Pan-Immune-Inflammation Value as a Predictor of Long-Term Outcomes in Patients with Urothelial Carcinoma of the Bladder: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
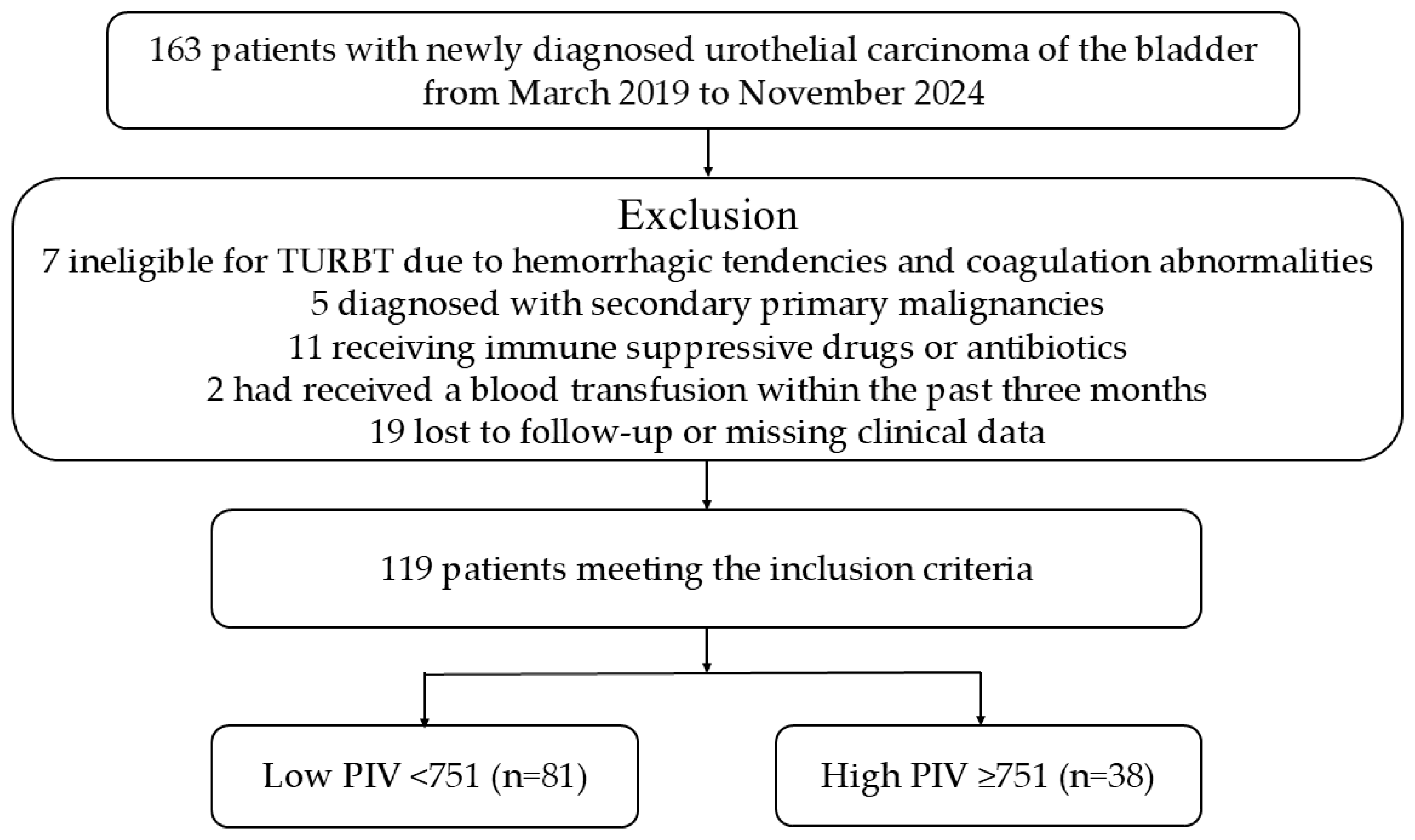
2.1. Participant Selection & Collection of the Data & Follow-Up
2.2. Ethical Statement
2.3. Statistical Analysis
3. Results
3.1. Cutoff Values of the Laboratory Parameters
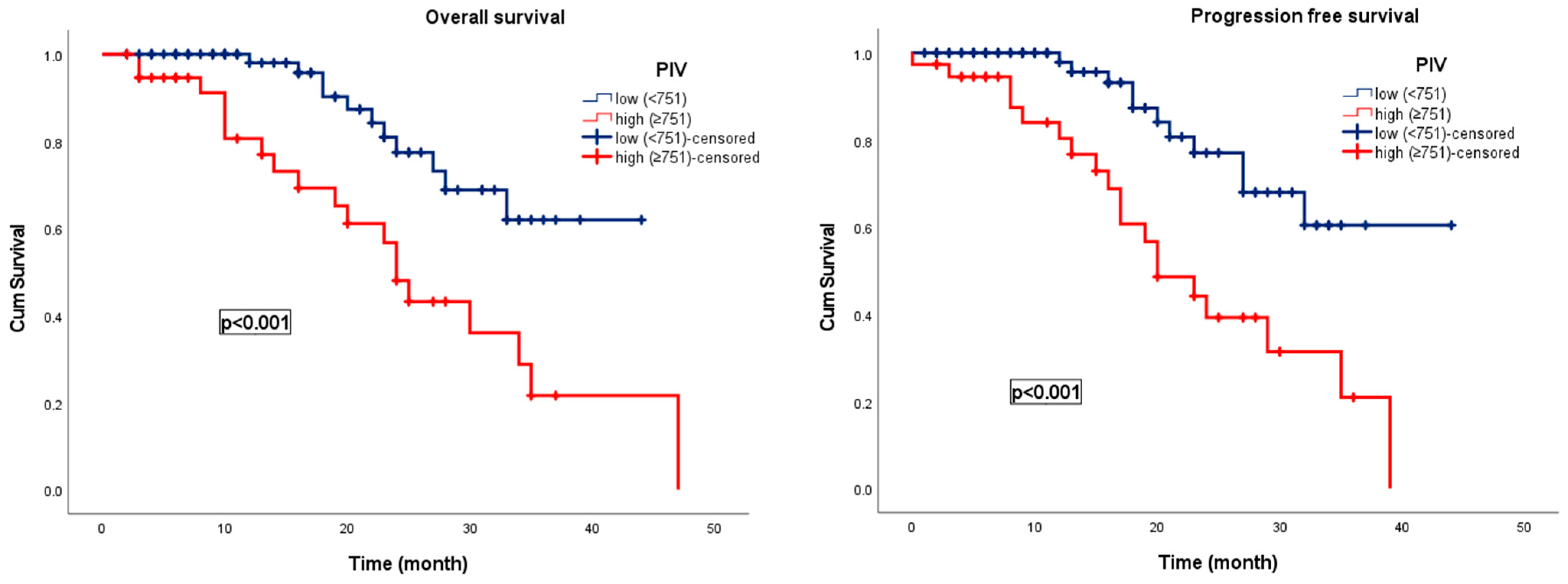
3.2. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UCB | Urothelial Carcinoma of the Bladder |
| NMIBC | Non-muscle-invasive Bladder Cancer |
| MIBC | Muscle-invasive Bladder Cancer |
| PIV | Pan-immune-inflammation Value |
| TNF | Tumor Necrosis Factor-Alpha |
| VEGF | Vascular Endothelial Growth Factor |
| PFS | Progression-free Survival |
| OS | Overall Survival |
| NLR | Neutrophil-to-lymphocyte Ratio |
| SII | Systemic Immune-inflammation Index |
| SIRI | Systemic Inflammation Response Index |
| PLR | Platelet-to-lymphocyte Ratio |
| TURBT | Transurethral Resection of the Bladder Tumor |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| BCG | Bacillus Calmette-Guérin |
| TNM | Tumor-Node-Metastasis |
| NK | Natural Killer |
References
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder Cancer: A Review. JAMA 2020, 324, 1980. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder Cancer. Nat. Rev. Dis. Primers 2023, 9, 58. [Google Scholar] [CrossRef]
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current Best Practice for Bladder Cancer: A Narrative Review of Diagnostics and Treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef]
- Guo, C.C.; Lee, S.; Lee, J.G.; Chen, H.; Zaleski, M.; Choi, W.; McConkey, D.J.; Wei, P.; Czerniak, B. Molecular Profile of Bladder Cancer Progression to Clinically Aggressive Subtypes. Nat. Rev. Urol. 2024, 21, 391–405. [Google Scholar] [CrossRef]
- Powles, T.; Catto, J.W.F.; Galsky, M.D.; Al-Ahmadie, H.; Meeks, J.J.; Nishiyama, H.; Vu, T.Q.; Antonuzzo, L.; Wiechno, P.; Atduev, V.; et al. Perioperative Durvalumab with Neoadjuvant Chemotherapy in Operable Bladder Cancer. N. Engl. J. Med. 2024, 391, 1773–1786. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Leyderman, M.; Chandrasekar, T.; Grivas, P.; Li, R.; Bhat, S.; Basnet, A.; Shapiro, O.; Jacob, J.; Daneshvar, M.A.; Kord, E.; et al. Metastasis Development in Non-Muscle-Invasive Bladder Cancer. Nat. Rev. Urol. 2025, 22, 375–386. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, A.G.; Bruins, H.M.; Carrion, A.; Cathomas, R.; Compérat, E.; Dimitropoulos, K.; Efstathiou, J.A.; Fietkau, R.; Kailavasan, M.; Lorch, A.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2025 Guidelines. Eur. Urol. 2025, 87, 582–600. [Google Scholar] [CrossRef]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value Is a New Prognostic Biomarker in Metastatic Colorectal Cancer: Results from a Pooled-Analysis of the Valentino and TRIBE First-Line Trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Lord, B.; Ambs, S. Immune Response and Inflammation in Cancer Health Disparities. Trends Cancer 2022, 8, 316–327. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Malanchi, I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Patysheva, M.; Frolova, A.; Larionova, I.; Afanas’ev, S.; Tarasova, A.; Cherdyntseva, N.; Kzhyshkowska, J. Monocyte Programming by Cancer Therapy. Front. Immunol. 2022, 13, 994319. [Google Scholar] [CrossRef]
- Dudiki, T.; Veleeparambil, M.; Zhevlakova, I.; Biswas, S.; Klein, E.A.; Ford, P.; Podrez, E.A.; Byzova, T.V. Mechanism of Tumor-Platelet Communications in Cancer. Circ. Res. 2023, 132, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Sheu, T.-T.; Chiang, B.-L. Lymphopenia, Lymphopenia-Induced Proliferation, and Autoimmunity. Int. J. Mol. Sci. 2021, 22, 4152. [Google Scholar] [CrossRef]
- Zhai, W.-Y.; Duan, F.-F.; Lin, Y.-B.; Lin, Y.-B.; Zhao, Z.-R.; Wang, J.-Y.; Rao, B.-Y.; Zheng, L.; Long, H. Pan-Immune-Inflammatory Value in Patients with Non-Small-Cell Lung Cancer Undergoing Neoadjuvant Immunochemotherapy. J. Inflamm. Res. 2023, 16, 3329–3339. [Google Scholar] [CrossRef]
- Qi, X.; Qiao, B.; Song, T.; Huang, D.; Zhang, H.; Liu, Y.; Jin, Q.; Yang, M.; Liu, D. Clinical Utility of the Pan-Immune-Inflammation Value in Breast Cancer Patients. Front. Oncol. 2023, 13, 1223786. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.A.; Kayikcioglu, E.; Unlu, A.; Acun, M.; Guzel, H.G.; Yavuz, R.; Ozgul, H.; Onder, A.H.; Ozturk, B.; Yildiz, M. Pan-Immune-Inflammation Value as a Novel Prognostic Biomarker for Advanced Pancreatic Cancer. Cureus 2024, 16, e71251. [Google Scholar] [CrossRef]
- Li, J.; Cao, D.; Huang, Y.; Xiong, Q.; Tan, D.; Liu, L.; Lin, T.; Wei, Q. The Prognostic and Clinicopathological Significance of Systemic Immune-Inflammation Index in Bladder Cancer. Front. Immunol. 2022, 13, 865643. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Cinar, N.B.; Avci, I.E.; Telli, E.; Uslubas, A.K.; Teke, K.; Dillioglugil, O. The Systemic Inflammation Response Index: An Independent Predictive Factor for Survival Outcomes of Bladder Cancer Stronger than Other Inflammatory Markers. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 256.e1–256.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ni, X.; Tang, G. Prognostic Role of Platelet-to-Lymphocyte Ratio in Patients With Bladder Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Du, P.; Yang, Y. The Clinical Use of Neutrophil-to-Lymphocyte Ratio in Bladder Cancer Patients: A Systematic Review and Meta-Analysis. Int. J. Clin. Oncol. 2017, 22, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, F.; Wu, Y.; Zhu, Y.; Jiang, Y.; Wu, Q.; Dong, Z.; Liu, K. Inflammation in Cancer: Therapeutic Opportunities from New Insights. Mol. Cancer 2025, 24, 51. [Google Scholar] [CrossRef]
- Topkan, E.; Kucuk, A.; Selek, U. Pretreatment Pan-Immune-Inflammation Value Efficiently Predicts Survival Outcomes in Glioblastoma Multiforme Patients Receiving Radiotherapy and Temozolomide. J. Immunol. Res. 2022, 2022, 1346094. [Google Scholar] [CrossRef]
- Liang, X.; Bu, J.; Jiang, Y.; Zhu, S.; Ye, Q.; Deng, Y.; Lu, W.; Liu, Q. Prognostic Significance of Pan-Immune-Inflammation Value in Hepatocellular Carcinoma Treated by Curative Radiofrequency Ablation: Potential Role for Individualized Adjuvant Systemic Treatment. Int. J. Hyperth. 2024, 41, 2355279. [Google Scholar] [CrossRef]
- Kayar, R.; Bastug, Y.; Tokuc, E.; Topaktas, R.; Akyurek, E.A.; Kayar, K.; Artuk, I.; Ozturk, M. Pan-Immune-Inflammation Value as a Prognostic Tool for Overall Survival and Disease-Free Survival in Non-Metastatic Muscle-Invasive Bladder Cancer. Int. Urol. Nephrol. 2023, 56, 509–518. [Google Scholar] [CrossRef]
- Russo, P.; Marino, F.; Rossi, F.; Bizzarri, F.P.; Ragonese, M.; Dibitetto, F.; Filomena, G.B.; Marafon, D.P.; Ciccarese, C.; Iacovelli, R.; et al. Is Systemic Immune-Inflammation Index a Real Non-Invasive Biomarker to Predict Oncological Outcomes in Patients Eligible for Radical Cystectomy? Medicina 2023, 59, 2063. [Google Scholar] [CrossRef]
- Russo, P.; Foschi, N.; Palermo, G.; Maioriello, G.; Lentini, N.; Iacovelli, R.; Ciccarese, C.; Ragonese, M.; Marino, F.; Bizzarri, F.P.; et al. SIRI as a Biomarker for Bladder Neoplasm: Utilizing Decision Curve Analysis to Evaluate Clinical Net Benefit. Urol. Oncol. Semin. Orig. Investig. 2025, 43, 393.e1–393.e8. [Google Scholar] [CrossRef]
| Variables | PIV | p | ||
|---|---|---|---|---|
| Low (<751) n, % | High (≥751) n, % | |||
| Age | <65 | 18 (22.2) | 4 (10.5) | 0.098 |
| ≥65 | 63 (77.8) | 34 (89.5) | ||
| Sex | Male | 72 (88.9) | 33 (86.8) | 0.481 |
| Female | 9 (11.1) | 5 (13.2) | ||
| ECOG PS | 0–1 | 59 (89.4) | 41 (77.4) | 0.059 |
| ≥2 | 7 (10.6) | 12 (22.6) | ||
| Comorbidity | No | 7 (9.9) | 6 (12.5) | 0.688 |
| Yes | 64 (90.1) | 42 (87.5) | ||
| Smoking status | No | 9 (11.1) | 12 (31.6) | 0.008 |
| Yes | 72 (88.9) | 26 (68.4) | ||
| Alcohol consumption | No | 69 (85.2) | 37 (97.4) | 0.039 |
| Yes | 12 (14.8) | 1 (2.6) | ||
| Intravesical BCG treatment | No | 50 (61.8) | 24 (63.2) | 0.158 |
| Yes | 31 (38.2) | 14 (36.8) | ||
| Transition from NMIBC to MIBC in clinical surveillance | No | 31 (86.1) | 18 (56.3) | 0.028 |
| Yes | 5 (13.9) | 14 (43.7) | ||
| Lymph node involvement | No | 63 (82.9) | 27 (77.1) | 0.282 |
| Yes | 13 (17.1) | 8 (22.9) | ||
| Distant metastasis | No | 70 (92.1) | 36 (94.7) | 0.148 |
| Yes | 6 (7.9) | 2 (5.3) | ||
| Tumor stage | pTa-Tis | 13 (19.1) | 6 (11.8) | 0.005 |
| pT1 | 35 (51.5) | 14 (27.5) | ||
| pT2 | 13 (19.1) | 19 (37.3) | ||
| pT3–4 | 7 (10.3) | 12 (23.5) | ||
| NLR | <3.16 | 70 (86.4) | 7 (18.4) | <0.001 |
| ≥3.16 | 11 (13.6) | 31 (81.6) | ||
| SII | <778 | 63 (77.8) | 1 (2.6) | <0.001 |
| ≥778 | 18 (22.2) | 37 (97.4) | ||
| SIRI | <1.26 | 44 (54.3) | 1 (2.6) | <0.001 |
| ≥1.26 | 37 (45.7) | 37 (97.4) | ||
| PLR | <165 | 65 (80.2) | 11 (28.9) | <0.001 |
| ≥165 | 16 (19.8) | 27 (71.1) | ||
| NLR | SII | SIRI | PLR | PIV | ||
|---|---|---|---|---|---|---|
| NLR | r | 1.000 | 0.656 | 0.467 | 0.469 | 0.663 |
| p | - | <0.001 | <0.001 | <0.001 | <0.001 | |
| SII | r | 0.656 | 1.000 | 0.445 | 0.636 | 0.703 |
| p | <0.001 | - | <0.001 | <0.001 | <0.001 | |
| SIRI | r | 0.467 | 0.445 | 1.000 | 0.298 | 0.497 |
| p | <0.001 | <0.001 | - | 0.001 | <0.001 | |
| PLR | r | 0.469 | 0.636 | 0.298 | 1.000 | 0.498 |
| p | <0.001 | <0.001 | 0.001 | - | <0.001 | |
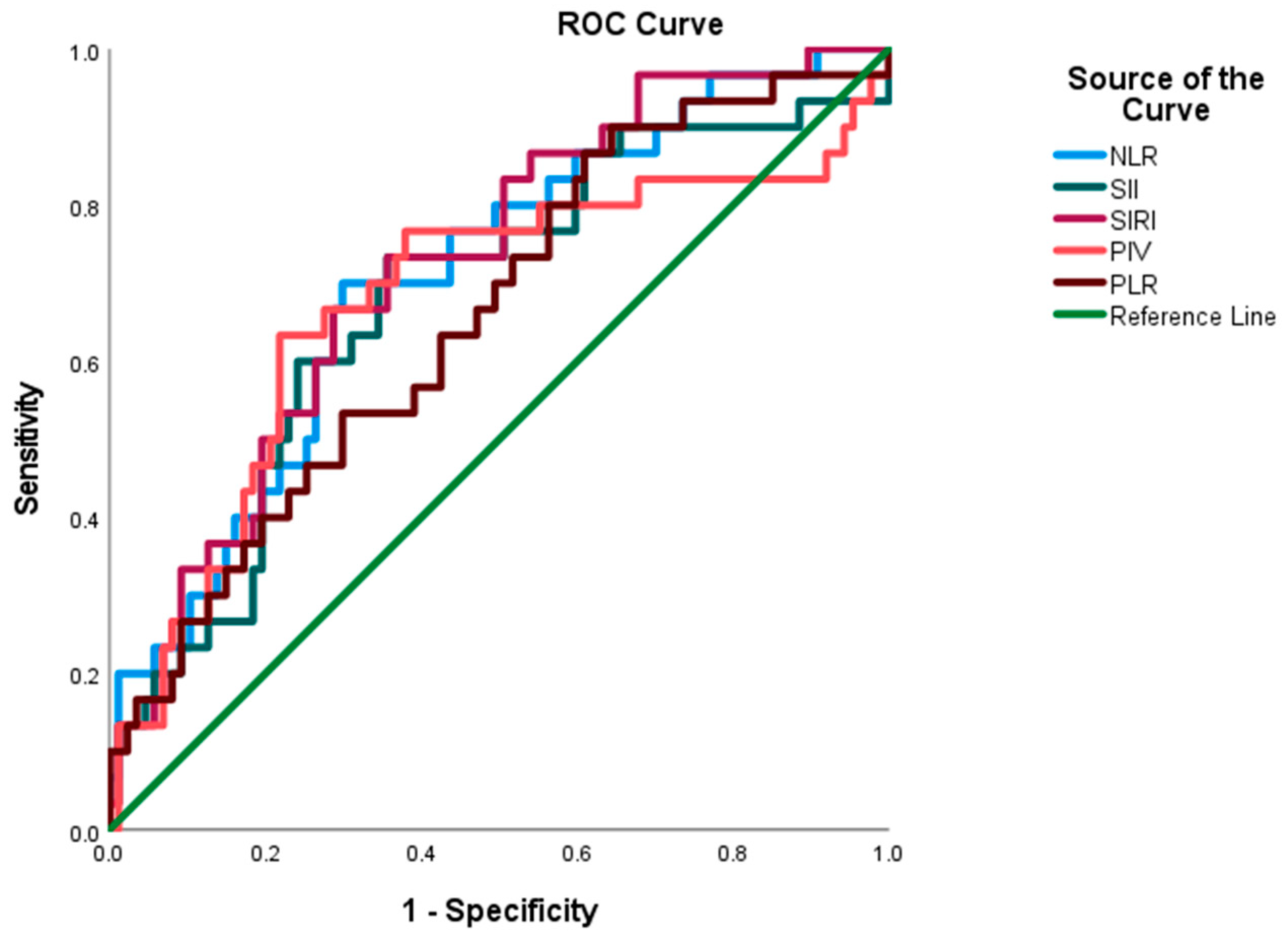
| AUC | Std. Error | 95% CI | p | Cut Off Value | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| NLR | 0.709 | 0.054 | 0.603 | 0.815 | 0.001 | 3.16 |
| SII | 0.678 | 0.059 | 0.563 | 0.793 | 0.004 | 778 |
| SIRI | 0.721 | 0.052 | 0.619 | 0.823 | 0.001 | 1.26 |
| PIV | 0.675 | 0.064 | 0.550 | 0.800 | 0.004 | 751 |
| PLR | 0.656 | 0.057 | 0.543 | 0.768 | 0.011 | 165 |
| (Univariate) Overall Survival | (Univariate) Progression Free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95.0% CI for HR | p | 95.0% CI for HR | p | |||||
| Lower | Upper | HR | Lower | Upper | |||||
| Age | 6.67 | 0.907 | 49.067 | 0.062 | Age | 6.063 | 0.825 | 44.579 | 0.077 |
| Sex | 0.697 | 0.165 | 2.935 | 0.623 | Sex | 0.719 | 0.171 | 3.031 | 0.653 |
| ECOG PS | 1.944 | 0.533 | 9.311 | 0.266 | ECOG PS | 2.251 | 0.689 | 8.112 | 0.314 |
| Comorbidity | 11.62 | 0.004 | 11.3 | 0.482 | Comorbidity | 22.058 | 0.007 | 733.483 | 0.455 |
| Smoking | 0.42 | 0.178 | 0.99 | 0.047 | Smoking | 0.5 | 0.213 | 1.176 | 0.112 |
| Alcoholism | 0.457 | 0.108 | 1.932 | 0.287 | Alcoholism | 0.358 | 0.083 | 1.539 | 0.167 |
| Intravesical BCG treatment | 1.911 | 0.863 | 4.232 | 0.11 | Intravesical BCG treatment | 1.777 | 0.805 | 3.923 | 0.155 |
| Lymph node involvement | 1.342 | 0.629 | 2.865 | 0.447 | Lymph node involvement | 1.542 | 0.726 | 3.274 | 0.26 |
| Distant metastasis | 0.61 | 0.183 | 2.036 | 0.421 | Distant metastasis | 0.644 | 0.192 | 2.155 | 0.475 |
| Tumor stage | 0.856 | 0.413 | 1.775 | 0.676 | Tumor stage | 0.863 | 0.405 | 1.838 | 0.702 |
| NLR | 2.263 | 1.079 | 4.747 | 0.031 | NLR | 2.114 | 1.015 | 4.401 | 0.045 |
| SII | 3.205 | 1.418 | 7.242 | 0.005 | SII | 3.238 | 1.437 | 7.295 | 0.005 |
| SIRI | 3.419 | 1.302 | 8.978 | 0.013 | SIRI | 3.238 | 1.236 | 8.482 | 0.017 |
| PIV | 3.3 | 1.556 | 6.998 | 0.002 | PIV | 3.533 | 1.676 | 7.448 | 0.001 |
| PLR | 2.505 | 1.196 | 5.245 | 0.015 | PLR | 2.374 | 1.135 | 4.967 | 0.022 |
| Tolerance | Variance Inflation Factor | |
|---|---|---|
| PIV | 0.42 | 2.38 |
| NLR | 0.36 | 2.77 |
| SII | 0.28 | 3.55 |
| SIRI | 0.21 | 4.72 |
| PLR | 0.25 | 3.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eren, A.E.; Aydin, A.A.; Aksaray, E.E.; Durak, A.; Unlu, A.; Islamoglu, M.E.; Ozturk, B.; Yildiz, M. Pan-Immune-Inflammation Value as a Predictor of Long-Term Outcomes in Patients with Urothelial Carcinoma of the Bladder: A Pilot Study. Curr. Oncol. 2025, 32, 534. https://doi.org/10.3390/curroncol32100534
Eren AE, Aydin AA, Aksaray EE, Durak A, Unlu A, Islamoglu ME, Ozturk B, Yildiz M. Pan-Immune-Inflammation Value as a Predictor of Long-Term Outcomes in Patients with Urothelial Carcinoma of the Bladder: A Pilot Study. Current Oncology. 2025; 32(10):534. https://doi.org/10.3390/curroncol32100534
Chicago/Turabian StyleEren, Ali Erhan, Asim Armagan Aydin, Eren Erdi Aksaray, Arda Durak, Ahmet Unlu, Mahmut Ekrem Islamoglu, Banu Ozturk, and Mustafa Yildiz. 2025. "Pan-Immune-Inflammation Value as a Predictor of Long-Term Outcomes in Patients with Urothelial Carcinoma of the Bladder: A Pilot Study" Current Oncology 32, no. 10: 534. https://doi.org/10.3390/curroncol32100534
APA StyleEren, A. E., Aydin, A. A., Aksaray, E. E., Durak, A., Unlu, A., Islamoglu, M. E., Ozturk, B., & Yildiz, M. (2025). Pan-Immune-Inflammation Value as a Predictor of Long-Term Outcomes in Patients with Urothelial Carcinoma of the Bladder: A Pilot Study. Current Oncology, 32(10), 534. https://doi.org/10.3390/curroncol32100534






