Impact of Bone-Modifying Agents on Post-Bone Metastasis Survival Across Cancer Types
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.-F.; Shen, J.; Li, X.; Rengan, R.; Silvestris, N.; Wang, M.; Derosa, L.; Zheng, X.; Belli, A.; Zhang, X.-L.; et al. Incidence of Patients with Bone Metastases at Diagnosis of Solid Tumors in Adults: A Large Population-Based Study. Ann. Transl. Med. 2020, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.K.; Wade, S.W.; Reich, A.; Pirolli, M.; Liede, A.; Lyman, G.H. Incidence of Bone Metastases in Patients with Solid Tumors: Analysis of Oncology Electronic Medical Records in the United States. BMC Cancer 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, M.; Jensen, A.Ø.; Jacobsen, J.B.; Cetin, K.; Fryzek, J.P.; Sørensen, H.T. Skeletal Related Events, Bone Metastasis and Survival of Prostate Cancer: A Population Based Cohort Study in Denmark (1999 to 2007). J. Urol. 2010, 184, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Sathiakumar, N.; Delzell, E.; Morrisey, M.A.; Falkson, C.; Yong, M.; Chia, V.; Blackburn, J.; Arora, T.; Kilgore, M.L. Mortality Following Bone Metastasis and Skeletal-Related Events among Men with Prostate Cancer: A Population-Based Analysis of US Medicare Beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011, 14, 177–183. [Google Scholar] [CrossRef]
- Yong, M.; Jensen, A.Ø.; Jacobsen, J.B.; Nørgaard, M.; Fryzek, J.P.; Sørensen, H.T. Survival in Breast Cancer Patients with Bone Metastases and Skeletal-Related Events: A Population-Based Cohort Study in Denmark (1999–2007). Breast Cancer Res. Treat. 2011, 129, 495–503. [Google Scholar] [CrossRef]
- Fujimoto, D.; Ueda, H.; Shimizu, R.; Kato, R.; Otoshi, T.; Kawamura, T.; Tamai, K.; Shibata, Y.; Matsumoto, T.; Nagata, K.; et al. Features and Prognostic Impact of Distant Metastasis in Patients with Stage IV Lung Adenocarcinoma Harboring EGFR Mutations: Importance of Bone Metastasis. Clin. Exp. Metastasis 2014, 31, 543–551. [Google Scholar] [CrossRef]
- Wardley, A.; Davidson, N.; Barrett-Lee, P.; Hong, A.; Mansi, J.; Dodwell, D.; Murphy, R.; Mason, T.; Cameron, D. Zoledronic Acid Significantly Improves Pain Scores and Quality of Life in Breast Cancer Patients with Bone Metastases: A Randomised, Crossover Study of Community vs Hospital Bisphosphonate Administration. Br. J. Cancer 2005, 92, 1869–1876. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Shen, C.-K.; Du, Y.-X.; Li, Z.-M.; Shi, X.; Wang, Y.; Wei, W.-J. Survival Outcome and Optimal Candidates of Primary Tumor Resection for Patients with Metastatic Medullary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2024, 109, 2979–2985. [Google Scholar] [CrossRef]
- Suresh Babu, M.C.; Garg, S.; Lakshmaiah, K.C.; Govind Babu, K.; Kumar, R.; Loknatha, D.; Abraham, L.J.; Rajeev, L.K.; Lokesh, K.N.; Rudresha, A.H.; et al. Colorectal Cancer Presenting as Bone Metastasis. J. Cancer Res. Ther. 2017, 13, 80–83. [Google Scholar]
- Lee, C.H.; Kang, M.; Kwak, C.; Ko, Y.H.; Kim, J.K.; Park, J.Y.; Bang, S.; Seo, S., II; Suh, J.; Song, W.; et al. Sites of Metastasis and Survival in Metastatic Renal Cell Carcinoma: Results from the Korean Renal Cancer Study Group Database. J. Korean Med. Sci. 2024, 39, 1–13. [Google Scholar] [CrossRef]
- Dunlop, D.D.; Hughes, S.L.; Manheim, L.M. Disability in Activities of Daily Living: Patterns of Change and a Hierarchy of Disability. Am. J. Public Health 1997, 87, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Neo, J.; Fettes, L.; Gao, W.; Higginson, I.J.; Maddocks, M. Disability in Activities of Daily Living among Adults with Cancer: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2017, 61, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Hirahata, M.; Imanishi, J.; Fujinuma, W.; Abe, S.; Inui, T.; Ogata, N.; Iimuro, S.; Fujita, R.; Sato, K.; Tokizaki, T.; et al. Cancer May Accelerate Locomotive Syndrome and Deteriorate Quality of Life: A Single-Centre Cross-Sectional Study of Locomotive Syndrome in Cancer Patients. Int. J. Clin. Oncol. 2023, 28, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Whitefield, S.; Ilan, M.B.; Lazarovici, T.S.; Friedlander-Barenboim, S.; Kassem, R.; Yarom, N. Medication-Related Osteonecrosis of the Jaw: A Cross-Sectional Study on the Prevalence of Cutaneous Manifestations and the Primary Care Physician’s Role in Its Early Diagnosis. Am. J. Med. 2024, 137, 266–272. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.-J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone Health in Cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Ebrahimpour, A.; Sadighi, M.; Hoveidaei, A.H.; Chehrassan, M.; Minaei, R.; Vahedi, H.; Mortazavi, S.M.J. Surgical Treatment for Bisphosphonate-Related Atypical Femoral Fracture: A Systematic Review. Arch. Bone Jt. Surg. 2021, 9, 283–296. [Google Scholar]
- Nisi, M.; Gennai, S.; Graziani, F.; Barone, A.; Izzetti, R. Clinical and Radiologic Treatment Outcomes of Implant Presence Tirggered-MRONJ: Systematic Review of Literature. Oral Dis. 2024, 30, 5255–5267. [Google Scholar] [CrossRef]
- Desautels, D.N.; Harlos, C.H.; Jerzak, K.J. Role of Bone-Modifying Agents in Advanced Cancer. Ann. Cardiothorac. Surg. 2020, 9, 1314–1323. [Google Scholar] [CrossRef]
- Chen, C.; Li, R.; Yang, T.; Ma, L.; Zhou, S.; Li, M.; Zhou, Y.; Cui, Y. Denosumab Versus Zoledronic Acid in the Prevention of Skeletal-Related Events in Vulnerable Cancer Patients: A Meta-Analysis of Randomized, Controlled Trials. Clin. Ther. 2020, 42, 1494–1507.e1. [Google Scholar] [CrossRef] [PubMed]
- Kohno, N.; Aogi, K.; Minami, H.; Nakamura, S.; Asaga, T.; Iino, Y.; Watanabe, T.; Goessl, C.; Ohashi, Y.; Takashima, S. Zoledronic Acid Significantly Reduces Skeletal Complications Compared with Placebo in Japanese Women with Bone Metastases from Breast Cancer: A Randomized, Placebo-Controlled Trial. J. Clin. Oncol. 2005, 23, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- O’Carrigan, B.; Wong, M.H.F.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and Other Bone Agents for Breast Cancer. Cochrane Database Syst. Rev. 2017, 2017, CD003474. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; De Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab Compared with Zoledronic Acid for the Treatment of Bone Metastases in Patients with Advanced Breast Cancer: A Randomized, Double-Blind Study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Pan, Y.; Jin, H.; Chen, W.; Yu, Z.; Ye, T.; Zheng, Y.; Weng, Z.; Wang, F. Docetaxel with or without Zoledronic Acid for Castration-Resistant Prostate Cancer. Int. Urol. Nephrol. 2014, 46, 2319–2326. [Google Scholar] [CrossRef]
- Qin, A.; Zhao, S.; Miah, A.; Wei, L.; Patel, S.; Johns, A.; Grogan, M.; Bertino, E.M.; He, K.; Shields, P.G.; et al. Bone Metastases, Skeletal-Related Events, and Survival in Patients with Metastatic Non–Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. J. Natl. Compr. Cancer Netw. 2021, 19, 915–921. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Hirsh, V.; Siena, S.; Henry, D.H.; Woll, P.J.; Manegold, C.; Solal-Celigny, P.; Rodriguez, G.; Krzakowski, M.; Mehta, N.D.; et al. Overall Survival Improvement in Patients with Lung Cancer and Bone Metastases Treated with Denosumab Versus Zoledronic Acid: Subgroup Analysis from a Randomized Phase 3 Study. J. Thorac. Oncol. 2012, 7, 1823–1829. [Google Scholar] [CrossRef]
- Kaku, T.; Oh, Y.; Sato, S.; Koyanagi, H.; Hirai, T.; Yuasa, M.; Yoshii, T.; Nakagawa, T.; Miyake, S.; Okawa, A. Incidence of Atypical Femoral Fractures in the Treatment of Bone Metastasis: An Alert Report. J. Bone Oncol. 2020, 23, 100301. [Google Scholar] [CrossRef]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in Cancer Survival, Mortality, and Incidence in Seven High-Income Countries 1995–2014 (ICBP SURVMARK-2): A Population-Based Study. Lancet. Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef]
- Hong, S.; Youk, T.; Lee, S.J.; Kim, K.M.; Vajdic, C.M. Bone Metastasis and Skeletal-Related Events in Patients with Solid Cancer: A Korean Nationwide Health Insurance Database Study. PLoS ONE 2020, 15, e0234927. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.; Christiansen, C.F.; Ulrichsen, S.P.; Rørth, M.R.; Sørensen, H.T. Survival after Bone Metastasis by Primary Cancer Type: A Danish Population-Based Cohort Study. BMJ Open 2017, 7, e016022. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Yamada, K.; Sugiura, T.; Hida, T.; Mitsudomi, T. Predictors of Survival in Patients with Bone Metastasis of Lung Cancer. Clin. Orthop. Relat. Res. 2008, 466, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, A.M.; Lori, E.; Bellini, M.I.; Bolis, E.; Lozza, P.; Castellani, L.; Saibene, A.M.; Pipolo, C.; Fuccillo, E.; Rosso, C.; et al. Advanced Differentiated Thyroid Cancer: A Complex Condition Needing a Tailored Approach. Front. Oncol. 2022, 12, 954759. [Google Scholar] [CrossRef]
- Kanaoka, K.; Sumikawa, H.; Oyamada, S.; Tamiya, A.; Inagaki, Y.; Taniguchi, Y.; Nakao, K.; Matsuda, Y.; Okishio, K. Osteoblastic Bone Reaction in Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Mutation Treated with Osimertinib. BMC Cancer 2023, 23, 834. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Gu, Y.; Sun, Z.; Qiu, Z.; Zhou, Y. Diagnostic Value of Whole-Body DWI With Background Body Suppression Plus Calculation of Apparent Diffusion Coefficient at 3 T Versus 18 F-FDG PET/CT for Detection of Bone Metastases. Am. J. Roentgenol. 2020, 214, 446–454. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yoshida, S.; Ishii, C.; Takahara, T.; Arita, Y.; Fukushima, H.; Tanaka, H.; Yokoyama, M.; Matsuoka, Y.; Fujii, Y. Metastatic Diffusion Volume Based on Apparent Diffusion Coefficient as a Prognostic Factor in Castration-Resistant Prostate Cancer. J. Magn. Reson. Imaging 2021, 54, 401–408. [Google Scholar] [CrossRef]


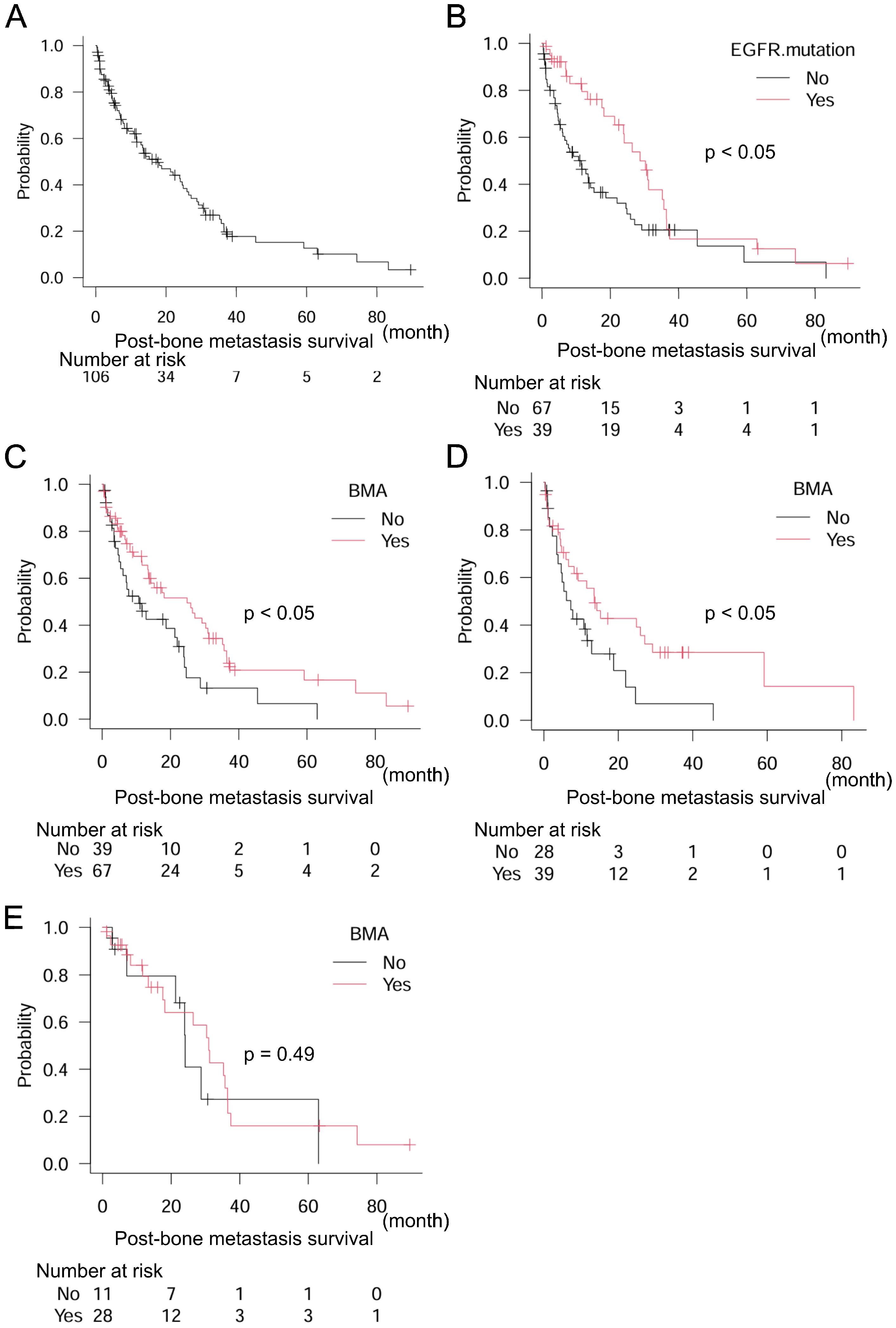
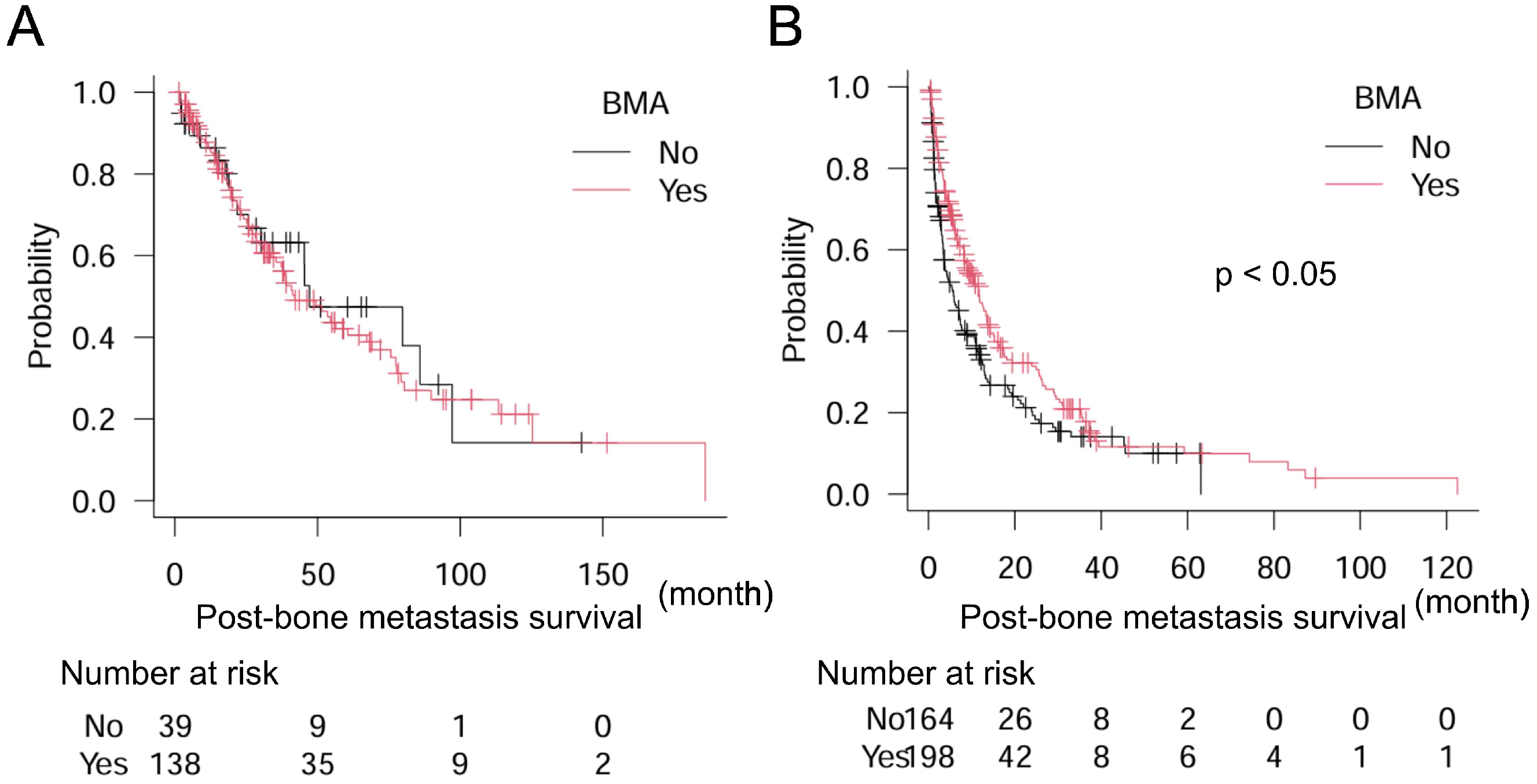
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | Number | Median Post-Bone Metastasis Survival (Months) | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Cancer Type | Thyroid | 11 | 97.2 | 0.22 | 0.07–0.72 | <0.05 | 0.22 | 0.07–0.70 | <0.05 |
| Breast | 84 | 51.5 | 0.39 | 0.27–0.57 | <0.001 | 0.45 | 0.30–0.66 | <0.001 | |
| Prostate | 48 | 47.2 | 0.34 | 0.21–0.54 | <0.001 | 0.36 | 0.22–0.59 | <0.001 | |
| Kidney | 34 | 38.8 | 0.48 | 0.29–0.79 | <0.005 | 0.47 | 0.28–0.78 | <0.005 | |
| NSCLC | 106 | 17.6 | Reference | Reference | |||||
| Bone and soft tissue | 27 | 11.9 | 1.04 | 0.61–1.76 | 0.89 | 0.93 | 0.54–1.59 | 0.78 | |
| Liver | 19 | 10.8 | 1.48 | 0.83–2.62 | 0.18 | 1.42 | 0.80–2.53 | 0.23 | |
| Colorectum | 30 | 8.8 | 1.56 | 0.96–2.54 | 0.07 | 1.44 | 0.88–2.35 | 0.15 | |
| SCLC | 18 | 8.6 | 1.44 | 0.78–2.65 | 0.25 | 1.23 | 0.66–2.28 | 0.52 | |
| Stomach | 18 | 6.9 | 2.26 | 1.29–3.95 | <0.005 | 2.28 | 1.29–4.03 | <0.005 | |
| Head and neck | 20 | 6.3 | 2.30 | 1.33–3.97 | <0.005 | 2.23 | 1.29–3.85 | <0.005 | |
| Pancreas | 23 | 5.5 | 2.26 | 1.36–3.75 | <0.005 | 2.30 | 1.38–3.84 | <0.005 | |
| Skin | 14 | 5.1 | 2.08 | 1.07–4.03 | <0.05 | 1.67 | 0.85–3.28 | 0.13 | |
| Biliary tract | 7 | 4.2 | 2.73 | 1.09–6.79 | <0.05 | 2.29 | 0.91–5.75 | 0.07 | |
| Uterus | 11 | 3.6 | 1.20 | 0.60–2.41 | 0.61 | 1.41 | 0.69–2.88 | 0.34 | |
| Urinary tract | 26 | 3.4 | 2.89 | 1.77–4.72 | <0.001 | 2.76 | 1.67–4.56 | <0.001 | |
| Esophagus | 22 | 2.9 | 2.94 | 1.76–4.90 | <0.001 | 2.55 | 1.51–4.30 | <0.001 | |
| Others | 21 | 8.3 | 1.11 | 0.65–1.89 | 0.70 | 1.06 | 0.62–1.81 | 0.83 | |
| BMA | No | 203 | 7.8 | Reference | Reference | ||||
| Yes | 336 | 21.9 | 0.64 | 0.51–0.79 | <0.001 | 0.72 | 0.57–0.91 | <0.01 | |
| Metastasis other than bone | No | 204 | 23.3 | Reference | Reference | ||||
| Yes | 335 | 14.1 | 1.40 | 1.12–1.75 | <0.005 | 1.19 | 0.94–1.51 | 0.15 | |
| Sex | Female | 235 | 25.7 | Reference | Reference | ||||
| Male | 304 | 11.9 | 1.39 | 1.13–1.72 | <0.005 | 1.15 | 0.89–1.48 | 0.29 | |
| Treatment for bone metastasis | No | 103 | 24.1 | Reference | Reference | ||||
| Yes | 436 | 13.6 | 1.39 | 1.05–1.84 | <0.05 | 1.33 | 0.99–1.78 | 0.059 | |
| Metastatic Site | Number | Median Post-Bone Metastasis Survival (Months) | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| None | 204 | 23.3 | Reference | ||
| Brain | 67 | 21.3 | 1.06 | 0.74–1.50 | 0.76 |
| Lung | 75 | 17.4 | 1.26 | 0.89–1.78 | 0.18 |
| Liver | 70 | 13.5 | 1.37 | 0.99–1.90 | 0.053 |
| More than two sites | 61 | 12.6 | 1.60 | 1.15–2.24 | <0.01 |
| Dissemination | 62 | 12.2 | 1.92 | 1.38–2.67 | <0.001 |
| Factor | Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|---|
| Cancer Type | Colorectum | Reference | ||
| Liver | 0.97 | 0.49–1.91 | 0.92 | |
| Pancreas | 1.43 | 0.76–2.68 | 0.26 | |
| Stomach | 1.46 | 0.75–2.86 | 0.27 | |
| Biliary tract | 1.68 | 0.63–4.52 | 0.3 | |
| Esophagus | 1.81 | 0.96–3.40 | 0.07 | |
| Factor | Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|---|
| BMA | No | Reference | ||
| Yes | 0.56 | 0.34–0.91 | <0.05 | |
| EGFR mutation | No | Reference | ||
| Yes | 0.55 | 0.34–0.90 | <0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamiya, H.; Nishino, K.; Kato, Y.; Nakahashi-Kato, R.; Kosuga-Tsujimoto, Y.; Kinoshita, S.; Suzuki, R.; Watanabe, M.; Wakamatsu, T.; Kakunaga, S.; et al. Impact of Bone-Modifying Agents on Post-Bone Metastasis Survival Across Cancer Types. Curr. Oncol. 2025, 32, 42. https://doi.org/10.3390/curroncol32010042
Tamiya H, Nishino K, Kato Y, Nakahashi-Kato R, Kosuga-Tsujimoto Y, Kinoshita S, Suzuki R, Watanabe M, Wakamatsu T, Kakunaga S, et al. Impact of Bone-Modifying Agents on Post-Bone Metastasis Survival Across Cancer Types. Current Oncology. 2025; 32(1):42. https://doi.org/10.3390/curroncol32010042
Chicago/Turabian StyleTamiya, Hironari, Kazumi Nishino, Yuji Kato, Reina Nakahashi-Kato, Yurika Kosuga-Tsujimoto, Shota Kinoshita, Rie Suzuki, Makiyo Watanabe, Toru Wakamatsu, Shigeki Kakunaga, and et al. 2025. "Impact of Bone-Modifying Agents on Post-Bone Metastasis Survival Across Cancer Types" Current Oncology 32, no. 1: 42. https://doi.org/10.3390/curroncol32010042
APA StyleTamiya, H., Nishino, K., Kato, Y., Nakahashi-Kato, R., Kosuga-Tsujimoto, Y., Kinoshita, S., Suzuki, R., Watanabe, M., Wakamatsu, T., Kakunaga, S., & Takenaka, S. (2025). Impact of Bone-Modifying Agents on Post-Bone Metastasis Survival Across Cancer Types. Current Oncology, 32(1), 42. https://doi.org/10.3390/curroncol32010042





