Doxycycline Plus Bortezomib-Containing Regimens for the Treatment of Light-Chain Amyloidosis in the Frontline Setting: Experience from the Amyloidosis Program of Calgary
Abstract
1. Introduction
2. Background
3. Materials and Methods
3.1. Study Aims
3.2. Treatment Regimes
3.3. Response Assessment
3.4. Statistics
4. Results
4.1. Hematologic Responses
4.2. Organ Response
4.3. Progression-Free and Overall Survival
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merlini, G.; Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Zhang, N.J.; Cherepanov, D.; Romanus, D.; Hughes, M.; Faller, D.V. Global epidemiology of amyloid light-chain amyloidosis. Orphanet J. Rare Dis. 2022, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, S.; Mohty, D.; Magne, J.; Lavergne, D.; Bordessoule, D.; Aboyans, V.; Jaccard, A. Incidence and prevalence of light chain amyloidosis: A population-based study. Blood 2017, 130 (Suppl. S1), 5577. [Google Scholar] [CrossRef]
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2015, 387, 2641–2654. [Google Scholar] [CrossRef]
- Staron, A.; Zheng, L.; Doros, G.; Connors, L.H.; Mendelson, L.M.; Joshi, T.; Sanchorawala, V. Marked progress in AL amyloidosis survival: A 40-year longitudinal natural history study. Blood Cancer J. 2021, 11, 139. [Google Scholar] [CrossRef]
- Sabinot, A.; Ghetti, G.; Pradelli, L.; Bellucci, S.; Lausi, A.; Palladini, G. State-of-the-art review on AL amyloidosis in Western Countries: Epidemiology, health economics, risk assessment and therapeutic management of a rare disease. Blood Rev. 2023, 59, 101040. [Google Scholar] [CrossRef]
- Mikhael, J.R.; Schuster, S.R.; Jimenez-Zepeda, V.H.; Bello, N.; Spong, J.; Reeder, C.B.; Stewart, A.K.; Bergsagel, P.L.; Fonseca, R. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood 2012, 119, 4391–4394. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Zepeda, V.H.; Duggan, P.; Neri, P.; Bahlis, N.J. Bortezomib-containing regimens for the treatment of newly diagnosed and relapsed amyloid light chain amyloidosis: A single-center experience. Clin. Lymphoma Myeloma Leuk. 2016, 16, e79–e84. [Google Scholar] [CrossRef]
- Palladini, G.; Merlini, G. How I treat AL amyloidosis. Blood 2022, 139, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Kyle, R.A.; Gertz, M.A.; Therneau, T.M.; Miller, W.L.; Chandrasekaran, K.; McConnell, J.P.; Burritt, M.F.; Jaffe, A.S. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003, 361, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Valero-Muñoz, M.; Wilson, R.M.; Bretón-Romero, R.; Croteau, D.; Seldin, D.C.; Sam, F. Doxycycline decreases amyloidogenic light chain-induced autophagy in isolated primary cardiac myocytes. Int. J. Cardiol. 2020, 321, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Wechalekar, A.D.; Whelan, C. Encouraging impact of doxycycline on early mortality in cardiac light chain (AL) amyloidosis. Blood Cancer J. 2017, 7, e546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ward, J.E.; Ren, R.; Toraldo, G.; SooHoo, P.; Guan, J.; O’Hara, C.; Jasuja, R.; Trinkaus-Randall, V.; Liao, R.; Connors, L.H.; et al. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood 2011, 118, 6610–6617. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Szabo, A.; Flynn, K.E.; Dhakal, B.; Chhabra, S.; Pasquini, M.C.; Weihrauch, D.; Hari, P.N. Adjuvant doxycycline to enhance antiamyloid effects: Results from the dual phase 2 trial. eClinicalMedicine 2020, 23, 100361. [Google Scholar] [CrossRef]
- Shen, K.N.; Fu, W.J.; Wu, Y.; Dong, Y.J.; Huang, Z.X.; Wei, Y.Q.; Li, C.R.; Sun, C.Y.; Chen, Y.; Miao, H.L.; et al. Doxycycline Combined With Bortezomib-Cyclophosphamide- Dexamethasone Chemotherapy for Newly Diagnosed Cardiac Light-Chain Amyloidosis: A Multicenter Randomized Controlled Trial. Circulation 2022, 145, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.R. Light Chain Amyloidosis: Epidemiology, Staging, and Prognostication. Methodist Debakey Cardiovasc. J. 2022, 18, 27–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palladini, G.; Dispenzieri, A.; Gertz, M.A.; Kumar, S.; Wechalekar, A.; Hawkins, P.N.; Schönland, S.; Hegenbart, U.; Comenzo, R.; Kastritis, E.; et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: Impact on survival outcomes. J. Clin. Oncol. 2012, 30, 4541–4549. [Google Scholar] [CrossRef]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef]
- Jimenez-Zepeda, V.H.; Lee, H.; Fine, N.; McCulloch, S.; Tay, J.; Duggan, P.; Neri, P.; Bahlis, N. Cyclophosphamide, bortezomib and methylprednisolone (CyBorMe) for the treatment of AL amyloidosis: Initial experience from a cingle Center. Indian J. Hematol. Blood Transfus. 2021, 37, 675–678. [Google Scholar] [CrossRef]
- Kastritis, E.; Palladini, G.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Sanchorawala, V.; Gibbs, S.; Mollee, P.; Venner, C.P.; et al. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N. Engl. J. Med. 2021, 385, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.S.; Kennel, S.J.; Williams, A.; Richey, T.; Stuckey, A.; Huang, Y.; Macy, S.; Donnell, R.; Barbour, R.; Seubert, P.; et al. AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils. PLoS ONE 2012, 7, e52686. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Landau, H.; Comenzo, R.L.; Seldin, D.; Weiss, B.; Zonder, J.; Merlini, G.; Schönland, S.; Walling, J.; Kinney, G.G.; et al. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J. Clin. Oncol. 2016, 34, 1097–1103. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | BCR + Doxycycline, n = 39 | BCR Alone, n = 25 | p Value |
|---|---|---|---|
| Age (median) | 68 | 64 | 0.3 |
| Gender | 0.4 | ||
| Male | 21 (53.8%) | 16 (64%) | |
| Female | 18 (46.1%) | 9 (36%) | |
| Hb (g/L) | 124 | 122 | 0.4 |
| Creatinine (µmol/L) | 96 | 80 | 0.5 |
| B2microglobulin (µmol/L) | 3.0 | 3.24 | 0.3 |
| Albumin (g/L) | 29 | 32 | 0.08 |
| Stage I | 2 (5.1%) | 0 (0%) | 0.2 |
| Stage II | 4 (10.2%) | 4 (16%) | |
| Stage III | 14 (35.8%) | 4 (16%) | |
| Stage IV | 17 (43.5%) | 14 (56%) | |
| Unknown | 2 (5.1%) | 3 (12%) | |
| LDH (IU/L) | 226 | 232 | 0.4 |
| BMPC (%) | 12 | 10 | 0.3 |
| NTproBNP ng/L | 2642 | 3246 | 0.4 |
| Troponin T ng/L | 53 | 65 | 0.4 |
| Light chain: | 0.6 | ||
| Kappa | 8 (20.5%) | 8 (32%) | |
| Lambda | 30 (76.9%) | 17 (68%) | |
| Biclonal | 1 (2.5%) | 0 | |
| Organ Involvement | |||
| Cardiac involvement | 39 (100%) | 25 (100%) | NS |
| Kidney involvement | 26 (66.6%) | 19 (76%) | 0.4 |
| Liver involvement | 7 (17.9%) | 2 (8%) | 0.2 |
| Nerve involvement | 4 (10.2%) | 5 (20%) | 0.2 |
| GI involvement | 8 (20.5%) | 5 (20%) | 0.9 |
| Lung involvement | 1 (2.5%) | 2 (8%) | 0.3 |
| Characteristic | BCR-D, n = 39 | BCR, n = 25 | p Value |
|---|---|---|---|
| Bortezomib-Containing Regimens | 0.001 | ||
| CyBorD | 20 (51.2%) | 20 (80%) | |
| CyBorMe | 19 (48.7%) | 2 (8%) | |
| CyBord plus clinical trial (no daratumumab) | 0 (0%) | 2 (8%) | |
| Other | 0 | 1 (4%) | |
| Median number of cycles of the BCR part | 4 | 6 | 0.6 |
| Hematological Response | |||
| Overall response rate | 35 (89.7%) | 21 (84%) | 0.4 |
| VGPR/CR | 19 (49%) | 15 (60%) | 0.3 |
| Complete response | 10 (25.6%) | 5 (20%) | 0.3 |
| dFLC at 1 month (median) | 69 | 50.5 | 0.5 |
| Time to first response (median) | 4 weeks | 4 weeks | 0.4 |
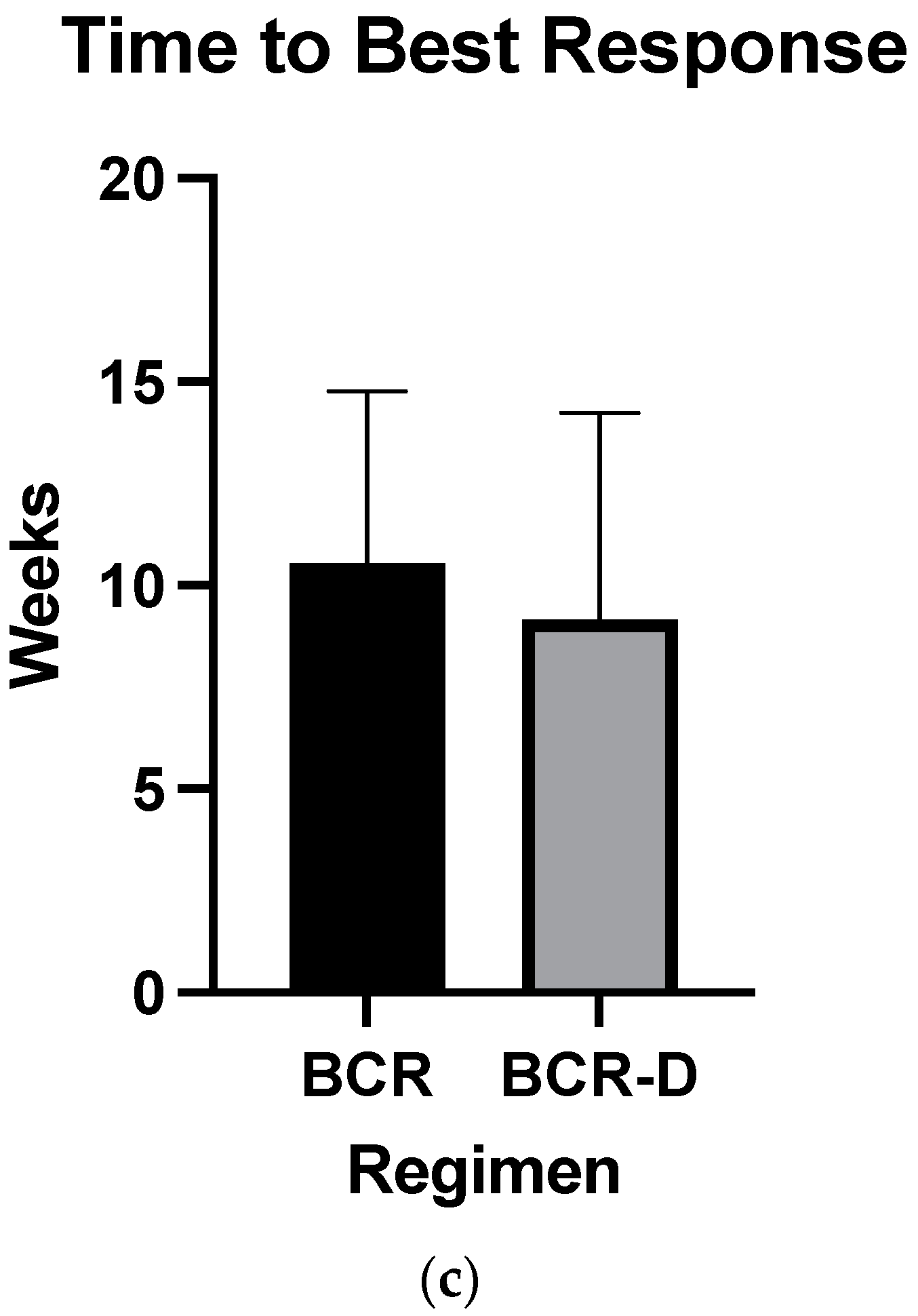
| Time to best response (median) | 12 weeks | 8 weeks | 0.2 |
| Organ Response | |||
| Overall organ response | 16 (41%) | 12 (48%) | 0.5 |
| Cardiac response | 15/39 (38.4%) | 14/25 (56%) | 0.1 |
| Renal response | 9/26 (34.6%) | 9/19 (47%) | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, E.; Fine, N.; McCulloch, S.; Tay, J.; Duggan, P.; Neri, P.; Bahlis, N.; Jimenez-Zepeda, V.H. Doxycycline Plus Bortezomib-Containing Regimens for the Treatment of Light-Chain Amyloidosis in the Frontline Setting: Experience from the Amyloidosis Program of Calgary. Curr. Oncol. 2024, 31, 5608-5616. https://doi.org/10.3390/curroncol31090415
Lewis E, Fine N, McCulloch S, Tay J, Duggan P, Neri P, Bahlis N, Jimenez-Zepeda VH. Doxycycline Plus Bortezomib-Containing Regimens for the Treatment of Light-Chain Amyloidosis in the Frontline Setting: Experience from the Amyloidosis Program of Calgary. Current Oncology. 2024; 31(9):5608-5616. https://doi.org/10.3390/curroncol31090415
Chicago/Turabian StyleLewis, Ellen, Nowell Fine, Sylvia McCulloch, Jason Tay, Peter Duggan, Paola Neri, Nizar Bahlis, and Victor H. Jimenez-Zepeda. 2024. "Doxycycline Plus Bortezomib-Containing Regimens for the Treatment of Light-Chain Amyloidosis in the Frontline Setting: Experience from the Amyloidosis Program of Calgary" Current Oncology 31, no. 9: 5608-5616. https://doi.org/10.3390/curroncol31090415
APA StyleLewis, E., Fine, N., McCulloch, S., Tay, J., Duggan, P., Neri, P., Bahlis, N., & Jimenez-Zepeda, V. H. (2024). Doxycycline Plus Bortezomib-Containing Regimens for the Treatment of Light-Chain Amyloidosis in the Frontline Setting: Experience from the Amyloidosis Program of Calgary. Current Oncology, 31(9), 5608-5616. https://doi.org/10.3390/curroncol31090415





