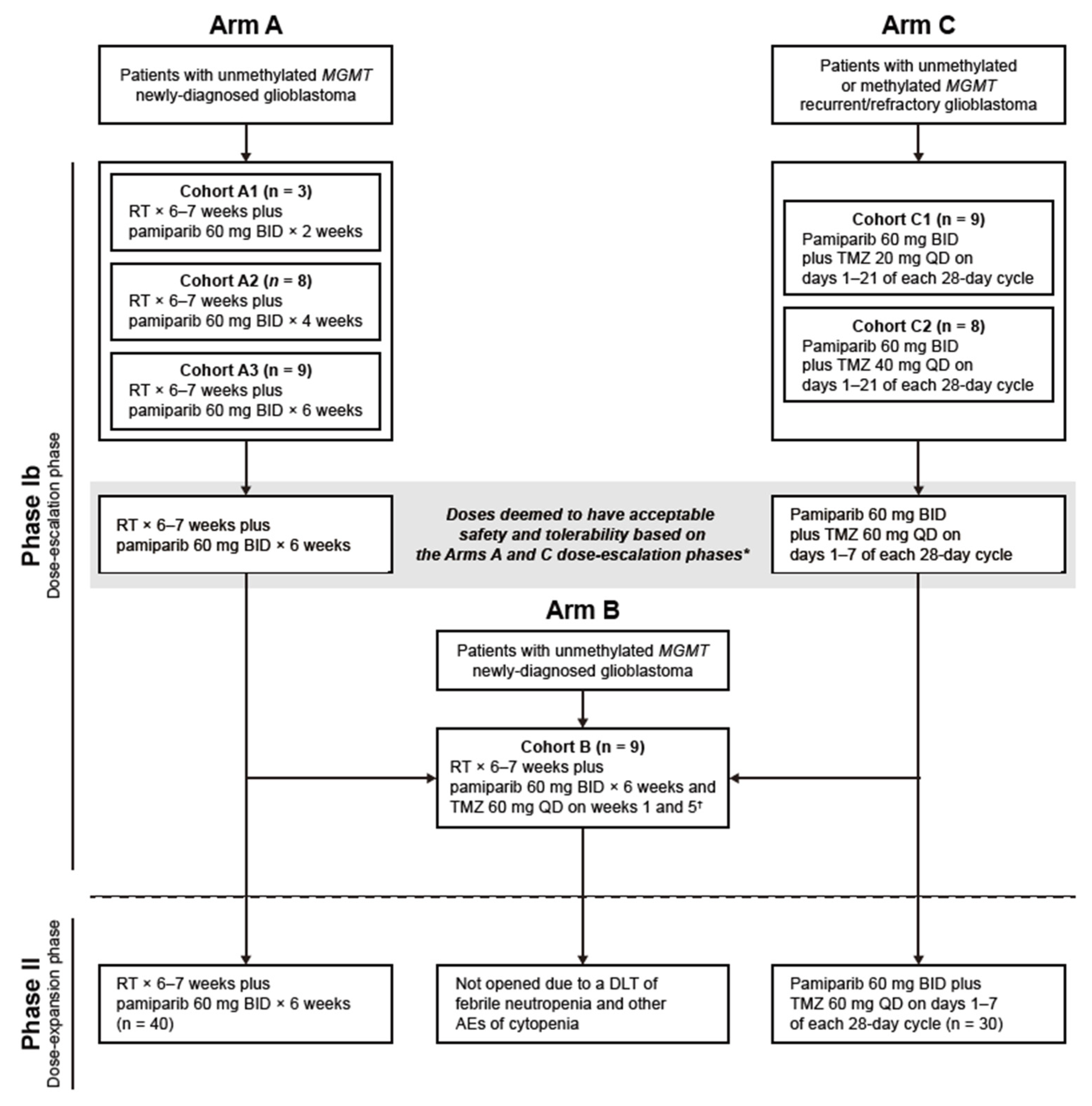
2.1. Study Design
2.2. Participants
2.3. Study Cohorts and Treatments
2.4. Endpoints
2.4.1. Primary and Secondary Endpoints: Phase Ib Study
2.4.2. Primary and Secondary Endpoints: Phase II Study
2.5. Assessments
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Treatment Exposure
3.3. Safety and Tolerability
3.4. Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| BID | Twice daily |
| CI | Confidence interval |
| CR | Complete response |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DCR | Disease control rate |
| DLT | Dose-limiting toxicity |
| DoR | Duration of response |
| ECOG PS | Eastern Cooperative Oncology Group performance status |
| EOT | End-of-treatment |
| IDH | Isocitrate dehydrogenase |
| MGMT | O-6-methylguanine-DNA methyltransferase |
| MRI | Magnetic resonance imaging |
| ORR | Objective response rate |
| OS | Overall survival |
| PARP | Poly (ADP-ribose) polymerase |
| PD | Progressive disease |
| PFS | Progression-free survival |
| PR | Partial response |
| QD | Once daily |
| RANO | Response Assessment in Neuro-Oncology |
| RT | Radiotherapy |
| SAE | Serious adverse event |
| SD | Stable disease |
| TEAE | Treatment-emergent adverse event |
| TMZ | Temozolomide |
| WHO | World Health Organization |
References
- Grochans, S.; Cybulska, A.M.; Siminska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of glioblastoma multiforme-literature review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro. Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Brain and Spinal Cord Tumor in Adults. Early Detection, Diagnosis, and Staging. Available online: https://www.cancer.org/cancer/brain-spinal-cord-tumors-adults/detection-diagnosis-staging/survival-rates.html (accessed on 5 January 2023).
- Smrdel, U.; Popovic, M.; Zwitter, M.; Bostjancic, E.; Zupan, A.; Kovac, V.; Glavac, D.; Bokal, D.; Jerebic, J. Long-term survival in glioblastoma: Methyl guanine methyl transferase (MGMT) promoter methylation as independent favourable prognostic factor. Radiol. Oncol. 2016, 50, 394–401. [Google Scholar] [CrossRef]
- Fernandes, C.; Costa, A.; Osorio, L.; Costa Lago, R.; Linhares, P.; Carvalho, B.; Caeiro, C. Current standards of care in glioblastoma therapy. In Glioblastoma; Vleeschouwer, S.D., Ed.; Codon Publications: Brisbane, Australia, 2017; pp. 197–242. [Google Scholar]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Gojo, I.; Beumer, J.H.; Pratz, K.W.; McDevitt, M.A.; Baer, M.R.; Blackford, A.L.; Smith, B.D.; Gore, S.D.; Carraway, H.E.; Showel, M.M.; et al. A phase 1 study of the PARP inhibitor veliparib in combination with temozolomide in acute myeloid leukemia. Clin. Cancer Res. 2017, 23, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Carducci, M.A.; Slovin, S.; Cetnar, J.; Qian, J.; McKeegan, E.M.; Refici-Buhr, M.; Chyla, B.; Shepherd, S.P.; Giranda, V.L.; et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Investig. New Drugs 2014, 32, 904–912. [Google Scholar] [CrossRef]
- Middleton, M.R.; Friedlander, P.; Hamid, O.; Daud, A.; Plummer, R.; Falotico, N.; Chyla, B.; Jiang, F.; McKeegan, E.; Mostafa, N.M.; et al. Randomized phase II study evaluating veliparib (ABT-888) with temozolomide in patients with metastatic melanoma. Ann. Oncol. 2015, 26, 2173–2179. [Google Scholar] [CrossRef]
- Plummer, R.; Stephens, P.; Aissat-Daudigny, L.; Cambois, A.; Moachon, G.; Brown, P.D.; Campone, M. Phase 1 dose-escalation study of the PARP inhibitor CEP-9722 as monotherapy or in combination with temozolomide in patients with solid tumors. Cancer Chemother. Pharmacol. 2014, 74, 257–265. [Google Scholar] [CrossRef]
- Su, J.M.; Thompson, P.; Adesina, A.; Li, X.N.; Kilburn, L.; Onar-Thomas, A.; Kocak, M.; Chyla, B.; McKeegan, E.; Warren, K.E.; et al. A phase I trial of veliparib (ABT-888) and temozolomide in children with recurrent CNS tumors: A pediatric brain tumor consortium report. Neuro-Oncol. 2014, 16, 1661–1668. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Hecht, J.R.; Konecny, G.E.; Goldman, J.W.; Sadeghi, S.; Chmielowski, B.; Singh, A.; Finn, R.S.; Martinez, D.; Yonemoto, L.; et al. Safety and efficacy results from a phase I dose-escalation trial of the PARP inhibitor talazoparib in combination with either temozolomide or irinotecan in patients with advanced malignancies. Cancer Res. 2016, 76, CT011. [Google Scholar] [CrossRef]
- Dziadkowiec, K.N.; Gasiorowska, E.; Nowak-Markwitz, E.; Jankowska, A. PARP inhibitors: Review of mechanisms of action and BRCA1/2 mutation targeting. Prz. Menopauzalny 2016, 15, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Lupo, B.; Trusolino, L. Inhibition of poly(ADP-ribosyl)ation in cancer: Old and new paradigms revisited. Biochim. Biophys. Acta 2014, 1846, 201–215. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA damage response in cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; O’Connor, M.J.; de Bono, J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 2016, 8, 362ps17. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Guo, Y.; Liu, Y.; Wang, H.; Gong, W.; Liu, Y.; Wang, X.; Gao, Y.; Yu, F.; Su, D.; et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia 2020, 22, 431–440. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Gao, F.; de Groot, J.F.; Koul, D.; Yung, W.K.A. PARP-mediated PARylation of MGMT is critical to promote repair of temozolomide-induced O6-methylguanine DNA damage in glioblastoma. Neuro-Oncol. 2021, 23, 920–931. [Google Scholar] [CrossRef]
- Higuchi, F.; Nagashima, H.; Ning, J.; Koerner, M.V.A.; Wakimoto, H.; Cahill, D.P. Restoration of temozolomide sensitivity by PARP inhibitors in mismatch repair deficient glioblastoma is independent of base excision repair. Clin. Cancer Res. 2020, 26, 1690–1699. [Google Scholar] [CrossRef]
- Zhou, J.X.; Feng, L.J.; Zhang, X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: A meta-analysis of randomized controlled trials. Drug Des. Dev. Ther. 2017, 11, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Bisht, P.; Kumar, V.U.; Pandey, R.; Velayutham, R.; Kumar, N. Role of PARP inhibitors in glioblastoma and perceiving challenges as well as strategies for successful clinical development. Front. Pharmacol. 2022, 13, 939570. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Waqar, S.N.; Krug, L.M.; Dowlati, A.; Hann, C.L.; Chiappori, A.; Owonikoko, T.K.; Woo, K.M.; Cardnell, R.J.; Fujimoto, J.; et al. Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J. Clin. Oncol. 2018, 36, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Kurian, K.M.; Williams, K.; Watts, C.; Jackson, A.; Carruthers, R.; Strathdee, K.; Cruickshank, G.; Dunn, L.; Erridge, S.; et al. Pharmacokinetics, safety, and tolerability of olaparib and temozolomide for recurrent glioblastoma: Results of the phase I OPARATIC trial. Neuro-Oncol. 2020, 22, 1840–1850. [Google Scholar] [CrossRef]
- Lesueur, P.; Lequesne, J.; Grellard, J.M.; Dugué, A.; Coquan, E.; Brachet, P.E.; Geffrelot, J.; Kao, W.; Emery, E.; Berro, D.H.; et al. Phase I/IIa study of concomitant radiotherapy with olaparib and temozolomide in unresectable or partially resectable glioblastoma: OLA-TMZ-RTE-01 trial protocol. BMC Cancer 2019, 19, 198. [Google Scholar] [CrossRef]
- Fulton, B.; Short, S.C.; James, A.; Nowicki, S.; McBain, C.; Jefferies, S.; Kelly, C.; Stobo, J.; Morris, A.; Williamson, A.; et al. PARADIGM-2: Two parallel phase I studies of olaparib and radiotherapy or olaparib and radiotherapy plus temozolomide in patients with newly diagnosed glioblastoma, with treatment stratified by MGMT status. Clin. Transl. Radiat. Oncol. 2018, 8, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Stefan, D.; Lesueur, P.; Lequesne, J.; Feuvret, L.; Bronnimann, C.; Castera, M.; Brachet, P.E.; Hrab, I.; Ducloie, M.; Lacroix, J.; et al. Olaparib, temozolomide, and concomitant radiotherapy for partially resected or biopsy-only glioblastoma first-line treatment: Results from the OLA-TMZ-RTE-01 phase I study. Clin. Cancer Res. 2025, 31, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, J.; Wang, J.; Lin, Z.; Yin, R.; Sun, W.; Zhou, Q.; Zhang, S.; Wang, D.; Shi, H.; et al. Pamiparib monotherapy for patients with germline BRCA1/2-mutated ovarian cancer previously treated with at least two lines of chemotherapy: A multicenter, open-label, phase II study. Clin. Cancer Res. 2022, 28, 653–661. [Google Scholar] [CrossRef]
- Xu, B.; Yin, Y.; Dong, M.; Song, Y.; Li, W.; Huang, X.; Wang, T.; He, J.; Mu, X.; Li, L.; et al. Pamiparib dose escalation in Chinese patients with non-mucinous high-grade ovarian cancer or advanced triple-negative breast cancer. Cancer Med. 2021, 10, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lickliter, J.D.M.L.; Mileshkin, L.; Voskoboynik, M.; Millward, M.; Freimund, A.; Meniawy, T.; Tang, T.; Wei, R.; Li, M.; Paton, V. Dose escalation/expansion study to investigate the safety, pharmacokinetics, food effect, and antitumor activity of BGB-290 in patients with advanced solid tumors. Ann. Oncol. 2017, 28, 123. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Stradella, A.; Johnson, M.; Goel, S.; Park, H.; Lakhani, N.; Arkenau, H.T.; Galsky, M.D.; Calvo, E.; Baz, V.; Moreno, V.; et al. Phase 1b study to assess the safety, tolerability, and clinical activity of pamiparib in combination with temozolomide in patients with locally advanced or metastatic solid tumors. Cancer Med. 2024, 13, e7385. [Google Scholar] [CrossRef]
- Barcellini, A.; Loap, P.; Murata, K.; Villa, R.; Kirova, Y.; Okonogi, N.; Orlandi, E. PARP inhibitors in combination with radiotherapy: To do or not to do? Cancers 2021, 13, 5380. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Gorlia, T.; van den Bent, M.J.; Vecht, C.J.; Steuve, J.; Brandes, A.A.; Platten, M.; A Kosch, M.; Hegi, M.E.; Lhermitte, B.; et al. Radiation therapy and concurrent plus adjuvant temsirolimus (CCI-779) versus chemoirradiation with temozolomide in newly diagnosed glioblastoma without methylation of the MGMT gene promoter. J. Clin. Oncol. 2014, 32, 2003. [Google Scholar] [CrossRef]
- Alnahhas, I.; Alsawas, M.; Rayi, A.; Palmer, J.D.; Raval, R.; Ong, S.; Giglio, P.; Murad, M.H.; Puduvalli, V. Characterizing benefit from temozolomide in MGMT promoter unmethylated and methylated glioblastoma: A systematic review and meta-analysis. Neuro-Oncol. Adv. 2020, 2, vdaa082. [Google Scholar] [CrossRef]
- Chen, C.; Xu, T.; Lu, Y.; Chen, J.; Wu, S. The efficacy of temozolomide for recurrent glioblastoma multiforme. Eur. J. Neurol. 2013, 20, 223–230. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Wen, P.Y.; Chang, S.M.; van den Bent, M.; Vogelbaum, M.A.; Li, G.; Li, S.; Kim, J.; Youssef, G.; Wick, W.; et al. Objective response rate (ORR) targets for recurrent glioblastoma clinical trials based on the historic association between ORR and median overall survival. Neuro-Oncol. 2023, 25, 1017–1028. [Google Scholar] [CrossRef]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Puduvalli, V.K.; Chamberlain, M.C.; van den Bent, M.J.; Carpentier, A.F.; Cher, L.M.; Mason, W.; Weller, M.; Hong, S.; Musib, L.; et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J. Clin. Oncol. 2010, 28, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.W.; Galanis, E.; Khasraw, M. PARP inhibitors in glioma: A review of therapeutic opportunities. Cancers 2022, 14, 1003. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Molenaar, R.J.; Verbaan, D.; Lamba, S.; Zanon, C.; Jeuken, J.W.; Boots-Sprenger, S.H.; Wesseling, P.; Hulsebos, T.J.; Troost, D.; van Tilborg, A.A.; et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro-Oncol. 2014, 16, 1263–1273. [Google Scholar] [CrossRef]
| Characteristic/ Demographic | Arm A: Dose-Escalation Phase | Arm A: Dose-Expansion Phase | Arm B: Dose-Escalation | Total Newly Diagnosed Glioblastoma (N = 69) | ||
|---|---|---|---|---|---|---|
| Pamiparib 2 Weeks + RT (N = 3) | Pamiparib 4 Weeks + RT (N = 8) | Pamiparib 6 Weeks + RT (N = 9) | Pamiparib 6 Weeks + RT (N = 40) | Pamiparib 6 Weeks + RT + TMZ 60 mg Weeks 1 and 5 (N = 9) | ||
| Median age, years (range) | 64.0 (50–65) | 65.5 (43–71) | 60.0 (42–68) | 58.0 (31–79) | 62.0 (45–77) | 61.0 (31–79) |
| Age group, n (%) | ||||||
| <65 years | 2 (66.7) | 3 (37.5) | 7 (77.8) | 28 (70.0) | 5 (55.6) | 45 (65.2) |
| ≥65 years | 1 (33.3) | 5 (62.5) | 2 (22.2) | 12 (30.0) | 4 (44.4) | 24 (34.8) |
| Sex, n (%) | ||||||
| Female | 0 (0.0) | 1 (12.5) | 2 (22.2) | 17 (42.5) | 4 (44.4) | 24 (34.8) |
| Race, n (%) | ||||||
| White | 3 (100.0) | 7 (87.5) | 7 (77.8) | 38 (95.0) | 9 (100.0) | 64 (92.8) |
| ECOG performance status, n (%) | ||||||
| 0 | 1 (33.3) | 2 (25.0) | 3 (33.3) | 13 (32.5) | 5 (55.6) | 24 (34.8) |
| 1 | 2 (66.7) | 6 (75.0) | 6 (66.7) | 27 (67.5) | 4 (44.4) | 45 (65.2) |
| Median time from initial diagnosis to study entry, weeks (range) | 4.1 (3–5) | 2.7 (2–6) | 3.7 (2–4) | 3.4 (1–8) | 3.1 (2–4) | 3.3 (1–8) |
| Median time since initial surgical resection, weeks (range) | 4.1 (3–5) | 2.9 (2–6) | 3.8 (2–4) | 3.4 (1–8) | 3.0 (1–4) | 3.3 (1–8) |
| Surgical status for initial diagnosis of glioblastoma, n (%) | ||||||
| Biopsy | 1 (33.3) | 2 (25.0) | 1 (11.1) | 2 (5.0) | 0 (0.0) | 6 (8.7) |
| Complete resection | 2 (66.7) | 3 (37.5) | 3 (33.3) | 21 (52.5) | 5 (55.6) | 34 (49.3) |
| Partial resection | 0 (0.0) | 3 (37.5) | 5 (55.6) | 16 (40.0) | 4 (44.4) | 28 (40.6) |
| Other (left temporal mass debulking) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.5) | 0 (0.0) | 1 (1.4) |
| Baseline corticosteroid use, n (%) | ||||||
| Yes | 3 (100.0) | 4 (50.0) | 6 (66.7) | 18 (45.0) | 5 (55.6) | 36 (52.2) |
| Characteristic/ Demographic | Arm C: Dose-Escalation Phase | Arm C: Dose-Expansion Phase | Total Recurrent/Refractory Glioblastoma (N = 47) | |
|---|---|---|---|---|
| Pamiparib + TMZ 20 mg Days 1–21 (N = 9) | Pamiparib + TMZ 40 mg Days 1–21 (N = 8) | Pamiparib + TMZ 60 mg Days 1–7 (N = 30) | ||
| Median age, years (range) | 53.0 (24–62) | 53.5 (25–67) | 58.0 (33–87) | 55.0 (24–87) |
| Age group, n (%) | ||||
| <65 years | 9 (100.0) | 7 (87.5) | 23 (76.7) | 39 (83.0) |
| ≥65 years | 0 (0.0) | 1 (12.5) | 7 (23.3) | 8 (17.0) |
| Sex, n (%) | ||||
| Female | 1 (11.1) | 4 (50.0) | 10 (33.3) | 15 (31.9) |
| Race, n (%) | ||||
| White | 7 (77.8) | 8 (100.0) | 27 (90.0) | 42 (89.4) |
| ECOG performance status, n (%) | ||||
| 0 | 2 (22.2) | 3 (37.5) | 10 (33.3) | 15 (31.9) |
| 1 | 7 (77.8) | 5 (62.5) | 20 (66.7) | 32 (68.1) |
| Median time from initial diagnosis to study entry, weeks (range) | 15.1 (12–108) | 24.2 (10–87) | 10.9 (5–91) | 14.2 (5–108) |
| Median time since initial surgical resection, weeks (range) | 15.1 (11–108) | 24.2 (10–87) | 10.7 (4–91) | 13.1 (4–108) |
| Surgical status for initial diagnosis of glioblastoma, n (%) | ||||
| Complete resection | 6 (66.7) | 7 (87.5) | 15 (50.0) | 28 (59.6) |
| Partial resection | 3 (33.3) | 1 (12.5) | 15 (50.0) | 19 (40.4) |
| Relapse status, n (%) | ||||
| First relapse | 7 (77.8) | 6 (75.0) | 27 (90.0) | 40 (85.1) |
| Second relapse | 2 (22.2) | 2 (25.0) | 3 (10.0) | 7 (14.9) |
| WHO grade at initial diagnosis of glioblastoma, n (%) | ||||
| Grade IV | 9 (100.0) | 6 (75.0) | 29 (96.7) | 44 (93.6) |
| MGMT promoter status, n (%) | ||||
| Methylated | 1 (11.1) | 3 (37.5) | 12 (40.0) | 16 (34.0) |
| Unmethylated | 7 (77.8) | 4 (50.0) | 18 (60.0) | 29 (61.7) |
| Prior use of TMZ, n (%) | ||||
| Yes | 9 (100.0) | 7 (87.5) | 30 (100.0) | 46 (97.9) |
| Baseline corticosteroid use, n (%) | ||||
| Yes | 5 (55.6) | 3 (37.5) | 17 (56.7) | 25 (53.2) |
| Events, N (%) | Newly Diagnosed Glioblastoma | Recurrent/Refractory Glioblastoma | |
|---|---|---|---|
| Arm A (N = 60) * | Arm B (N = 9) † | Arm C (N = 47) ‡ | |
| Patients with ≥1 TEAE | 60 (100.0) | 9 (100.0) | 46 (97.9) |
| TEAEs of grade ≥3 | 33 (55.0) | 4 (44.4) | 31 (66.0) |
| Treatment-emergent SAEs | 22 (36.7) | 2 (22.2) | 18 (38.3) |
| TEAE leading to death | 3 (5.0) | 0 (0.0) | 1 (2.1) |
| TEAE leading to treatment discontinuation of pamiparib only | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| TEAE leading to treatment discontinuation of RT only | 1 (1.7) | 0 (0.0) | – |
| TEAE leading to treatment discontinuation of TMZ only | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| TEAE leading to dose modification of pamiparib only | 12 (20.0) | 2 (22.2) | 13 (27.7) |
| Leading to dose interruption | 12 (20.0) | 2 (22.2) | 13 (27.7) |
| Leading to dose reduction | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| TEAE leading to dose modification of RT only | 4 (6.7) | 0 (0.0) | – |
| Leading to dose interruption | 4 (6.7) | 0 (0.0) | – |
| Leading to dose reduction | 0 (0.0) | 0 (0.0) | – |
| TEAE leading to dose modification of TMZ only | 3 (5.0) | 1 (11.1) | 4 (8.5) |
| Leading to dose interruption | 2 (3.3) | 1 (11.1) | 3 (6.4) |
| Leading to dose reduction | 1 (1.7) | 0 (0.0) | 2 (4.3) |
| TEAE related to pamiparib only | 38 (63.3) | 3 (33.3) | 11 (23.4) |
| TEAE related to RT only | 39 (65.0) | 6 (66.7) | – |
| TEAE related to TMZ only | 5 (8.3) | 5 (55.6) | 16 (34.0) |
| TEAE related to pamiparib only grade ≥3 | 5 (8.3) | 1 (11.1) | 0 (0.0) |
| TEAE related to RT only grade ≥3 | 2 (3.3) | 1 (11.1) | – |
| TEAE related to TMZ only grade ≥3 | 0 (0.0) | 0 (0.0) | 3 (6.4) |
| Serious TEAE related to pamiparib only | 3 (5.0) | 0 (0.0) | 1 (2.1) |
| Serious TEAE related to RT only | 1 (1.7) | 0 (0.0) | – |
| Serious TEAE related to TMZ only | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Treatment-related TEAEs leading to death | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Treatment-related TEAE leading to treatment discontinuation of pamiparib only | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Treatment-related TEAE leading to treatment discontinuation of RT only | 0 (0.0) | 0 (0.0) | – |
| Treatment-related TEAE leading to treatment discontinuation of TMZ only | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Treatment-related TEAE leading to dose modification of pamiparib only | 7 (11.7) | 2 (22.2) | 10 (21.3) |
| Leading to dose interruption | 7 (11.7) | 2 (22.2) | 10 (21.3) |
| Leading to dose reduction | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Treatment-related TEAE leading to dose modification of RT only | 1 (1.7) | 0 (0.0) | – |
| Leading to dose interruption | 1 (1.7) | 0 (0.0) | – |
| Leading to dose reduction | 0 (0.0) | 0 (0.0) | – |
| Treatment-related TEAE leading to dose modification of TMZ only | 3 (5.0) | 1 (11.1) | 4 (8.5) |
| Leading to dose interruption | 2 (3.3) | 1 (11.1) | 3 (6.4) |
| Leading to dose reduction | 1 (1.7) | 0 (0.0) | 2 (4.3) |
| Endpoint | Arm A: Dose-Escalation Phase | Arm A: Dose-Expansion Phase | Arm B: Dose-Escalation | |||
|---|---|---|---|---|---|---|
| Pamiparib 2 Weeks + RT | Pamiparib 4 Weeks + RT | Pamiparib 6 Weeks + RT | Pamiparib 6 Weeks + RT | Pamiparib 6 Weeks + RT + TMZ 60 mg Weeks 1 and 5 | Total Newly Diagnosed Glioblastoma | |
| Disease Response Analyses (Unconfirmed; Efficacy Analysis Set *) | ||||||
| N | 3 | 6 | 7 | 32 | 5 | 53 |
| Best response per RANO criteria, n (%) | ||||||
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 0 | 3 (50.0) | 0 | 3 (9.4) | 0 | 6 (11.3) |
| SD | 2 (66.7) | 3 (50.0) | 3 (42.9) | 19 (59.4) | 4 (80.0) | 31 (58.5) |
| PD | 1 (33.3) | 0 | 4 (57.1) | 10 (31.3) | 1 (20.0) | 16 (30.2) |
| Modified disease control rate (CR, PR, or SD at EOT visit), n (%) [95% CI] | 2 (66.7) [9.4–99.2] | 6 (100.0) [54.1–100.0] | 3 (42.9) [9.9–81.6] | 21 (65.6) [46.8–81.4] | 4 (80.0) [28.4–99.5] | 36 (67.9) [53.7–80.1] |
| ORR, n (%) [95% CI] | 0 [0.0–70.8] | 3 (50.0) [11.8–88.2] | 0 [0.0–41.0] | 3 (9.4) [2.0–25.0] | 0 [0.0–52.2] | 6 (11.3) [4.3–23.0] |
| Clinical benefit rate (CR + PR + durable † SD), n (%) [95% CI] | 0 [0.0–70.8] | 4 (66.7) [22.3–95.7] | 0 [0.0–41.0] | 13 (40.6) [23.7–59.4] | 2 (40.0) [5.3–85.3] | 19 (35.8) [23.1–50.2] |
| DoR per RANO criteria | ||||||
| Events, n (%) | 0 | 1 (33.3) | 0 | 3 (100.0) | 0 | 4 (66.7) |
| Median, months (95% CI) ‡ | NA | 6.4 (NE–NE) | NA | 3.8 (1.18–10.32) | NA | 5.1 (1.18–10.32) |
| Survival analyses (safety analysis set §) | ||||||
| N | 3 | 8 | 9 | 40 | 8 | 68 |
| PFS | ||||||
| Median, months (95% CI) | 3.1 (2.8–3.3) | 8.9 (3.8–11.6) | 2.6 (2.1–NE) | 4.4 (3.3–6.2) | 5.8 (2.4–6.5) | 4.4 (3.4–6.1) |
| Event-free rate, % (95% CI) # | ||||||
| 6 months | 0.0 (NE–NE) | 66.7 (5.4–94.5) | NE (NE–NE) | 42.7 (25.6–58.7) | 38.1 (6.1–71.6) | 39.5 (25.9–52.7) |
| 12 months | 0.0 (NE–NE) | 0.0 (NE–NE) | NE (NE–NE) | 6.6 (1.2–18.9) | 0.0 (NE–NE) | 3.5 (0.3–13.7) |
| OS | ||||||
| Median, months (95% CI) ¶ | 14.5 (13.9–15.0) | 13.4 (4.1–20.2) | 10.3 (4.4–19.8) | 12.7 (9.8–14.4) | 14.2 (8.0–NE) | 12.8 (10.2–14.2) |
| Event-free rate, % (95% CI) # | ||||||
| 6 months | 100.0 (NE–NE) | 85.7 (33.4–97.9) | 66.7 (28.2–87.8) | 89.3 (74.0–95.9) | 100.0 (NE–NE) | 87.3 (76.3–93.5) |
| 12 months | 100.0 (NE–NE) | 57.1 (17.2–83.7) | 33.3 (7.8–62.3) | 54.1 (37.0–68.5) | 71.4 (25.8–92.0) | 55.0 (41.9–66.4) |
| Endpoint | Arm C: Dose- Escalation Phase | Arm C: Dose- Expansion Phase | Total Recurrent/ Refractory Glioblastoma | |
|---|---|---|---|---|
| Pamiparib + TMZ 20 mg Days 1–21 | Pamiparib + TMZ 40 mg Days 1–21 | Pamiparib + TMZ 60 mg Days 1–7 | ||
| Disease Response Analyses (Unconfirmed; Efficacy Analysis Set *) | ||||
| N | 9 | 7 | 28 | 44 |
| Best response per RANO criteria, n (%) | ||||
| CR | 0 | 0 | 0 | 0 |
| PR | 0 | 2 (28.6) | 4 (14.3) | 6 (13.6) |
| SD | 5 (55.6) | 3 (42.9) | 4 (14.3) | 12 (27.3) |
| PD | 4 (44.4) | 2 (28.6) | 18 (64.3) | 24 (54.5) |
| NE | 0 | 0 | 2 (7.1) | 2 (4.5) |
| ORR, n (%) [95% CI] | 0 [0.0–33.6] | 2 (28.6) [3.7–71.0] | 4 (14.3) [4.0–32.7] | 6 (13.6) [5.2–27.4] |
| Disease control rate (CR + PR + SD), n (%) [95% CI] | 5 (55.6) [21.2–86.3] | 5 (71.4) [29.0–96.3] | 8 (28.6) [13.2–48.7] | 18 (40.9) [26.3–56.8] |
| Clinical benefit rate (CR + PR + durable † SD), n (%) [95% CI] | 0 [0.0–33.6] | 2 (28.6) [3.7–71.0] | 5 (17.9) [6.1–36.9] | 7 (15.9) [6.6–30.1] |
| DoR per RANO criteria | ||||
| Events, n (%) | 0 | 2 (100.0) | 2 (50.0) | 4 (66.7) |
| Median, months (95% CI) ‡ | NA | 6.6 (2.0–11.2) | 12.7 (5.6–NE) | 11.1 (2.0–NE) |
| Survival analyses (safety analysis set §) | ||||
| N | 9 | 8 | 30 | 47 |
| PFS | ||||
| Median, months (95% CI) ¶ | 1.8 (0.8–3.4) | 2.7 (0.7–7.4) | 1.9 (1.5–1.9) | 1.9 (1.7–2.3) |
| Event-free rate, % (95% CI) # | ||||
| 6 months | 0.0 (NE–NE) | 28.6 (4.1–61.2) | 19.6 (7.3–36.3) | 17.2 (7.6–30.1) |
| 12 months | 0.0 (NE–NE) | 14.3 (0.7–46.5) | 19.6 (7.3–36.3) | 14.8 (6.0–27.2) |
| OS | ||||
| Median, months (95% CI) ¶ | 6.0 (2.6–9.8) | 8.6 (3.0–NE) | 7.8 (6.2–10.7) | 7.3 (6.2–9.8) |
| Event-free rate, % (95% CI) # | ||||
| 6 months | 50.0 (15.2–77.5) | 62.5 (22.9–86.1) | 75.4 (55.2–87.5) | 68.5 (52.6–80.0) |
| 12 months | 0.0 (NE–NE) | 16.7 (0.9–50.8) | 26.4 (11.7–43.7) | 20.0 (9.5–33.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowski, A.F.; Shih, K.; Giglio, P.; Colman, H.; Wen, P.Y.; Campian, J.L.; Butowski, N.; Cloughesy, T.; Zhu, Z.; Gisin, V.; et al. Phase Ib/II Study of Pamiparib Plus Radiation Therapy and/or Temozolomide in Adult Patients with Treatment-Naïve or Recurrent/Refractory Glioblastoma. Curr. Oncol. 2025, 32, 541. https://doi.org/10.3390/curroncol32100541
Piotrowski AF, Shih K, Giglio P, Colman H, Wen PY, Campian JL, Butowski N, Cloughesy T, Zhu Z, Gisin V, et al. Phase Ib/II Study of Pamiparib Plus Radiation Therapy and/or Temozolomide in Adult Patients with Treatment-Naïve or Recurrent/Refractory Glioblastoma. Current Oncology. 2025; 32(10):541. https://doi.org/10.3390/curroncol32100541
Chicago/Turabian StylePiotrowski, Anna F., Kent Shih, Pierre Giglio, Howard Colman, Patrick Y. Wen, Jian Li Campian, Nicholas Butowski, Timothy Cloughesy, Zhaoyin Zhu, Vitaliy Gisin, and et al. 2025. "Phase Ib/II Study of Pamiparib Plus Radiation Therapy and/or Temozolomide in Adult Patients with Treatment-Naïve or Recurrent/Refractory Glioblastoma" Current Oncology 32, no. 10: 541. https://doi.org/10.3390/curroncol32100541
APA StylePiotrowski, A. F., Shih, K., Giglio, P., Colman, H., Wen, P. Y., Campian, J. L., Butowski, N., Cloughesy, T., Zhu, Z., Gisin, V., & Badruddoja, M. (2025). Phase Ib/II Study of Pamiparib Plus Radiation Therapy and/or Temozolomide in Adult Patients with Treatment-Naïve or Recurrent/Refractory Glioblastoma. Current Oncology, 32(10), 541. https://doi.org/10.3390/curroncol32100541






