Real-World Prevalence, Treatment Patterns, and Economic Impact of EGFR- and ALK-Targeted Therapies in Non-Small Cell Lung Cancer: A Nationwide Analysis from Greece
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Study Cohort, Outcomes and Definitions
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Prescribing Prevalence of TKI Based Treatment
3.2. Treatment Utilization Patterns
3.3. Cost of First Line Tyrosine Kinase Inhibitor Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bironzo, P.; Reale, M.L.; Sperone, T.; Tabbò, F.; Caglio, A.; Listì, A.; Passiglia, F.; Di Maio, M.; Righi, L.; Bussolino, F.; et al. Clinical and Molecular Features of Epidermal Growth Factor Receptor (EGFR) Mutation Positive Non-Small-Cell Lung Cancer (NSCLC) Patients Treated with Tyrosine Kinase Inhibitors (TKIs): Predictive and Prognostic Role of Co-Mutations. Cancers 2021, 13, 2425. [Google Scholar] [CrossRef]
- Gainor, J.F.; Varghese, A.M.; Ou, S.-H.I.; Kabraji, S.; Awad, M.M.; Katayama, R.; Pawlak, A.; Mino-Kenudson, M.; Yeap, B.Y.; Riely, G.J. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: An analysis of 1,683 patients with non–small cell lung cancer. Clin. Cancer Res. 2013, 19, 4273–4281. [Google Scholar] [CrossRef]
- Passiglia, F.; Bironzo, P.; Bertaglia, V.; Listì, A.; Garbo, E.; Scagliotti, G.V. Optimizing the clinical management of EGFR-mutant advanced non-small cell lung cancer: A literature review. Transl. Lung Cancer Res. 2022, 11, 935–949. [Google Scholar] [CrossRef]
- Addeo, A.; Tabbò, F.; Robinson, T.; Buffoni, L.; Novello, S. Precision medicine in ALK rearranged NSCLC: A rapidly evolving scenario. Crit. Rev. Oncol./Hematol. 2018, 122, 150–156. [Google Scholar] [CrossRef]
- Hellenic Society of Medical Oncology (HESMO). Therapeutic Protocols—10th Edition 2024. Available online: https://www.hesmo.gr/images/%CE%98%CE%B5%CF%81%CE%B1%CF%80%CE%B5%CF%85%CF%84%CE%B9%CE%BA%CE%B1%CC%81_%CE%A0%CF%81%CF%89%CF%84%CE%BF%CC%81%CE%BA%CE%BF%CE%BB%CE%BB%CE%B1_%CE%95%CE%9F%CE%A0%CE%95-10%CE%B7_%CE%95%CC%81%CE%BA%CE%B4%CE%BF%CF%83%CE%B7.pdf (accessed on 26 June 2025).
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, N.; Daaboul, N. Lung Cancer: Targeted Therapy in 2025. Curr. Oncol. 2025, 32, 146. [Google Scholar] [CrossRef]
- Dalurzo, M.L.; Avilés-Salas, A.; Soares, F.A.; Hou, Y.; Li, Y.; Stroganova, A.; Öz, B.; Abdillah, A.; Wan, H.; Choi, Y.L. Testing for EGFR Mutations and ALK Rearrangements in Advanced Non-Small-Cell Lung Cancer: Considerations for Countries in Emerging Markets. OncoTargets Ther. 2021, 14, 4671–4692. [Google Scholar] [CrossRef]
- Goulart, B.H.L.; Chennupati, S.; Fedorenko, C.R.; Ramsey, S.D. Access to Tyrosine Kinase Inhibitors and Survival in Patients with Advanced EGFR(+) and ALK(+) Positive Non-small-cell Lung Cancer Treated in the Real-World. Clin. Lung Cancer 2021, 22, e723–e733. [Google Scholar] [CrossRef]
- Gourzoulidis, G.; Kastanioti, C.; Mavridoglou, G.; Kotsilieris, T.; Voudigaris, D.; Tzanetakos, C. Does Real-World Evidence of the Economic Burden of Lung Cancer in Greece Exist? A Systematic Review of the Literature. Curr. Oncol. 2025, 32, 130. [Google Scholar] [CrossRef]
- Kanavos, V.; Tzouma, A.M.; Fontrier, K.; Souliotis, K. Implementing health technology assessment (HTA) in Greece: Myths, reality andcautionary tales. Arch. Hell. Med. 2019, 36, 444–451. [Google Scholar]
- Liatis, S.; Dafoulas, G.E.; Kani, C.; Politi, A.; Litsa, P.; Sfikakis, P.P.; Makrilakis, K. The prevalence and treatment patterns of diabetes in the Greek population based on real-world data from the nation-wide prescription database. Diabetes Res. Clin. Pract. 2016, 118, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Sfikakis, P.P.; Bournia, V.K.; Sidiropoulos, P.; Boumpas, D.T.; Drosos, A.A.; Kitas, G.D.; Konstantonis, G.; Liossis, S.N.; Manoussakis, M.N.; Sakkas, L.; et al. Biologic treatment for rheumatic disease: Real-world big data analysis from the Greek country-wide prescription database. Clin. Exp. Rheumatol. 2017, 35, 579–585. [Google Scholar]
- Greek Ministry of Health. Drug Price Bulletin. Available online: https://www.moh.gov.gr/articles/times-farmakwn (accessed on 26 July 2025).
- Greek Ministry of Health. Catalogue of Reimbursable Medicines. Available online: https://www.moh.gov.gr/articles/times-farmakwn/epitroph-aksiologhshs-kai-apozhmiwshs-farmakwn/13369-anathewrhsh-katalogoy-apozhmioymenwn-farmakwn-toy-arthroy-251-toy-n-4512-2018-opws-tropopoihthhke-me-to-arthro-24-toy-n-4633-2019 (accessed on 8 August 2025).
- Chayab, L.; Leighl, N.B.; Tadrous, M.; Warren, C.M.; Wong, W.W.L. Trends in Real-World Clinical Outcomes of Patients with Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small Cell Lung Cancer (NSCLC) Receiving One or More ALK Tyrosine Kinase Inhibitors (TKIs): A Cohort Study in Ontario, Canada. Curr. Oncol. 2024, 32, 13. [Google Scholar] [CrossRef]
- Moulson, R.; Law, J.; Sacher, A.; Liu, G.; Shepherd, F.A.; Bradbury, P.; Eng, L.; Iczkovitz, S.; Abbie, E.; Elia-Pacitti, J.; et al. Real-World Outcomes of Patients with Advanced Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer in Canada Using Data Extracted by Large Language Model-Based Artificial Intelligence. Curr. Oncol. 2024, 31, 1947–1960. [Google Scholar] [CrossRef]
- Bailey, H.; Lee, A.; Eccles, L.; Yuan, Y.; Burlison, H.; Forshaw, C.; Varol, N. Treatment patterns and outcomes of patients with metastatic non-small cell lung cancer in five European countries: A real-world evidence survey. BMC Cancer 2023, 23, 603. [Google Scholar] [CrossRef]
- Mountzios, G.; Koumarianou, A.; Bokas, A.; Mavroudis, D.; Samantas, E.; Fergadis, E.G.; Linardou, H.; Katsaounis, P.; Athanasiadis, E.; Karamouzis, M.V.; et al. A Real-World, Observational, Prospective Study to Assess the Molecular Epidemiology of Epidermal Growth Factor Receptor (EGFR) Mutations upon Progression on or after First-Line Therapy with a First- or Second-Generation EGFR Tyrosine Kinase Inhibitor in EGFR Mutation-Positive Locally Advanced or Metastatic Non-Small Cell Lung Cancer: The ‘LUNGFUL’ Study. Cancers 2021, 13, 3172. [Google Scholar] [CrossRef]
- Gibson, A.J.W.; Box, A.; Dean, M.L.; Elegbede, A.A.; Hao, D.; Sangha, R.; Bebb, D.G. Retrospective Real-World Outcomes for Patients With ALK-Rearranged Lung Cancer Receiving ALK Receptor Tyrosine Kinase Inhibitors. JTO Clin. Res. Rep. 2021, 2, 100157. [Google Scholar] [CrossRef]
- Lampaki, S.; Mountzios, G.; Georgoulias, V.; Rapti, A.; Xanthakis, I.; Baka, S.; Mavroudis, D.; Samantas, E.; Athanasiadis, E.; Zagouri, F.; et al. Real-world management patterns in EGFR-mutant advanced non-small-cell lung cancer before first-line adoption of osimertinib: The REFLECT study in Greece. Future Oncol. 2022, 18, 3151–3164. [Google Scholar] [CrossRef]
- Gourzoulidis, G.; Zisimopoulou, O.; Liavas, A.; Tzanetakos, C. Lorlatinib as a first-line treatment of adult patients with anaplastic lymphoma kinase-positive advanced non-small cell lung cancer: A cost-effectiveness analysis in Greece. Expert. Rev. Pharmacoecon. Outcomes Res. 2024, 24, 375–385. [Google Scholar] [CrossRef]
- Gourzoulidis, G.; Zisimopoulou, O.; Boubouchairopoulou, N.; Michailidi, C.; Lowry, C.; Tzanetakos, C.; Kourlaba, G. Cost-effectiveness Analysis of Lorlatinib in Patients Previously Treated with Anaplastic Lymphoma Kinase Inhibitors for Non-small Cell Lung Cancer in Greece. J. Health Econ. Outcomes Res. 2022, 9, 50–57. [Google Scholar] [CrossRef]
- Bote-de Cabo, H.; Siringo, M.; Conde, E.; Hernández, S.; López-Ríos, F.; Castelo-Loureiro, A.; García-Lorenzo, E.; Baena, J.; Herrera, M.; Enguita, A.B.; et al. Clinical Utility of Combined Tissue and Plasma Next-Generation Sequencing in Patients With Advanced, Treatment-Naïve NSCLC. JTO Clin. Res. Rep. 2025, 6, 100778. [Google Scholar] [CrossRef]
- Athanasakis, K.; Karampli, E.; Agorastos, G.; Kyriopoulos, I. Sustainability and Resilience in the Greek Health System. Partnership for Health System Sustainability and Resilience. Available online: https://www3.weforum.org/docs/WEF_PHSSR_Greece_2023.pdf (accessed on 7 August 2025).
- Souliotis, K.; Golna, C.; Golnas, P.; Markakis, I.A.; Linardou, H.; Sifaki-Pistolla, D.; Hatziandreou, E. Lung Cancer Screening in Greece: A Modelling Study to Estimate the Impact on Lung Cancer Life Years. Cancers 2022, 14, 5484. [Google Scholar] [CrossRef]
- Pan, X.; Togka, K.; Ten Berge, H.; de Jong, L.; Groen, H.; Postma, M.J.; Zervas, E.; Gkiozos, I.; Foroulis, C.; Tavernaraki, K.; et al. Lung cancer screening with volume computed tomography is cost-effective in Greece. PLoS ONE 2025, 20, e0316351. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Makras, P.; Athanasakis, K.; Bournia, V.K.; Mathioudakis, K.; Tsolakidis, A.; Kassi, E.; Kaltsas, G.; Mitrou, P.; Sfikakis, P.P. Prevalence and patterns of anti-osteoporotic drug use based on 2019 real-world nationwide data in Greece. Arch. Osteoporos. 2022, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzis, C.; Grigoriadou, E.; Boziki, M.K.; Kesidou, E.; Siafis, S.; Moysiadis, T.; Tsakona, D.; Thireos, E.; Nikolaidis, I.; Pourzitaki, C.; et al. The Administrative Prevalence of Multiple Sclerosis in Greece on the Basis of a Nationwide Prescription Database. Front. Neurol. 2020, 11, 1012. [Google Scholar] [CrossRef] [PubMed]
- de Joode, K.; Dumoulin, D.W.; Engelen, V.; Bloemendal, H.J.; Verheij, M.; van Laarhoven, H.W.M.; Dingemans, I.H.; Dingemans, A.C.; van der Veldt, A.A.M. Impact of the coronavirus disease 2019 pandemic on cancer treatment: The patients’ perspective. Eur. J. Cancer 2020, 136, 132–139. [Google Scholar] [CrossRef] [PubMed]

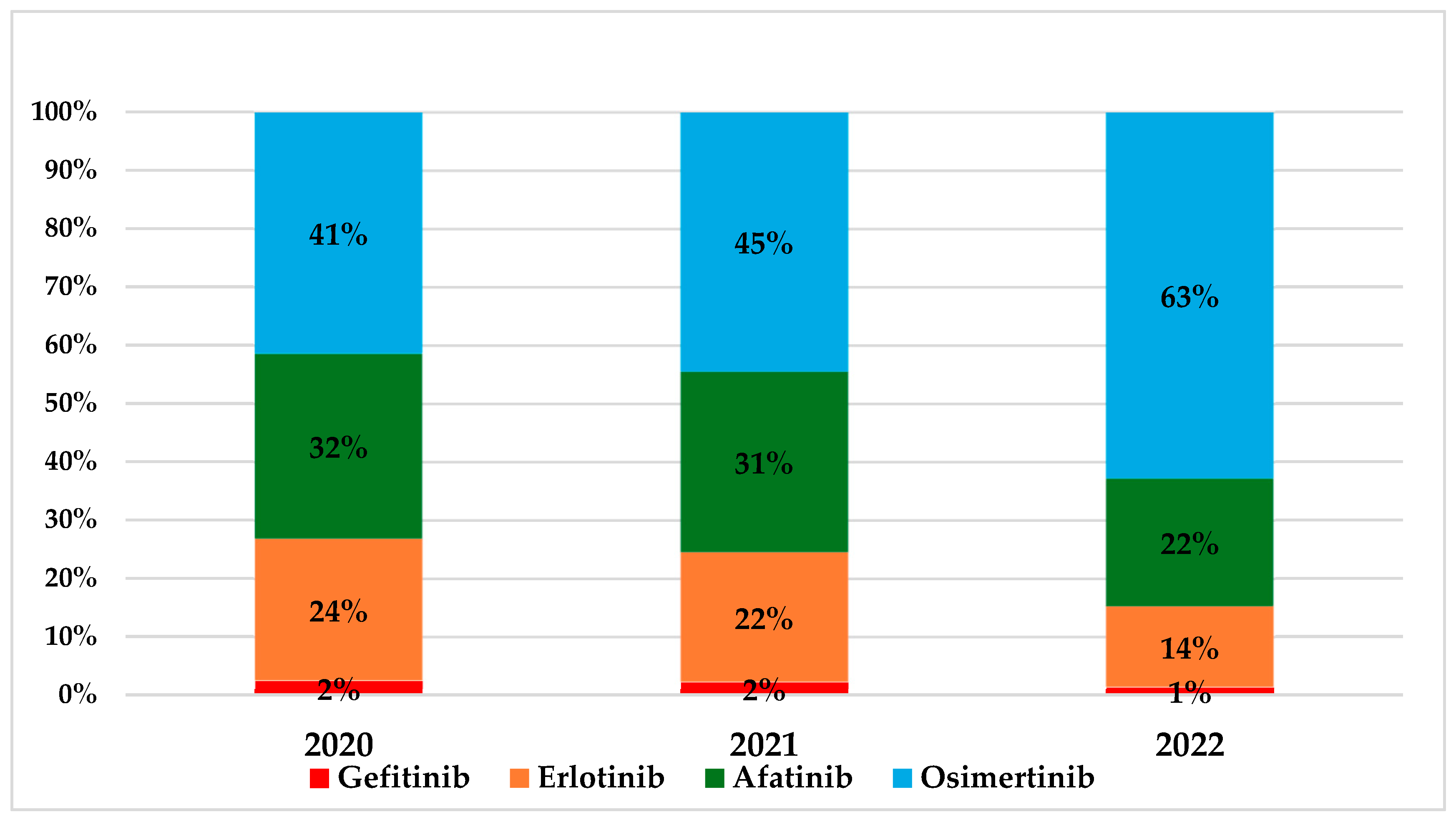

| Age Groups | Number of EGFR-Positive NSCLC Patients | Prevalence of the EGFR-Positive NSCLC Patients in the Greek Population | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Male | Females | Total | Prevalence (/100,000) Male 95% CI | Prevalence (/100,000) Females 95% CI | Prevalence (/100,000) Total 95% CI | ||
| 18–39 | 6 | 2 | 8 | 0.23 (0.05–0.42) | 0.16 (−0.06–0.39) | 0.21 (0.06–0.36) | 0.659 |
| 40–49 | 21 | 26 | 47 | 2.63 (1.51–3.76) | 3.26 (2.00–4.51) | 2.94 (2.10–3.79) | 0.467 |
| 50–59 | 50 | 68 | 118 | 6.63 (4.80–8.47) | 9.50 (7.35–11.65) | 8.10 (6.68–9.52) | 0.161 |
| 60–69 | 130 | 160 | 290 | 20.63 (17.08–24.18) | 23.04 (19.47–26.61) | 21.89 (19.37–24.41) | 0.349 |
| 70–79 | 200 | 215 | 415 | 43.87 (37.79–49.94) | 39.81 (34.49–45.13) | 41.67 (37.66–45.67) | 0.323 |
| 80 and older | 150 | 160 | 310 | 49.87 (41.30–57.03) | 34.71 (29.33–40.09) | 40.47 (35.96–44.97) | 0.226 |
| Total | 557 | 631 | 1188 | 10.09 (9.25–10.92) | 13.99 (12.89–15.07) | 11.84 (11.16–12.51) | 0.149 |
| Age Groups | Number of ALK-Positive NSCLC Patients | Prevalence of the ALK-Positive NSCLC Patients in the Greek Population | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Male | Females | Total | Prevalence (/100,000) Male 95% CI | Prevalence (/100,000) Females 95% CI | Prevalence (/100,000) Total 95% CI | ||
| 18–39 | 6 | 3 | 9 | 0.23 (0.05–0.42) | 0.24 (−0.03–0.52) | 0.24 (0.08–0.39) | 0.947 |
| 40–49 | 11 | 14 | 25 | 1.38 (0.56–2.19) | 1.75 (0.84–2.67) | 1.57 (0.95–2.18) | 0.550 |
| 50–59 | 20 | 29 | 49 | 2.65 (1.49–3.82) | 3.67 (2.34–5.01) | 3.17 (2.29–4.06) | 0.262 |
| 60–69 | 30 | 33 | 63 | 4.76 (3.06–6.46) | 4.75 (3.13–6.37) | 4.76 (3.58–5.93) | 0.994 |
| 70–79 | 41 | 28 | 69 | 8.99 (6.24–11.75) | 5.18 (3.26–7.10) | 6.93 (5.29–8.56) | 0.023 |
| 80 and older | 11 | 20 | 31 | 3.61 (1.47–5.74) | 4.34 (2.44–6.24) | 4.05 (2.62–5.47) | 0.621 |
| Total | 119 | 127 | 246 | 2.15 (1.77–2.54) | 2.81 (2.32–3.30) | 2.45 (2.14–2.76) | 0.369 |
| Age Groups | Gefitinib | Erlotinib | Afatinib | Osimertinib | ||||
|---|---|---|---|---|---|---|---|---|
| 2020 | Male | Females | Male | Females | Male | Females | Male | Females |
| 18–39 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 |
| 40–49 | 0 | 1 | 1 | 3 | 1 | 1 | 4 | 6 |
| 50–59 | 2 | 1 | 0 | 4 | 4 | 5 | 12 | 10 |
| 60–69 | 1 | 1 | 10 | 12 | 12 | 16 | 18 | 20 |
| 70–79 | 1 | 1 | 19 | 23 | 22 | 24 | 25 | 30 |
| 80 and older | 1 | 1 | 12 | 16 | 22 | 23 | 20 | 21 |
| Total | 5 | 5 | 42 | 58 | 61 | 69 | 82 | 88 |
| Age Groups | Male | Females | Male | Females | Male | Females | Male | Females |
| 2021 | ||||||||
| 18–39 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 40–49 | 0 | 0 | 4 | 1 | 5 | 3 | 3 | 5 |
| 50–59 | 1 | 0 | 1 | 8 | 4 | 7 | 10 | 16 |
| 60–69 | 1 | 1 | 6 | 15 | 12 | 16 | 20 | 21 |
| 70–79 | 3 | 1 | 15 | 19 | 23 | 28 | 30 | 24 |
| 80 and older | 1 | 1 | 9 | 12 | 12 | 14 | 27 | 23 |
| Total | 6 | 3 | 35 | 55 | 57 | 68 | 91 | 89 |
| Age Groups | Male | Females | Male | Females | Male | Females | Male | Females |
| 2022 | ||||||||
| 18–39 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 40–49 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 5 |
| 50–59 | 0 | 0 | 1 | 3 | 1 | 2 | 14 | 12 |
| 60–69 | 1 | 1 | 5 | 8 | 14 | 18 | 30 | 31 |
| 70–79 | 1 | 1 | 10 | 14 | 14 | 17 | 37 | 33 |
| 80 and older | 1 | 0 | 4 | 6 | 7 | 8 | 34 | 35 |
| Total | 3 | 2 | 20 | 32 | 36 | 46 | 119 | 116 |
| Age Groups | Crizotinib | Ceritinib | Alectinib | Lorlatinib | ||||
|---|---|---|---|---|---|---|---|---|
| 2020 | Male | Females | Male | Females | Male | Females | Male | Females |
| 18–39 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 40–49 | 2 | 2 | 3 | 1 | 0 | 0 | 0 | 0 |
| 50–59 | 4 | 6 | 0 | 8 | 0 | 0 | 0 | 0 |
| 60–69 | 3 | 5 | 9 | 1 | 0 | 0 | 0 | 0 |
| 70–79 | 11 | 10 | 3 | 7 | 0 | 0 | 0 | 0 |
| 80 and older | 4 | 3 | 0 | 2 | 0 | 0 | 0 | 0 |
| Total | 27 | 26 | 15 | 20 | 0 | 0 | 0 | 0 |
| Age Groups | Male | Females | Male | Females | Male | Females | Male | Females |
| 2021 | ||||||||
| 18–39 | 3 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| 40–49 | 1 | 3 | 0 | 1 | 2 | 4 | 0 | 0 |
| 50–59 | 3 | 1 | 0 | 0 | 6 | 5 | 0 | 0 |
| 60–69 | 4 | 1 | 1 | 1 | 9 | 14 | 0 | 0 |
| 70–79 | 1 | 1 | 2 | 0 | 3 | 3 | 0 | 0 |
| 80 and older | 0 | 1 | 1 | 1 | 5 | 4 | 0 | 0 |
| Total | 12 | 8 | 4 | 3 | 27 | 30 | 0 | 0 |
| Age Groups | Male | Females | Male | Females | Male | Females | Male | Females |
| 2022 | ||||||||
| 18–39 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 40–49 | 1 | 1 | 0 | 1 | 0 | 3 | 0 | 0 |
| 50–59 | 1 | 2 | 1 | 1 | 7 | 3 | 0 | 1 |
| 60–69 | 2 | 1 | 0 | 2 | 2 | 8 | 1 | 0 |
| 70–79 | 2 | 0 | 2 | 0 | 14 | 7 | 0 | 0 |
| 80 and older | 0 | 1 | 0 | 0 | 0 | 8 | 0 | 0 |
| Total | 6 | 6 | 3 | 4 | 24 | 29 | 1 | 1 |
| EGFR TKIs | |||
|---|---|---|---|
| Treatment | 2020 | 2021 | 2022 |
| Gefitinib | 131,329 | 102,228 | 52,818 |
| Erlotinib | 734,907 | 589,702 | 316,867 |
| Afatinib | 1,303,616 | 1,197,318 | 775,012 |
| Osimertinib | 9,320,131 | 8,535,158 | 10,732,643 |
| Total cost per treatment per year (€) | 11,489,983 | 10,424,407 | 11,877,340 |
| EGFR TKIs | |||
| Treatment | 2020 | 2021 | 2022 |
| Gefitinib | 13,133 | 11,359 | 10,564 |
| Erlotinib | 7349 | 6552 | 6094 |
| Afatinib | 10,028 | 9579 | 9451 |
| Osimertinib | 54,824 | 47,418 | 45,671 |
| Cost per patient per year (€) | 28,024 | 25,803 | 31,758 |
| ALK TKIs | |||
| Treatment | 2020 | 2021 | 2022 |
| Crizotinib | 2,081,949 | 771,661 | 418,496 |
| Ceritinib | 1,369,425 | 273,885 | 273,885 |
| Alectinib | 0 | 2,714,004 | 2,523,548 |
| Lorlatinib | 0 | 0 | 85,105 |
| Total cost per treatment per year (€) | 3,451,374 | 3,759,550 | 3,301,034 |
| ALK TKIs | |||
| Treatment | 2020 | 2021 | 2022 |
| Crizotinib | 39,282 | 38,583 | 34,875 |
| Ceritinib | 39,126 | 39,126 | 39,126 |
| Alectinib | 0 | 47,614 | 47,614 |
| Lorlatinib | 0 | 0 | 42,552 |
| Cost per patient per year (€) | 39,220 | 44,757 | 44,609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gourzoulidis, G.; Kastanioti, C.; Mavridoglou, G.; Kotsilieris, T.; Tsolakidis, A.; Mathioudakis, K.; Voudigaris, D.; Tzanetakos, C. Real-World Prevalence, Treatment Patterns, and Economic Impact of EGFR- and ALK-Targeted Therapies in Non-Small Cell Lung Cancer: A Nationwide Analysis from Greece. Curr. Oncol. 2025, 32, 542. https://doi.org/10.3390/curroncol32100542
Gourzoulidis G, Kastanioti C, Mavridoglou G, Kotsilieris T, Tsolakidis A, Mathioudakis K, Voudigaris D, Tzanetakos C. Real-World Prevalence, Treatment Patterns, and Economic Impact of EGFR- and ALK-Targeted Therapies in Non-Small Cell Lung Cancer: A Nationwide Analysis from Greece. Current Oncology. 2025; 32(10):542. https://doi.org/10.3390/curroncol32100542
Chicago/Turabian StyleGourzoulidis, George, Catherine Kastanioti, George Mavridoglou, Theodore Kotsilieris, Anastasios Tsolakidis, Konstantinos Mathioudakis, Dikaios Voudigaris, and Charalampos Tzanetakos. 2025. "Real-World Prevalence, Treatment Patterns, and Economic Impact of EGFR- and ALK-Targeted Therapies in Non-Small Cell Lung Cancer: A Nationwide Analysis from Greece" Current Oncology 32, no. 10: 542. https://doi.org/10.3390/curroncol32100542
APA StyleGourzoulidis, G., Kastanioti, C., Mavridoglou, G., Kotsilieris, T., Tsolakidis, A., Mathioudakis, K., Voudigaris, D., & Tzanetakos, C. (2025). Real-World Prevalence, Treatment Patterns, and Economic Impact of EGFR- and ALK-Targeted Therapies in Non-Small Cell Lung Cancer: A Nationwide Analysis from Greece. Current Oncology, 32(10), 542. https://doi.org/10.3390/curroncol32100542






