Quality of Life Determinants in Patients with Metastatic Prostate Cancer: Insights from a Cross-Sectional Questionnaire-Based Study
Abstract
1. Introduction
2. Methodology
2.1. Study Design
2.2. Assessment of HRQOL
2.3. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Quality of Life
3.3. Factors Affecting HRQoL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 28 January 2024).
- Sayegh, N.; Swami, U.; Agarwal, N. Recent Advances in the Management of Metastatic Prostate Cancer. JCO Oncol. Pract. 2022, 18, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Leaning, D.; Kaur, G.; Morgans, A.K.; Ghouse, R.; Mirante, O.; Chowdhury, S. Treatment landscape and burden of disease in metastatic castration-resistant prostate cancer: Systematic and structured literature reviews. Front. Oncol. 2023, 13, 1240864. [Google Scholar] [CrossRef]
- Megari, K. Quality of life in chronic disease patients. Health Psychol. Res. 2013, 1, e27. [Google Scholar] [CrossRef]
- Chu, D.; Popovic, M.; Chow, E.; Cella, D.; Beaumont, J.L.; Lam, H.; Nguyen, J.; Giovanni, J.D.; Pulenzas, N.; Bedard, G.; et al. Development, characteristics and validity of the EORTC QLQ-PR25 and the FACT-P for assessment of quality of life in prostate cancer patients. J. Comp. Eff. Res. 2014, 3, 523–531. [Google Scholar] [CrossRef]
- Ratti, M.M.; Gandaglia, G.; Sisca, E.S.; Derevianko, A.; Alleva, E.; Beyer, K.; Moss, C.; Barletta, F.; Scuderi, S.; Omar, M.I.; et al. A Systematic Review to Evaluate Patient-Reported Outcome Measures (PROMs) for Metastatic Prostate Cancer According to the COnsensus-Based Standard for the Selection of Health Measurement INstruments (COSMIN) Methodology. Cancers 2022, 14, 5120. [Google Scholar] [CrossRef] [PubMed]
- Esper, P.; Mo, F.; Chodak, G.; Sinner, M.; Cella, D.; Pienta, K.J. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy-prostate instrument. Urology 1997, 50, 920–928. [Google Scholar] [CrossRef]
- Cella, D.; Nichol, M.B.; Eton, D.; Nelson, J.B.; Mulani, P. Estimating Clinically Meaningful Changes for the Functional Assessment of Cancer Therapy—Prostate: Results from a Clinical Trial of Patients with Metastatic Hormone-Refractory Prostate Cancer. Value Health 2009, 12, 124–129. [Google Scholar] [CrossRef]
- Sharma, A.; Garg, G.; Sadasukhi, N.; Sadasukhi, T.C.; Gupta, H.L.; Gupta, M.; Malik, S.; Patel, K.; Sinha, R.J. A prospective longitudinal study to evaluate bone health, implication of FRAX tool and impact on quality of life (FACT-P) in advanced prostate cancer patients. Am. J. Clin. Exp. Urol. 2021, 9, 211–220. [Google Scholar] [PubMed]
- Sullivan, P.W.; Nelson, J.B.; Mulani, P.M.; Sleep, D. Quality of life as a potential predictor for morbidity and mortality in patients with metastatic hormone-refractory prostate cancer. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2006, 15, 1297–1306. [Google Scholar] [CrossRef]
- Roy, S.; Morgan, S.C.; Spratt, D.E.; MacRae, R.M.; Grimes, S.; Malone, J.; Mukherjee, D.; Malone, S. Association of Baseline Patient-reported Health-related Quality of Life Metrics with Outcome in Localised Prostate Cancer. Clin. Oncol. 2022, 34, e61–e68. [Google Scholar] [CrossRef]
- Morgans, A.K.; Chen, Y.; Jarrard, D.F.; Carducci, M.; Liu, G.; Eisenberger, M.; Plimack, E.R.; Bryce, A.; Garcia, J.A.; Dreicer, R.; et al. Association between baseline body mass index and survival in men with metastatic hormone-sensitive prostate cancer: ECOG-ACRIN CHAARTED E3805. Prostate 2022, 82, 1176–1185. [Google Scholar] [CrossRef]
- 66th Annual Report, 2021–2022. All India Institute of Medical Sciences, New Delhi—110029. Available online: https://www.aiims.edu/images/pdf/annual_reports/english.pdf (accessed on 3 March 2024).
- India 2020: Reference Annual. Profile—Literacy—Know India: National Portal of India. Publications Division, Ministry of Information and Broadcasting, Government of India, 2020. Available online: https://pmindiaun.gov.in/public_files/assets/pdf/India_2020_REFERENCEANNUAL.pdf (accessed on 2 March 2024).
- Misra, A.; Chowbey, P.; Makkar, B.M.; Vikram, N.K.; Wasir, J.S.; Chadha, D.; Joshi, S.R.; Sadikot, S.; Gupta, R.; Gulati, S.; et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J. Assoc. Physicians India. 2009, 57, 163–170. [Google Scholar]
- Hussain, M.; Tombal, B.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltso, E.; Kalebasty, A.R.; Bögemann, M.; Ye, D.; et al. Darolutamide Plus Androgen-Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer by Disease Volume and Risk Subgroups in the Phase III ARASENS Trial. J. Clin. Oncol. 2023, 41, 3595–3607. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Quality of life in cancer patients receiving palliative care. Indian. J. Palliat. Care 2010, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, A.; van den Bergh, R.C.N.; Martini, A.; Marra, G.; Valerio, M.; Tsaur, I.; Heidegger, I.; Kasivisvanathan, V.; Kesch, C.; Preisser, F.; et al. Assessment of Health-Related Quality of Life in Patients with Advanced Prostate Cancer—Current State and Future Perspectives. Cancers 2021, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Ganguli, A.; Turnbull, J.; Rohay, J.; Morlock, R. US Population Reference Values for Health-Related Quality of Life Questionnaires Based on Demographics of Patients with Prostate Cancer. Adv. Ther. 2022, 39, 3696–3710. [Google Scholar] [CrossRef]
- Han, J.; Yang, D.L.; Liu, J.H.; Li, B.H.; Kan, Y.; Ma, S.; Mao, L.J. Family-centered psychological support helps improve illness cognition and quality of life in patients with advanced prostate cancer. Zhonghua Nan Ke Xue Natl. J. Androl. 2020, 26, 505–512. [Google Scholar]
- Gotto, G.; Drachenberg, D.E.; Chin, J.; Casey, R.; Fradet, V.; Sabbagh, R.; Shayegan, B.; Rendon, R.A.; Danielson, B.; Camacho, F.; et al. Real-world evidence in patient-reported outcomes (PROs) of metastatic castrate-resistant prostate cancer (mCRPC) patients treated with abiraterone acetate + prednisone (AA+P) across Canada: Final results of COSMiC. Can. Urol. Assoc. J. 2020, 14. Available online: https://cuaj.ca/index.php/journal/article/view/6388 (accessed on 29 January 2024). [CrossRef]
- Wu, E.Q.; Mulani, P.; Farrell, M.H.; Sleep, D. Mapping FACT-P and EORTC QLQ-C30 to Patient Health Status Measured by EQ-5D in Metastatic Hormone-Refractory Prostate Cancer Patients. Value Health 2007, 10, 408–414. [Google Scholar] [CrossRef]
- Jacob, J.; Palat, G.; Verghese, N.; Chandran, P.; Rapelli, V.; Kumari, S.; Malhotra, C.; Teo, I.; Finkelstein, E. Health-related quality of life and its socio-economic and cultural predictors among advanced cancer patients: Evidence from the APPROACH cross-sectional survey in Hyderabad-India. BMC Palliat. Care 2019, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, V.C.; Martin, P.; Lewandowski, P.A. Cancer cachexia: Impact, mechanisms and emerging treatments. J. Cachexia Sarcopenia Muscle 2013, 4, 95–109. [Google Scholar] [CrossRef]
- Montgomery, B.; Nelson, P.S.; Vessella, R.; Kalhorn, T.; Hess, D.; Corey, E. Estradiol suppresses tissue androgens and prostate cancer growth in castration resistant prostate cancer. BMC Cancer 2010, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Kroenke, C.H.; Bradshaw, P.T.; Chen, W.Y.; Prado, C.M.; Weltzien, E.K.; Castillo, A.L.; Caan, B.J. Postdiagnosis Weight Change and Survival Following a Diagnosis of Early-Stage Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jiralerspong, S.; Goodwin, P.J. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J. Clin. Oncol. 2016, 34, 4203–4216. [Google Scholar] [CrossRef]
- Xu, M.C.; Huelster, H.L.; Hatcher, J.B.; Avulova, S.; Stocks, B.T.; Glaser, Z.A.; Moses, K.A.; Silver, H.J. Obesity is Associated with Longer Survival Independent of Sarcopenia and Myosteatosis in Metastatic and/or Castrate-Resistant Prostate Cancer. J. Urol. 2021, 205, 800–805. [Google Scholar] [CrossRef]
- Beaumont, J.L.; Butt, Z.; Li, R.; Cella, D. Meaningful differences and validity for the NCCN/FACT-P Symptom Index: An analysis of the ALSYMPCA data. Cancer 2019, 125, 1877–1885. [Google Scholar] [CrossRef]
- James, C.; Brunckhorst, O.; Eymech, O.; Stewart, R.; Dasgupta, P.; Ahmed, K. Fear of cancer recurrence and PSA anxiety in patients with prostate cancer: A systematic review. Support. Care Cancer 2022, 30, 5577–5589. [Google Scholar] [CrossRef] [PubMed]
- Assayag, J.; Kim, C.; Chu, H.; Webster, J. The prognostic value of Eastern Cooperative Oncology Group performance status on overall survival among patients with metastatic prostate cancer: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1194718. [Google Scholar] [CrossRef]
- Jeon, H.; Eo, W.; Shim, B.; Kim, S.; Lee, S. Prognostic Value of Functional Assessment of Cancer Therapy-General (FACT-G) in Advanced Non-Small-Cell Lung Cancer Treated with Korean Medicine. Evid. Based Complement. Altern. Med. 2020, 2020, 2845401. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.C.; Murphy, R.F.; Matar, H.E.; Almerie, M.Q. Quality of life in patients with prostate cancer: Development and application of a hybrid assessment method. Prostate Cancer Prostatic Dis. 2009, 12, 72–77. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Characteristic | 1M | Overall, N = 84 |
|---|---|---|
| Age, Median (IQR) | 66 (59–71) | |
| Area, n (%) | ||
| Rural | 29 (35) | |
| Urban | 55 (65) | |
| Education, n (%) | ||
| Illiterate | 8 (9.5) | |
| Schooling | 47 (56) | |
| Graduate and above | 29 (35) | |
| Duration of Disease (Months), Median (IQR) | 10 (12) | 11 (4–38) |
| Comorbidities, n (%) | ||
| Diabetes Mellitus, n (%) | 15 (18) | |
| Hypertension, n (%) | 29 (35) | |
| Hypothyroidism, n (%) | 2 (2.4) | |
| Coronary Artery Disease, n (%) | 5 (6.0) | |
| Chronic Kidney Disease, n (%) | 1 (1.2) | |
| Stroke and TIAs, n (%) | 2 (2.4) | |
| Other (AIDS, Hepatitis B, Ca Bladder, Hernia), n (%) | 4 (4.8) | |
| Number of Comorbidities, n (%) | ||
| None | 44 (52) | |
| 1 Comorbidity | 29 (35) | |
| >1 Comorbidity | 11 (13) | |
| Tobacco (Smokeless), n (%) | 49 (58) | |
| Smoking, n (%) | 30 (36) | |
| Alcohol, n (%) | 25 (30) | |
| Type of Metastasis, n (%) | 5 (6.0) | |
| Bony metastasis | 22 (28) | |
| Visceral metastasis | 9 (11) | |
| Bony and Visceral metastasis | 48 (61) | |
| Accessibility to AIIMS (kilometers), Median (IQR) | 2 (2.4) | 70 (21–400) |
| BMI, n (%) | ||
| Underweight (< 18.5) | 6 (7.1) | |
| Normal (18.5–22.9) | 25 (30) | |
| Overweight (23–24.9) | 14 (17) | |
| Obese (> 25) | 39 (46) | |
| ECOG Status, n (%) | 4 (4.8) | |
| ECOG (0–1) | 47 (59) | |
| ECOG (2–3) | 33 (41) | |
| PSA (ng/mL), Median (IQR) | 3 (3.6) | 7 (1–71) |
| Gleason Score, n (%) | 6 (7.1) | |
| 6 and 7, Low and Medium Risk | 19 (24) | |
| 8, High Risk | 23 (29) | |
| 9 and 10, High Risk | 36 (46) | |
| Castration Sensitivity, n (%) | 5 (6.0) | |
| Castration-Sensitive, CSPC | 39 (49) | |
| Castration-Resistant, CRPC | 40 (51) | |
| Type of ADT, n (%) | 6 (7.1) | |
| Medical | 24 (31) | |
| Surgical | 54 (69) | |
| Risk-Stratification, n (%) | 5 (6.0) | |
| Low-risk | 28 (35) | |
| High-risk | 51 (65) | |
| Burden of Disease, n (%) ¶ | 5 (6.0) | |
| Low-volume | 30 (38) | |
| High-volume | 49 (62) | |
| Number of Treatment Lines, n (%) | 5 (6.0) | |
| 1 Line of Treatment | 47 (59) | |
| >1 Lines of Treatment | 32 (41) | |
| 1st Line Treatment Received, n (%) | 10 (12) | |
| ADT + Abiraterone | 53 (72) | |
| ADT + Docetaxel | 17 (23) | |
| ADT + Enzalutamide | 2 (2.7) | |
| ADT + Fosfosterol | 2 (2.7) | |
| Treatment at the Time of Assessment, n (%) | 10 (12) | |
| ADT + Abiraterone | 51 (69) | |
| ADT + Docetaxel | 17 (23) | |
| ADT + Enzalutamide | 5 (6.8) | |
| ADT + Cabazitaxel | 1 (1.4) | |
| Duration of 1st Line Treatment (Months), Median (IQR) | 13 (15) | 12 (5–21) |
| Current Status, n (%) | ||
| Alive | 45 (54) | |
| Dead | 39 (46) |
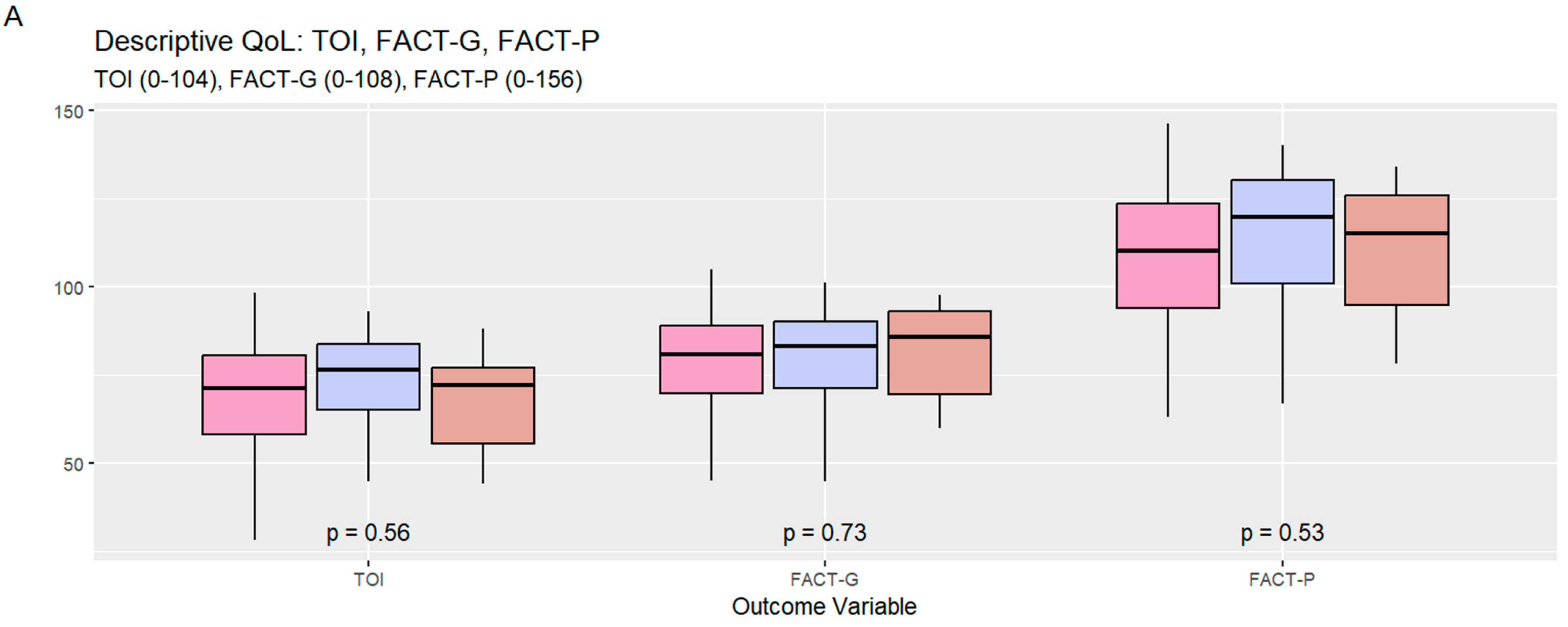
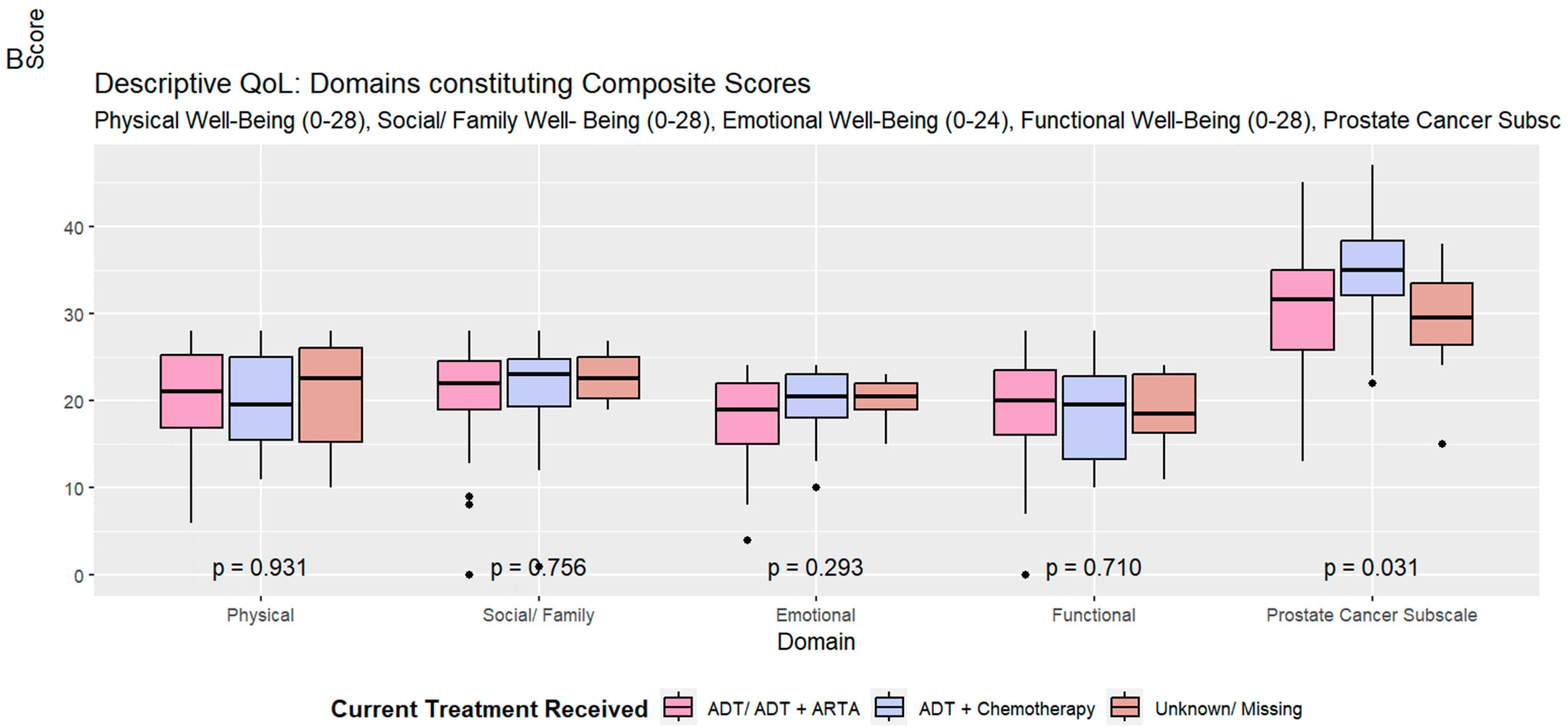
| Characteristic | Overall, N = 84 | ADT + ARTA, N = 56 | ADT + Chemotherapy, N = 18 | Unknown/Missing, N = 10 | p-Value 1 |
|---|---|---|---|---|---|
| Physical Well-Being (0–28) | 0.93 | ||||
| Mean (SD) | 20.41 (5.69) | 20.37 (5.64) | 20.31 (5.75) | 20.82 (6.48) | |
| Social/Family Well-Being (0–28) | 0.76 | ||||
| Mean (SD) | 21.24 (5.46) | 20.98 (5.46) | 21.29 (6.63) | 22.60 (2.74) | |
| Emotional Well-Being (0–24) | 0.29 | ||||
| Mean (SD) | 18.70 (4.39) | 18.10 (4.70) | 19.78 (3.90) | 20.10 (2.64) | |
| Functional Well-Being (0–28) | 0.71 | ||||
| Mean (SD) | 18.90 (5.60) | 19.14 (5.77) | 18.43 (5.69) | 18.40 (4.88) | |
| Prostate Cancer Subscale (0–48) | 0.031 | ||||
| Mean (SD) | 31.02 (7.10) | 30.15 (7.09) | 34.82 (6.36) | 29.06 (6.57) | |
| FACT-P TOI (Trial Outcome Index) (0–104) | 0.56 | ||||
| Mean (SD) | 70.33 (15.16) | 69.66 (15.49) | 73.56 (14.33) | 68.28 (15.43) | |
| FACT-G (General Cancer) (0–108) | 0.73 | ||||
| Mean (SD) | 79.25 (15.02) | 78.59 (15.27) | 79.81 (15.23) | 81.92 (14.33) | |
| FACT-P (Prostate Cancer) (0–156) | 0.53 | ||||
| Mean (SD) | 110.27 (20.18) | 108.74 (20.46) | 114.62 (20.02) | 110.98 (19.63) |
| Characteristic | TOI | FACT-G | FACT-P | |||
|---|---|---|---|---|---|---|
| Beta (95% CI) 1 | p-Value | Beta (95% CI) 1 | p-Value | Beta (95% CI) 1 | p-Value | |
| Age | −0.14 (−0.51 to 0.23) | 0.44 | −0.05 (−0.42 to 0.32) | 0.78 | −0.17 (−0.67 to 0.32) | 0.49 |
| Area | 0.72 | 0.95 | 0.82 | |||
| Rural | — | — | — | |||
| Urban | 1.3 (−5.7 to 8.2) | −0.21 (−7.1 to 6.7) | −1.0 (−10 to 8.2) | |||
| Accessibility to AIIMS with respect to Median Distance | 0.13 | 0.92 | 0.60 | |||
| Lesser/Equal | — | — | — | |||
| Greater | −5.1 (−12 to 1.6) | −0.33 (−7.0 to 6.3) | −2.3 (−11 to 6.6) | |||
| Education | 0.97 | 0.67 | 0.93 | |||
| Illiterate | — | — | — | |||
| Schooling | 0.36 (−11 to 12) | 2.6 (−8.9 to 14) | 1.2 (−14 to 17) | |||
| Graduate and above | 1.2 (−11 to 13) | 4.9 (−7.1 to 17) | 2.6 (−14 to 19) | |||
| Duration of Disease with respect to Median Duration | 0.88 | 0.63 | 0.61 | |||
| Lesser/Equal | — | — | — | |||
| Greater | 0.55 (−6.6 to 7.8) | 1.7 (−5.4 to 8.9) | 2.4 (−7.1 to 12) | |||
| Number of Comorbidities | 0.63 | 0.81 | 0.87 | |||
| None | — | — | — | |||
| 1 Comorbidity | 0.65 (−6.6 to 7.9) | 2.3 (−4.9 to 9.5) | 1.2 (−8.5 to 11) | |||
| >1 Comorbidity | −4.4 (−15 to 5.8) | 0.33 (−9.8 to 10) | −2.7 (−16 to 11) | |||
| Tobacco (Smokeless) | −2.6 (−9.3 to 4.1) | 0.44 | −0.68 (−7.3 to 6.0) | 0.84 | −0.66 (−9.6 to 8.3) | 0.88 |
| Alcohol | 0.54 (−6.7 to 7.8) | 0.88 | 2.4 (−4.7 to 9.6) | 0.50 | 0.93 (−8.7 to 11) | 0.85 |
| Type of Metastasis | 0.23 | 0.48 | 0.32 | |||
| Bony metastasis | — | — | — | |||
| Visceral metastasis | −10 (−22 to 1.5) | −7.2 (−19 to 4.7) | −12 (−28 to 3.7) | |||
| Bony and Visceral metastasis | −3.2 (−11 to 4.4) | −1.4 (−9.1 to 6.3) | −3.7 (−14 to 6.5) | |||
| Obese: BMI > 25 | 6.8 (0.31 to 13) | 0.040 | 6.5 (0.05 to 13) | 0.048 | 9.6 (1.0 to 18) | 0.028 |
| ECOG | 0.072 | 0.44 | 0.36 | |||
| ECOG (0–1) | — | — | — | |||
| ECOG (2–3) | −6.2 (−13 to 0.57) | −2.7 (−9.5 to 4.1) | −4.2 (−13 to 4.9) | |||
| PSA | −0.01 (−0.01 to 0.00) | 0.16 | −0.01 (−0.02 to 0.00) | 0.029 | −0.01 (−0.02 to 0.00) | 0.062 |
| Gleason Score Category | 0.96 | 0.44 | 0.79 | |||
| 6 and 7, Low and Medium Risk | — | — | — | |||
| 8, High Risk | −0.36 (−9.8 to 9.1) | −4.0 (−13 to 5.3) | −2.9 (−16 to 9.8) | |||
| 9 and 10, High Risk | 0.75 (−7.9 to 9.4) | 1.1 (−7.5 to 9.7) | 0.78 (−11 to 12) | |||
| Castration Sensitivity | 0.15 | 0.40 | 0.30 | |||
| Castration-Sensitive, CSPC | — | — | — | |||
| Castration-Resistant, CRPC | −5.0 (−12 to 1.9) | −2.9 (−9.7 to 3.9) | −4.9 (−14 to 4.3) | |||
| Type of ADT Received | 0.53 | 0.67 | 0.85 | |||
| Medical | — | — | — | |||
| Surgical | −2.4 (−10 to 5.2) | 1.6 (−5.9 to 9.1) | 0.95 (−9.1 to 11) | |||
| Burden of Disease | 0.37 | 0.24 | 0.24 | |||
| Low-volume | — | — | — | |||
| High-volume | −3.2 (−10 to 3.9) | −4.2 (−11 to 2.8) | −5.6 (−15 to 3.9) | |||
| Number of Treatment Lines Received | 0.60 | 0.72 | 0.73 | |||
| 1 Line of Treatment | — | — | — | |||
| >1 Lines of Treatment | −1.9 (−8.9 to 5.2) | −1.3 (−8.2 to 5.7) | −1.7 (−11 to 7.8) | |||
| 1st Line Treatment Received | 0.053 | 0.055 | 0.065 | |||
| Abiraterone | — | — | — | |||
| Docetaxel | −9.7 (−18 to −1.6) | −9.8 (−18 to −1.7) | −12 (−23 to −1.1) | |||
| Enzalutamide | 5.5 (−16 to 27) | 3.5 (−18 to 25) | 7.3 (−21 to 36) | |||
| Fosfosterol | 12 (−9.0 to 33) | 12 (−8.8 to 33) | 18 (−10 to 47) | |||
| Duration of First-Line Treatment Received | 0.28 (0.04 to 0.52) | 0.020 | 0.19 (−0.06 to 0.43) | 0.13 | 0.31 (−0.02 to 0.63) | 0.063 |
| Current Treatment Received | 0.35 | 0.77 | 0.29 | |||
| ADT + ARTA | — | — | — | |||
| ADT + Chemotherapy | 3.9 (−4.3 to 12) | 1.2 (−7.0 to 9.5) | 5.9 (−5.1 to 17) | |||
| Characteristic | TOI | FACT-G | FACT-P | |||
|---|---|---|---|---|---|---|
| Beta (95% CI) 1 | p-Value | Beta (95% CI) 1 | p-Value | Beta (95% CI) 1 | p-Value | |
| Age | −0.28 (−0.71 to 0.15) | 0.20 | −0.17 (−0.62 to 0.28) | 0.45 | −0.40 (−0.99 to 0.20) | 0.19 |
| Number of Comorbidities | 0.35 | 0.96 | 0.69 | |||
| None | — | — | — | |||
| 1 Comorbidity | −3.0 (−11 to 5.3) | −0.54 (−9.1 to 8.1) | −2.2 (−14 to 9.2) | |||
| >1 Comorbidity | −7.9 (−19 to 3.5) | −1.7 (−14 to 10) | −6.5 (−22 to 9.1) | |||
| Type of Metastasis | 0.31 | 0.60 | 0.55 | |||
| Bony metastasis | — | — | — | |||
| Visceral metastasis | −10 (−23 to 3.1) | −5.8 (−19 to 7.8) | −9.5 (−27 to 8.4) | |||
| Bony and Visceral metastasis | −2.0 (−10 to 6.1) | 0.44 (−8.0 to 8.8) | −1.3 (−12 to 9.8) | |||
| Obese: BMI > 25 | 8.2 (1.2 to 15) | 0.022 | 7.2 (−0.12 to 14) | 0.054 | 11 (1.6 to 21) | 0.023 |
| ECOG | −4.9 (−12 to 2.6) | 0.19 | −2.7 (−10 to 5.1) | 0.49 | −3.1 (−13 to 7.2) | 0.55 |
| PSA | −0.06 (−0.14 to 0.03) | 0.19 | −0.08 (−0.17 to 0.00) | 0.061 | −0.09 (−0.21 to 0.03) | 0.12 |
| 1st Line Treatment Received | 0.11 | 0.13 | 0.15 | |||
| Abiraterone | — | — | — | |||
| Docetaxel | −11 (−20 to −1.9) | −11 (−20 to −1.8) | −14 (−26 to −1.8) | |||
| Enzalutamide | 4.9 (−26 to 35) | 2.8 (−29 to 35) | 8.4 (−34 to 50) | |||
| Fosfosterol | −3.7 (−28 to 20) | 4.7 (−20 to 30) | 1.0 (−32 to 34) | |||
| Duration of First-Line Treatment Received | 0.14 (−0.16 to 0.44) | 0.35 | 0.04 (−0.27 to 0.35) | 0.81 | 0.12 (−0.29 to 0.53) | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, C.; Gupta, H.; Nagpal, C.; Sahoo, R.K.; Sharma, A.; Gangadharaiah, B.B.; Tansir, G.; Panaiyadiyan, S.; Shamim, S.A.; Kaushal, S.; et al. Quality of Life Determinants in Patients with Metastatic Prostate Cancer: Insights from a Cross-Sectional Questionnaire-Based Study. Curr. Oncol. 2024, 31, 4940-4954. https://doi.org/10.3390/curroncol31090366
Mittal C, Gupta H, Nagpal C, Sahoo RK, Sharma A, Gangadharaiah BB, Tansir G, Panaiyadiyan S, Shamim SA, Kaushal S, et al. Quality of Life Determinants in Patients with Metastatic Prostate Cancer: Insights from a Cross-Sectional Questionnaire-Based Study. Current Oncology. 2024; 31(9):4940-4954. https://doi.org/10.3390/curroncol31090366
Chicago/Turabian StyleMittal, Chetanya, Hardik Gupta, Chitrakshi Nagpal, Ranjit K. Sahoo, Aparna Sharma, Bharat B. Gangadharaiah, Ghazal Tansir, Sridhar Panaiyadiyan, Shamim A. Shamim, Seema Kaushal, and et al. 2024. "Quality of Life Determinants in Patients with Metastatic Prostate Cancer: Insights from a Cross-Sectional Questionnaire-Based Study" Current Oncology 31, no. 9: 4940-4954. https://doi.org/10.3390/curroncol31090366
APA StyleMittal, C., Gupta, H., Nagpal, C., Sahoo, R. K., Sharma, A., Gangadharaiah, B. B., Tansir, G., Panaiyadiyan, S., Shamim, S. A., Kaushal, S., Das, C. J., Haresh, K. P., Seth, A., Nayak, B., & Batra, A. (2024). Quality of Life Determinants in Patients with Metastatic Prostate Cancer: Insights from a Cross-Sectional Questionnaire-Based Study. Current Oncology, 31(9), 4940-4954. https://doi.org/10.3390/curroncol31090366






