Abstract
Neuroblastoma is a pediatric cancer with significant clinical heterogeneity. Despite extensive efforts, it is still difficult to cure children with high-risk neuroblastoma. Immunotherapy is a promising approach to treat children with this devastating disease. We have previously reported that macrophages are important effector cells in high-risk neuroblastoma. In this perspective article, we discuss the potential function of the macrophage inhibitory receptor SIRPA in the homeostasis of tumor-associated macrophages in high-risk neuroblastoma. The ligand of SIRPA is CD47, known as a “don’t eat me” signal, which is highly expressed on cancer cells compared to normal cells. CD47 is expressed on both tumor and stroma cells, whereas SIRPA expression is restricted to macrophages in high-risk neuroblastoma tissues. Notably, high SIRPA expression is associated with better disease outcome. According to the current paradigm, the interaction between CD47 on tumor cells and SIRPA on macrophages leads to the inhibition of tumor phagocytosis. However, data from recent clinical trials have called into question the use of anti-CD47 antibodies for the treatment of adult and pediatric cancers. The restricted expression of SIRPA on macrophages in many tissues argues for targeting SIRPA on macrophages rather than CD47 in CD47/SIRPA blockade therapy. Based on the data available to date, we propose that disruption of the CD47-SIRPA interaction by anti-CD47 antibody would shift the macrophage polarization status from M1 to M2, which is inferred from the 1998 study by Timms et al. In contrast, the anti-SIRPA F(ab’)2 lacking Fc binds to SIRPA on the macrophage, mimics the CD47-SIRPA interaction, and thus maintains M1 polarization. Anti-SIRPA F(ab’)2 also prevents the binding of CD47 to SIRPA, thereby blocking the “don’t eat me” signal. The addition of tumor-opsonizing and macrophage-activating antibodies is expected to enhance active tumor phagocytosis.
1. Introduction
Neuroblastoma is a common pediatric solid tumor of neural crest origin. Neuroblastoma exhibits significant clinical heterogeneity. While some neuroblastomas are low risk and curable, high-risk neuroblastomas progress relentlessly despite the current multimodal high-intensity therapy. The Children’s Oncology Group defines high-risk neuroblastomas using several established clinical indicators, including the International Neuroblastoma Risk Group Staging System, age at diagnosis, MYCN amplification status, International Neuroblastoma Pathology Classification, the presence or absence of segmental chromosomal aberrations, and ploidy [1]. Based on this classification, about 50% of high-risk cases are MYCN amplified, and the rest are non-MYCN amplified. Due to the resistance to current therapy, long-term survival of high-risk neuroblastoma patients has remained below 50% for three decades. Moreover, the current therapy for high-risk neuroblastoma causes considerable adverse side effects. Alternative and effective treatments with low toxicity are needed to treat these patients. Immunotherapy has shown great promise for the treatment of adult tumors [2,3]. However, using immunotherapy as an approach to treat high-risk neuroblastoma has its own challenges. High-risk neuroblastoma cells lack classical human leukocyte antigen (HLA) class I expression [4], and therefore, CD8+ T cell-mediated immune surveillance is considered ineffective for disease control. In addition, the infiltration of NK cells indicated by the expression of NK-specific marker genes is the lowest among immune cells present in high-risk neuroblastoma tissues [5,6]. Consistent with these observations, high-risk neuroblastoma cells do not express the immune checkpoint molecule PD-L1 [5] that controls the activity of PD-1 positive CD8+ T and NK cells [7,8]. Conversely, the majority of PD-L1 positive cells found in high-risk neuroblastoma tissues are macrophages [5]. Subsequently, we have reported macrophages as important immune effector cells in high-risk neuroblastoma [6].
Macrophages are professional phagocytic cells and the major phagocyte population resident in most normal tissues at homeostasis. The activation and phagocytic function of macrophages are tightly regulated to avoid host tissue damage and their self-engagement. Macrophages express a wide spectrum of receptors, which are essential to their immune functions and their vital homeostatic role [9]. Therapeutic strategies for malignant diseases have also exploited the presence of activating and inhibitory receptors on macrophages to promote macrophage-mediated tumor clearance [10].
2. Plasticity of Macrophages and Heterogeneity of Tumor-Associated Macrophages
Tumor-associated macrophages (TAMs) arise primarily from bone marrow-derived monocytes, which are recruited to the tumor microenvironment (TME) by the tumor or stroma-derived chemokines [11,12]. As the tumors grow, the TME greatly influences the phenotype of TAMs. The plasticity of macrophages represents their ability to adjust their phenotype in response to various environmental cues, such as cytokines, tissue metabolites, and interactions with other cells [9]. The plasticity of macrophages also gives rise to various subpopulations of macrophages with different phenotypes and functions [9], and the functional effect of the same type of macrophages can be beneficial or detrimental to the host depending on the disease context. Classically activated M1 macrophages exhibit anti-tumor immunity, but they are key mediators involved in immunopathological processes of several autoimmune diseases [9,13]. Alternatively activated M2 macrophages that are important in tissue injury can phagocytose debris, promote wound healing, and antagonize destructive inflammation, but they are pro-tumor in cancers [14]. Although macrophages are relatively abundant in the TME of most solid tumors, they are considered mostly in the immunosuppressive form or with the pro-tumor M2 phenotype [15]. Preserving the anti-tumor M1 TAMs in the TME is thus a key strategy for immunotherapy against solid tumors. In fact, significant efforts have been made in the reprogramming of TAMs from pro-tumor M2 to anti-tumor M1. However, these therapeutic strategies have only shown limited efficacy in human clinical trials against solid tumors [16].
This report is our effort to further explore the role of macrophages as immune effector cells in high-risk neuroblastoma. Below, we will describe the polarization status of macrophages versus their phagocytic capacity in high-risk neuroblastoma at diagnosis and the CD47-SIRPA axis in cancer immunotherapy. In addition, we will present our perspective on (i) the potential function of the macrophage inhibitory receptor signal regulatory protein alpha (SIRPA or SIRPα) in the homeostasis of tumor-associated macrophages in high-risk neuroblastoma, (ii) how to optimally enhance tumor phagocytosis by macrophages in high-risk neuroblastoma by targeting SIRPA and activating the macrophages, and (iii) recent clinical trials with anti-CD47 antibodies in human adult and pediatric cancers.
3. Macrophage Polarization Status and Phagocytic Capacity in High-Risk Neuroblastoma
The immune microenvironment is unique to individual tumors among high-risk neuroblastomas [5,6], and thus, immunotherapy requires a comprehensive understanding of the composition and phenotypic states of intratumoral immune cells. Of these, macrophages are among the most relevant immune cells in high-risk neuroblastoma because of their predominant presence in the tumor [6]. We were particularly interested in the phenotype and the phagocytic capacity of TAMs in high-risk neuroblastoma. Previously, we reported that most M2-like TAMs could still function as anti-tumor phagocytes in high-risk neuroblastomas at diagnosis [6]. To further explore this, we performed additional analyses on the gene expression profile dataset (n = 176) of high-risk neuroblastomas collected at diagnosis [17,18]. As shown in Figure S1, the expression of cytokines, lymphokines, and enzymes for bioresponse modification that influenced the polarization of TAMs were detected in tumor tissues. Furthermore, expression levels of M2 polarization-related genes (CSF1, IL33, IL34, TGFB1, VEGFA, ARG2) were higher than those of M1 polarization-related genes (IL1B, IL6, TNF, NOS2) (Figure S1A). Most hypoxia signature genes reported [19] were also expressed at elevated levels (Figure S1B). These observations suggest that macrophages in high-risk neuroblastoma tissues are in the M2-dominant state. In addition, as shown in Figure S1C, the expression of both CD68 and CD163 was significantly correlated with the expression of phagocytosis-related genes [20,21] and macrophage activation marker genes (CD38 and CXCL10) [22,23], suggesting that macrophages in high-risk neuroblastoma tissues at diagnosis retain their phagocytic capacity. Therefore, therapeutic strategies that promote tumor phagocytosis by TAMs beyond diagnosis are essential.
4. The CD47-SIRPA Axis in Cancer Immunotherapy
SIRPA is a macrophage inhibitory receptor, which has a restricted expression pattern to myeloid and neuronal cells [24,25]. SIRPA is also known by a variety of names such as BIT, CD172a, MFR, and SHPS-1 [25,26]. SIRPA is expressed in all myeloid cells including monocytes, macrophages, neutrophils, a subset of dendritic cells, and microglia [24,27,28]. The ligand of SIRPA is CD47, which is known as a “don’t eat me” signal and is highly expressed on cancer cells compared with normal cells. Based on the current paradigm, the interaction between CD47 on tumor cells and SIRPA on macrophages leads to inhibition of tumor phagocytosis [29,30,31,32]. Others have reported the therapeutic efficacy of CD47 blockade with anti-CD47 antibody on various cancers in preclinical models [33,34,35,36,37,38]. In fact, among the cancer types studied, leukemia is the most susceptible to the anti-CD47 antibody treatment [33,35]. In addition, it has been reported that CD47/SIRPA blockade with either SIRPα variants acting as an analogue of anti-CD47 antibody or anti-SIRPA antibody does not effectively induce phagocytosis on its own and requires additional tumor-opsonizing antibodies to be efficacious [39,40,41]. Consistent with this notion, it has been shown recently that the CD47/SIRPA blockade therapy with anti-CD47 antibody in xenograft models of high-risk neuroblastoma requires an additional opsonizing antibody against neuroblastoma, such as anti-GD2 antibody, to be efficacious [42]. As will be described below, recent clinical trials with anti-CD47 antibodies have revealed new challenges regarding the CD47-SIRPA axis in immunotherapy.
5. The CD47-SIRPA Axis in High-Risk Neuroblastoma
5.1. CD47 Expression in High-Risk Neuroblastoma
To determine the cell types expressing CD47 in high-risk neuroblastoma tissues, we performed immunohistochemical analysis on six high-risk neuroblastoma specimens. Our data showed that both stroma cells and tumor cells express CD47. In addition, among the six high-risk neuroblastoma tumors examined, we observed a difference in CD47 staining in high-risk neuroblastoma with MYCN amplification vs. those without MYCN amplification. As shown in Figure 1A, among high-risk neuroblastoma with MYCN amplification, weak cell membrane staining of CD47 was detected and high CD47 expression was detected on neurites of the tumor cells. In contrast, among non-MYCN amplified tumors, high expression of CD47 was detected on both cell membrane and neurites of the tumor cells (Figure 1A). CD47 staining was also detected in the vasculature of high-risk neuroblastoma (Figure S2). For comparison, we examined CD47 expression in favorable histology neuroblastoma. Weak cell membrane staining of CD47 and strong CD47 staining on neurites of the favorable tumor cells were observed (Figure S3).
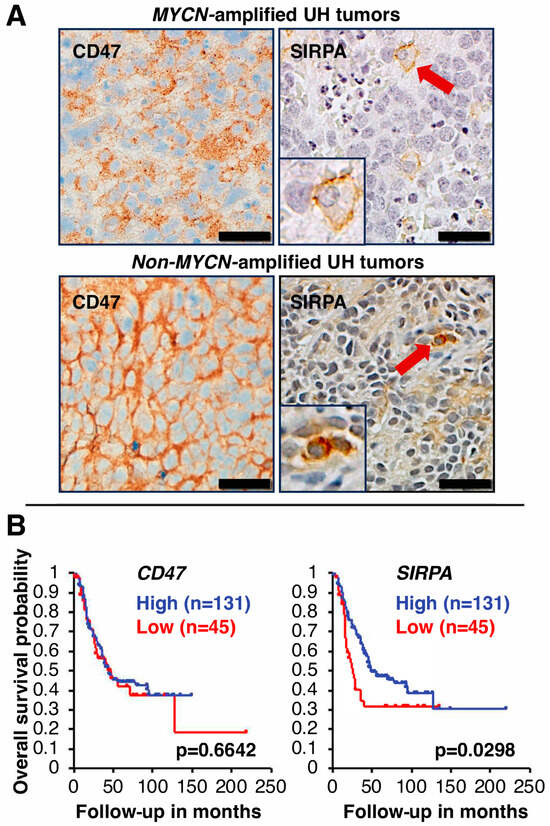
Figure 1.
(A) CD47 and SIRPA expression in high-risk neuroblastoma tissues. Immunohistochemical analysis was performed on high-risk neuroblastoma specimens (three tumors with MYCN amplification and the other three tumors without MYCN amplification). Representative images are presented. In the high-risk tumor with MYCN amplification, weak cell membrane staining of CD47 was detected, and high CD47 expression was detected on neurites of the tumor cells. In contrast, high expression of CD47 was detected on both cell membrane and neurites of the tumor cells without MYCN amplification. Some blood vessels were also stained positive for CD47 (see Figure S2). Source of anti-CD47 antibody: Clone SP279 (Abcam). SIRPA expression was restricted to macrophages in high-risk neuroblastoma tissues. Source of anti-SIRPA antibody: Clone D6I3M (Cell Signaling Technology). Red arrows indicate SIRPA-positive macrophages in tumor tissues. Insets show images of a fully differentiated macrophage (the upper panel) and an early/differentiating macrophage (the lower panel) with 2-fold magnification of the original. Scale bars: 20 µm. UH: unfavorable histology. (B) Clinical implication of CD47 and SIRPA expression in high-risk neuroblastoma. Survival of high-risk neuroblastoma patients with high or low expression of CD47 or SIRPA was analyzed by the R2 Genomics Analysis and Visualization Platform (http://r2.amc.nl, (accessed on 4 November 2023) [43] using the high-risk subset of the SEQC dataset [17,18]. The cohort was split into two groups based on gene expression levels, with the cutoff for the first group set at the first quartile. There was no correlation between CD47 expression and outcome of high-risk neuroblastoma. In contrast, high SIRPA expression was significantly associated with better outcome of high-risk neuroblastoma.
5.2. SIRPA Expression in High-Risk Neuroblastoma
We next investigated the expression of SIRPA in high-risk neuroblastoma tissues by immunohistochemical analysis (Figure 1A). In contrast to CD47 expression, among the six high-risk neuroblastoma tumors examined, SIRPA expression was mainly observed in macrophages. Macrophages expressing SIRPA were rather dispersed throughout tumor tissues, but a higher density of smaller SIRPA-positive macrophages at necrotic areas was noticed, suggesting that these macrophages were newly recruited from the circulation in response to tumor-derived chemotactic factors [5,44]. In favorable histology tumor tissues, we also observed SIRPA-positive macrophages (Figure S3).
5.3. Prognostic Significance of CD47 and SIRPA Expression in High-Risk Neuroblastoma
To evaluate the prognostic significance of CD47 and SIRPA expression in high-risk neuroblastoma, Kaplan–Meier survival analysis was performed on the high-risk neuroblastoma cohort [17,18]. As described above, CD47 was widely expressed in various cell types (tumor cells and stroma cells) in high-risk neuroblastoma tissues. As expected, there was no correlation between CD47 expression and disease outcome of high-risk neuroblastoma (Figure 1B). In contrast, the survival analysis showed that 131 cases with high SIRPA expression (hazard ratio = 0.61) exhibited significantly better outcome (p = 0.0298) than those (n = 45) with low SIRPA expression (hazard ratio = 1.63) (Figure 1B). Based on these data, patients bearing tumors with high SIRPA expression had better outcome likely due to more tumor-infiltrating macrophages in the TME, and furthermore, these macrophages were still in the M1-like TAM status and/or M2 TAMs that were able to phagocytose the tumor as suggested by our previous study [6] and Figure S1C.
It should be mentioned that the prognostic significance of SIRPA expression is inconsistent across different cancer types. It has been reported that high SIRPA expression is a favorable factor in colorectal cancer [45], but it is associated with poor prognosis in diffuse large B-cell lymphoma, follicular lymphoma, non-small cell lung cancer, and esophageal carcinoma [46,47,48,49]. A plausible explanation for these opposing findings is that the M1/M2 TAM ratio and/or the tumor-phagocytotic capacity of TAMs in colorectal cancer would likely be higher than those of the second group of tumors where high SIRPA expression correlates with poor outcome.
5.4. A Missing Piece of the Puzzle
The observations described above led to the following two questions. First, why is there a differential therapeutic efficacy of the CD47/SIRPA blockade in leukemia vs. solid tumors? Second, what is the underlying mechanism for high expression of an inhibitory receptor on macrophages (i.e., SIRPA) being associated with a better disease outcome in high-risk neuroblastoma? Notably, the study by Timms et al. [50] published in 1998 sheds light on the answers to these questions. These authors have shown that upon interaction with CD47, SIRPA recruits phosphatases SHP1/2 to its cytoplasmic domain, and SIRPA-SHP1/2 together bind to CSF1R, forming the multi-molecular complex (SIRPA-SHP1/2-CSF1R) to inhibit the CSF1R signaling [50]. To date, CSF1R activation via CSF1 or IL-34 is known to be required for the M1 to M2 polarization of macrophages [51,52,53,54,55]. The finding by Timms et al. was ahead of its time because the role of CSF1R signaling in M2 polarization was not yet recognized. Because of this, the significance of the original finding by Timms et al. with respect to the macrophage polarization has been left unacknowledged for decades. Consequently, the mechanism underlying the CD47/SIRPA blockade is not fully understood.
5.5. A Perspective on the Biological Significance of the CD47-SIRPA Axis in Cancer Immunotherapy and Influences of the CD47/SIRPA Blockade on Macrophage Polarization
Based on the current paradigm, disruption of the CD47-SIRPA interaction by either anti-CD47 or anti-SIRPA antibody would inhibit the “don’t eat me” signal and thus promote tumor phagocytosis. However, the current paradigm cannot explain the following. First, the CD47/SIRPA blockade cannot effectively induce phagocytosis on its own when the cancer cells reside in the tumor mass in which TAMs exist. Second, the CD47 blockade is more efficacious in leukemia. Notably, leukemia cells circulate naturally in the blood and will be destroyed by macrophages in the spleen upon administration of the CD47 blockade treatment. To gain further insight into this dilemma and to explain the clinical observation shown in Figure 1B, we incorporate the observation made by Timms et al. [50] and hypothesize that engagement of SIRPA by its partner in some forms (e.g., CD47, anti-SIRPA F(ab’)2 lacking FcγR binding) is critical to M1 polarization. Disruption of the CD47-SIRPA interaction by anti-CD47 antibody shifts macrophage polarization from M1 to M2 status, resulting in insufficient tumor phagocytosis (Figure 2C). In contrast, the anti-SIRPA F(ab’)2 binds to SIRPA on macrophages, prevents engagement of CD47 with SIRPA, which in turn blocks the “don’t eat me” signal. Moreover, engagement of SIRPA by the anti-SIRPA F(ab’)2 preserves the capacity of macrophage as M1 TAMs (Figure 2D). Additional macrophage-activating signals via tumor-opsonizing antibodies, such as anti-GD2, would augment active tumor phagocytosis by the M1 TAMs.
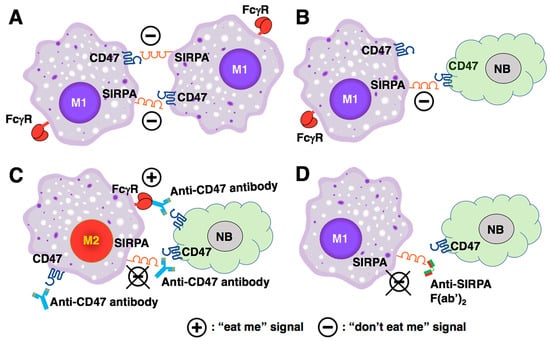
Figure 2.
Biological significance of the CD47-SIRPA axis in macrophage homeostasis and the influences of CD47/SIRPA blockade on macrophage polarization. (A) Under normal physiological conditions, macrophages co-express CD47 and SIRPA. The CD47-SIRPA interaction facilitates macrophages clustering but also prevents macrophages from self-destruction [26]. Moreover, the CD47-SIRPA interaction preserves the phagocytic capacity of M1-like macrophages by inhibiting the CSF1R signaling (see also (D)). (B) By engaging SIRPA on macrophages, CD47-positive tumor cells send a “don’t eat me” signal to the macrophages. (C) Anti-CD47 antibody inhibits the “don’t eat me” signal. However, disruption of the CD47-SIRPA interaction by the anti-CD47 antibody leads to M2 polarization because the SIRPA engagement is lost. Although anti-CD47 antibody can mediate the tumor killing by ADCP via its binding to Fcγ receptor on the macrophage, the tumor phagocytosis is insufficient due to the M2 TAMs’ status. In addition, the anti-CD47 antibody that binds to CD47 on the macrophages can also bind to Fcγ receptors on another macrophage via its Fc portion, which in turn can induce the self-engagement and self-destruction of the macrophages. (D) Anti-SIRPA F(ab’)2 binds to SIRPA on macrophage, prevents engagement of CD47 to SIRPA, which in turn blocks the “don’t’ eat me” signal. Furthermore, engagement of SIRPA by anti-SIRPA F(ab’)2 preserves the capacity of macrophage as M1 TAMs. NB: neuroblastoma.
5.6. SLAMF7 as a Potential Target to Activate TAMs in High-Risk Neuroblastoma
It is known that pro-tumor M2 TAMs could persist under the influence of TME-derived TGFβ and glucocorticoid [56,57,58,59], for which anti-SIRPA F(ab’)2 cannot block their actions. In fact, TGFB1 expression was detected in high-risk neuroblastoma tissues (Figure S1A), and most neuroblastomas arise in adrenal glands that produce glucocorticoid. To overcome this, we propose to further activate macrophages by engaging SLAMF7 by F(ab’)2 of the clinically relevant anti-SLAMF7 antibody elotuzumab [60,61]. SLAMF7 is expressed in a variety of immune cells including macrophages [62]. Our previous study [6] and Figure 3 suggest that SLAMF7 is a new therapeutic target for high-risk neuroblastoma. As shown in Figure 3, the immunohistochemistry analysis revealed that SLAMF7 expression was detected on macrophages and a restricted number of high-risk neuroblastoma cells. Moreover, the right image of Figure 3 shows that the SLAMF7-positive high-risk neuroblastoma cells are seen as clusters in the tumor specimen. Within this cluster of the tumor cells, there is a SLAMF7-positive macrophage indicated by the red arrow. The data in Figure 3 are consistent with our previous gene expression analysis which indicates that ~60% of high-risk neuroblastoma likely expressed some levels of SLAMF7 [6]. Because homotypic interaction is a mode of action of SLAMF7, the expression of SLAMF7 on both macrophages and some high-risk neuroblastoma cells supports the idea that SLAMF7 is a potential therapeutic target for killing the tumor cells.
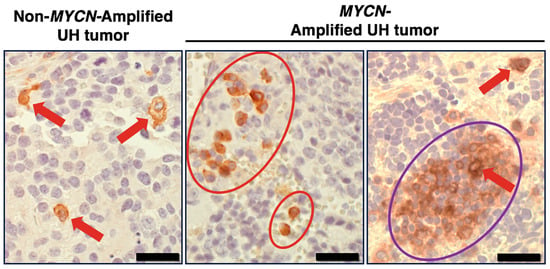
Figure 3.
SLAMF7 expression in high-risk neuroblastoma tissues. Immunohistochemical analysis was performed on high-risk neuroblastoma specimens (three tumors with MYCN amplification and the other three tumors without MYCN amplification). Representative images are presented. In the high-risk tumors examined, SLAMF7 expression was found on macrophages and a restricted number of high-risk neuroblastoma cells. The anti-SLAMF7 antibody, clone EPR21155 (Abcam) was used in the immunohistochemical analysis. Red arrows and red circles indicate macrophages stained by anti-SLAMF7 antibody. The purple circle indicates the SLAMF7-positive high-risk neuroblastoma cells, which are seen as clusters in some tumor specimens. Within this cluster of the tumor cells, there was a SLAMF7-positive macrophage, indicated by the red arrow. UH: unfavorable histology. Scale bars: 20 µm.
5.7. A Proposed Combination Antibody-Based Therapy for High-Risk Neuroblastoma
Figure 4 illustrates a proposed antibody-based therapy and its underlying mechanisms to optimally enhance tumor phagocytosis by macrophages in high-risk neuroblastoma. Notably, TAMs in high-risk neuroblastoma tissues highly expressed the macrophage-activating Fcγ receptor genes FCGR2A and FCGR3A (Figure S1D), and therefore, antibody-mediated approaches are plausible for treating high-risk neuroblastoma patients. As shown in Figure 4, anti-SIRPA F(ab’)2 inhibits the “don’t eat me” signal mediated by the CD47-SIRPA axis, blocks M2 polarization, and preserves M1-like TAMs. To further activate macrophages, anti-SLAMF7 F(ab’)2 can be included as an additional therapeutic agent. To directly target the high-risk neuroblastoma cells, the tumor-bound anti-GD2 antibody will be included as a therapeutic of the combination. Anti-GD2 antibody can mediate tumor killing by macrophages via antibody-dependent cellular phagocytosis (ADCP). Anti-GD2 antibodies are currently used in the standard of care for maintenance therapy in patients with high-risk neuroblastoma post autologous stem cell transplant [63]. These include the dinutuximab [64] and humanized 3F8 or naxitamab [65]. In addition, hu14.18K322A is currently being tested in clinical trials [66].
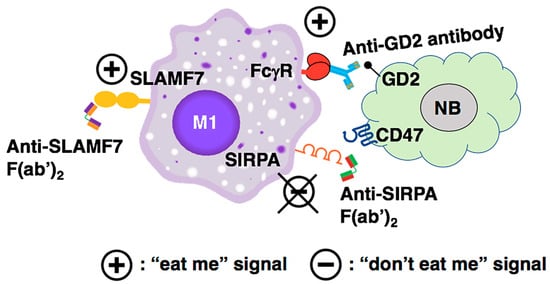
Figure 4.
The mechanism underlying the proposed antibody-mediated immunotherapy to optimally promote tumor phagocytosis by macrophages against high-risk neuroblastoma cells. Anti-SIRPA F(ab’)2 inhibits the “don’t eat me” signal mediated by the CD47-SIRPA axis, blocks M2 polarization, and preserves M1-like TAMs. To further activate macrophages, the anti-SLAMF7 F(ab’)2 can be included as an additional therapeutic agent. To directly target the high-risk neuroblastoma cells, the tumor-bound anti-GD2 antibody is included as a therapeutic of the combination. Anti-GD2 antibody can mediate tumor killing by the macrophages via ADCP.
6. Recent Clinical Trials with Anti-CD47 Antibody Magrolimab in Human Adult and Pediatric Cancers
The current development on the first-generation anti-CD47 antibody magrolimab highlights challenges in targeting the CD47-SIRPA axis for cancer immunotherapy. It has been reported that magrolimab and some other anti-CD47 antibodies in clinical trials cause hemagglutination and platelet aggregation. Furthermore, adverse effects and a lack of survival benefit compared to standard of care have been observed in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) trials. Based on these findings, the FDA has placed all magrolimab studies in AML and MDS on full clinical hold (https://www.targetedonc.com/view/fda-halts-clinical-studies-of-magrolimab-in-aml-mds, (accessed on 15 February 2024)). Gilead Sciences, Inc. the manufacturer of magrolimab, announced that it has paused enrollment globally in the magrolimab solid tumor studies because FDA requested a partial clinical hold on these trials. For the pediatric oncology, the phase 1 National Cancer Institute-sponsored study (NCT04751383) of magrolimab and dinutuximab (Unituxin) in patients with relapsed/refractory neuroblastoma or relapsed osteosarcoma was also suspended due to unacceptable toxicities (https://clinicaltrials.gov/study/NCT04751383, (accessed on 27 March 2024)). Although cancer cells tend to overexpress CD47, most normal cells, including hematopoietic cells, endothelial and epithelial cells, and fibroblasts (Human Protein Atlas) [62], express CD47 as well, which may have resulted in the observed toxicity of magrolimab therapy.
7. Anti-SIRPA Antibodies as Alternatives to Target the CD47-SIRPA Axis
In contrast to CD47, SIRPA expression is restricted to macrophages in many tissues (Human Protein Atlas) [62]. To avoid toxicity of anti-CD47 antibodies due to the ubiquitous expression of CD47, one could target the SIRPA protein on macrophages to inhibit the “don’t eat me” signal, block M2 polarization, and preserve M1-like TAMs (Figure 2D). In fact, several anti-SIRPA antibodies have been in development for cancer immunotherapy [67] and tested in human clinical trials (see Table S1). In addition, several clinically relevant anti-SIRPA antibodies are “pan-allelic” and can bind to most SIRPA variants [68,69,70,71] existing in human populations [69].
Importantly, when antibodies are used to target specific molecules on the macrophage (i.e., SIRPA in this case), the Fc portion of the antibody could also bind to FcγRs on macrophages. This could in turn cause [1] self-engagement among macrophages, and [2] the competition between anti-SIRPA antibodies and other therapeutic antibodies (e.g., anti-GD2) for FcγR binding, which would dampen the therapeutic efficacy of the tumor-opsonizing antibodies. To circumvent this problem, most clinically relevant anti-SIRPA antibodies have been engineered to include mutations in the IgG1 Fc portion to inhibit or reduce their binding to FcγRs [72]. Some anti-SIRPA antibodies are made with IgG2 and IgG4 isoforms that exhibit a naturally low-affinity binding to FcγRs [73,74,75]. Alternatively, the use of anti-SIRPA F(ab’)2 lacking the Fc portion as a therapeutic agent would be a valid option.
In addition to anti-SIRPA F(ab’)2 and the engineered anti-SIRPA antibodies mentioned above, a recombinant CD47 extracellular domain (ED) targeting SIRPA on macrophages could be an alternative therapeutic. However, the natural affinity of CD47 ED against SIRPA is relatively low (a low μM KD range) [76]. Therefore, a free form of the native CD47 ED (not expressed on the cell surface as multivalent forms) would not be suitable for therapeutic applications. Ho et al. have addressed this problem and identified several CD47 ED variants with increased affinities against SIRPA by several hundred-fold [77]. To this end, a high-affinity CD47 ED-silent Fc fusion protein may also be an alternative therapeutic agent to target the CD47-SIRPA axis and effectively promote macrophage-mediated tumor cell phagocytosis.
8. Discussion
Macrophages were discovered as primary immune cells that are always present and ready to engulf and digest microorganisms, providing the first line of defense against infection. To date, macrophages are emerging as an attractive target and focus of cancer immunotherapy due to their relative abundance in the TME of solid tumors, their plasticity, and the numerous receptors expressed on their cell surface [78]. In this article, we discuss our perspective on the CD47/SIRPA blockade therapy in high-risk neuroblastoma and the dual role of the inhibitory receptor SIRPA on macrophages.
As mentioned above, the study by Timms et al. has shown that upon engagement of CD47 with SIRPA on macrophages, the CSF1R signaling is inhibited [50]. To date, CSF1R activation via CSF1 or IL-34 is known to be required for the M1 to M2 polarization of macrophages [51,52,53,54,55]. Therefore, engagement of SIRPA on macrophages by CD47 on tumor cells not only suppresses phagocytosis function of macrophages but also elicits the second signal, which would block M2 polarization and preserve M1 TAMs. In this perspective article, we propose anti-SIRPA F(ab’)2, which mimics the engagement of CD47 against SIRPA on macrophages and preserves anti-tumor M1 TAMs by blocking M2 polarization (Figure 2D). Due to the smaller size of its F(ab’)2, anti-SIRPA F(ab’)2 can penetrate tumor tissues more efficiently [79]. Small molecules such as anti-SIRPA scFv (single-chain fragment variable) can also easily penetrate the tumor tissue. However, in vivo instability issues of scFv have been reported [80]. Although anti-SIRPA antibodies have been used previously as therapeutics against cancers in preclinical models, including a mouse neuroblastoma model [40,41,81], a comparison between the therapeutic efficacy of anti-SIRPA F(ab’)2 and the intact anti-SIRPA antibody has not been explored.
Because SIRPA expression is rather restricted to macrophages in many tissues [62], anti-SIRPA F(ab’)2 may provide both safety and efficacy. Anti-SIRPA F(ab’)2 may also have the advantage over small molecule inhibitors of targeting pathways involved in M2 polarization. For example, PI3Kγ inhibitors have been considered for preventing M2 polarization by blocking downstream of the CSF1/CSF1R pathway. However, PI3Kγ is expressed in a variety of immune cells [62], and therefore, these inhibitors could adversely affect the general immune status of the host. Moreover, PI3Kγ inhibitors could constrain the M1-polarizing CSF2/CSF2R signaling, which also utilizes PI3Kγ as one of the downstream signaling effectors [82].
9. Conclusions
Based on collective data from others and our group, we suggest that disruption of the CD47-SIRPA interaction by anti-CD47 antibody promotes M2 polarization, and counterintuitively, we propose that anti-SIRPA would lead to preserving M1. In addition, we hypothesize that an effective and less toxic cure for high-risk neuroblastoma should target SIRPA on macrophages and not CD47 on the tumor cells. In essence, the proposed approach will focus on how to make the immune cells (i.e., macrophages) eradicate the tumor cells. We have also proposed various combination treatments for high-risk neuroblastoma. The three-component combination treatment includes anti-SIRPA F(ab’)2, F(ab’)2 of elotuzumab as the anti-SLAMF7 antibody, and an anti-GD2 antibody, such as dinutuximab and naxitamab (Figure 4). We expect that this combination would effectively promote tumor clearance by macrophages in high-risk neuroblastoma and other GD2-positive solid tumors. Nonetheless, it is equally important to investigate the therapeutic efficacy of the two-component combination treatments: (anti-SIRPA F(ab’)2 and F(ab’)2 of elotuzumab) and (anti-SIRPA F(ab’)2 and anti-GD2 antibody). This is because a balance between maximum efficacy and minimum side effects is essential to cancer immunotherapy. The availability of clinically relevant humanized anti-SIRPA antibodies (see Table S1) has made the proposed therapeutic approach feasible since all three antibodies are already in use in clinics to treat cancer patients. Lastly, because of the heterogeneity of high-risk neuroblastoma, we anticipate that antibody-mediated approaches would further require specific combinations with engineered adoptive cell therapies to treat all high-risk neuroblastoma patients.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/curroncol31060243/s1: Figure S1. Macrophage polarization and phagocytic capacity in high-risk neuroblastoma tissues; Figure S2. CD47 expression on vascular cells of high-risk neuroblastoma tissues; Figure S3. CD47 and SIRPA expression in favorable neuroblastoma; Table S1. Anti-SIRPα antibodies tested in human clinical trials for malignant diseases. References [83,84,85,86,87,88,89,90,91] are cited in the Supplementary Materials.
Author Contributions
X.X.T. and N.I. designed the study, generated and analyzed the data, and conceptualized, wrote, and edited the manuscript. H.S. generated and analyzed the IHC data and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
There is no external funding for this study.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (Stanford University IRB#: 50930).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study under Stanford University IRB#: 45458.
Data Availability Statement
Publicly available datasets were analyzed in this study. This dataset is found at NCBI GEO, GSE62564.
Conflicts of Interest
There are no conflicts of interest.
References
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef]
- Sharma, P.; Siddiqui, B.A.; Anandhan, S.; Yadav, S.S.; Subudhi, S.K.; Gao, J.; Goswami, S.; Allison, J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021, 11, 838–857. [Google Scholar] [CrossRef]
- Finck, A.V.; Blanchard, T.; Roselle, C.P.; Golinelli, G.; June, C.H. Engineered cellular immunotherapies in cancer and beyond. Nat. Med. 2022, 28, 678–689. [Google Scholar] [CrossRef]
- Wölfl, M.; Jungbluth, A.A.; Garrido, F.; Cabrera, T.; Meyen-Southard, S.; Spitz, R.; Ernestus, K.; Berthold, F. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol. Immunother. 2005, 54, 400–406. [Google Scholar] [CrossRef]
- Tang, X.X.; Shimada, H.; Ikegaki, N. Clinical Relevance of CD4 Cytotoxic T Cells in High-Risk Neuroblastoma. Front. Immunol. 2021, 12, 650427. [Google Scholar] [CrossRef]
- Tang, X.X.; Shimada, H.; Ikegaki, N. Macrophage-mediated anti-tumor immunity against high-risk neuroblastoma. Genes Immun. 2022, 23, 129–140. [Google Scholar] [CrossRef]
- Chamoto, K.; Yaguchi, T.; Tajima, M.; Honjo, T. Insights from a 30-year journey: function, regulation and therapeutic modulation of PD1. Nat. Rev. Immunol. 2023, 23, 682–695. [Google Scholar] [CrossRef]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Feng, M.; Jiang, W.; Kim, B.Y.S.; Zhang, C.C.; Fu, Y.X.; Weissman, I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 568–586. [Google Scholar] [CrossRef]
- Bied, M.; Ho, W.W.; Ginhoux, F.; Bleriot, C. Roles of macrophages in tumor development: a spatiotemporal perspective. Cell Mol. Immunol. 2023, 20, 983–992. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Tumor-associated macrophages. Curr. Biol. 2020, 30, R246–R248. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, M.; Jia, S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front. Immunol. 2023, 14, 1080310. [Google Scholar] [CrossRef]
- Kuninaka, Y.; Ishida, Y.; Ishigami, A.; Nosaka, M.; Matsuki, J.; Yasuda, H.; Kofuna, A.; Kimura, A.; Furukawa, F.; Kondo, T. Macrophage polarity and wound age determination. Sci. Rep. 2022, 12, 20327. [Google Scholar] [CrossRef]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef]
- Jahchan, N.S.; Mujal, A.M.; Pollack, J.L.; Binnewies, M.; Sriram, V.; Reyno, L.; Krummel, M.F. Tuning the Tumor Myeloid Microenvironment to Fight Cancer. Front. Immunol. 2019, 10, 1611. [Google Scholar] [CrossRef]
- Su, Z.; Fang, H.; Hong, H.; Shi, L.; Zhang, W.; Zhang, W.; Zhang, Y.; Dong, Z.; Lancashire, L.J.; Bessarabova, M.; et al. An investigation of biomarkers derived from legacy microarray data for their utility in the RNA-seq era. Genome Biol. 2014, 15, 523. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Y.; Hertwig, F.; Thierry-Mieg, J.; Zhang, W.; Thierry-Mieg, D.; Wang, J.; Furlanello, C.; Devanarayan, V.; Cheng, J.; et al. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome Biol. 2015, 16, 133. [Google Scholar] [CrossRef]
- Yang, L.; Forker, L.; Irlam, J.J.; Pillay, N.; Choudhury, A.; West, C.M.L. Validation of a hypoxia related gene signature in multiple soft tissue sarcoma cohorts. Oncotarget. 2018, 9, 3946–3955. [Google Scholar] [CrossRef]
- Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Mao, Y.; Finnemann, S.C. Regulation of phagocytosis by Rho GTPases. Small GTPases 2015, 6, 89–99. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Sathyapalan, T.; Diboun, I.; Atkin, S.L.; Butler, A.E. Identification of macrophage activation-related biomarkers in obese type 2 diabetes that may be indicative of enhanced respiratory risk in COVID-19. Sci. Rep. 2021, 11, 6428. [Google Scholar] [CrossRef]
- Amici, S.A.; Young, N.A.; Narvaez-Miranda, J.; Jablonski, K.A.; Arcos, J.; Rosas, L.; Papenfuss, T.L.; Torrelles, J.B.; Jarjour, W.N.; Guerau-de-Arellano, M. CD38 Is Robustly Induced in Human Macrophages and Monocytes in Inflammatory Conditions. Front. Immunol. 2018, 9, 1593. [Google Scholar] [CrossRef]
- Adams, S.; van der Laan, L.J.; Vernon-Wilson, E.; Renardel de Lavalette, C.; Döpp, E.A.; Dijkstra, C.D.; Simmons, D.L.; van den Berg, T.K. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J. Immunol. 1998, 161, 1853–1859. [Google Scholar] [CrossRef]
- Barclay, A.N. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr. Opin. Immunol. 2009, 21, 47–52. [Google Scholar] [CrossRef]
- Han, X.; Sterling, H.; Chen, Y.; Saginario, C.; Brown, E.J.; Frazier, W.A.; Lindberg, F.P.; Vignery, A. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J. Biol. Chem. 2000, 275, 37984–37992. [Google Scholar] [CrossRef]
- Seiffert, M.; Cant, C.; Chen, Z.; Rappold, I.; Brugger, W.; Kanz, L.; Brown, E.J.; Ullrich, A.; Bühring, H.J. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood 1999, 94, 3633–3643. [Google Scholar] [CrossRef]
- Veillette, A.; Thibaudeau, E.; Latour, S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 1998, 273, 22719–22728. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Chen, Z.; Sures, I.; Wang, H.; Schilling, J.; Ullrich, A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 1997, 386, 181–186. [Google Scholar] [CrossRef]
- Fujioka, Y.; Matozaki, T.; Noguchi, T.; Iwamatsu, A.; Yamao, T.; Takahashi, N.; Tsuda, M.; Takada, T.; Kasuga, M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell Biol. 1996, 16, 6887–6899. [Google Scholar] [CrossRef]
- Tsai, R.K.; Discher, D.E. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 2008, 180, 989–1003. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D., Jr.; van Rooijen, N.; Weissman, I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Jan, M.; Weissman-Tsukamoto, R.; Zhao, F.; Park, C.Y.; Weissman, I.L.; Majeti, R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011, 71, 1374–1384. [Google Scholar] [CrossRef]
- Chao, M.P.; Tang, C.; Pachynski, R.K.; Chin, R.; Majeti, R.; Weissman, I.L. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood 2011, 118, 4890–4901. [Google Scholar] [CrossRef]
- Kim, D.; Wang, J.; Willingham, S.B.; Martin, R.; Wernig, G.; Weissman, I.L. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012, 26, 2538–2545. [Google Scholar] [CrossRef]
- Edris, B.; Weiskopf, K.; Volkmer, A.K.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Liu, J.; Majeti, R.; West, R.B.; Fletcher, J.A.; et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc. Natl. Acad. Sci. USA 2012, 109, 6656–6661. [Google Scholar] [CrossRef]
- Weiskopf, K.; Ring, A.M.; Ho, C.C.; Volkmer, J.P.; Levin, A.M.; Volkmer, A.K.; Ozkan, E.; Fernhoff, N.B.; van de Rijn, M.; Weissman, I.L.; et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013, 341, 88–91. [Google Scholar] [CrossRef]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A.; et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef]
- Weiskopf, K.; Ring, A.M.; Guo, N.; Schnorr, P.J.; Maute, R.L.; Volkmer, J.-P.; Weissman, I.L. Direct SIRPa Blockade Augments Macrophage Responses to Therapeutic Anticancer Antibodies. Blood 2014, 124, 2729. [Google Scholar] [CrossRef]
- Theruvath, J.; Menard, M.; Smith, B.A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344. [Google Scholar] [CrossRef]
- Koster, J.; Volckmann, R.; Zwijnenburg, D.; Molenaar, P.; Versteeg, R. Abstract 2490: R2: Genomics analysis and visualization platform. Cancer Res. 2019, 79, 2490. [Google Scholar] [CrossRef]
- Green, C.E.; Liu, T.; Montel, V.; Hsiao, G.; Lester, R.D.; Subramaniam, S.; Gonias, S.L.; Klemke, R.L. Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS ONE 2009, 4, e6713. [Google Scholar] [CrossRef]
- Sugimura-Nagata, A.; Koshino, A.; Inoue, S.; Matsuo-Nagano, A.; Komura, M.; Riku, M.; Ito, H.; Inoko, A.; Murakami, H.; Ebi, M.; et al. Expression and Prognostic Significance of CD47-SIRPA Macrophage Checkpoint Molecules in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 2690. [Google Scholar] [CrossRef]
- Kazama, R.; Miyoshi, H.; Takeuchi, M.; Miyawaki, K.; Nakashima, K.; Yoshida, N.; Kawamoto, K.; Yanagida, E.; Yamada, K.; Umeno, T.; et al. Combination of CD47 and signal-regulatory protein-α constituting the “don’t eat me signal” is a prognostic factor in diffuse large B-cell lymphoma. Cancer Sci. 2020, 111, 2608–2619. [Google Scholar] [CrossRef]
- Chen, Y.P.; Kim, H.J.; Wu, H.; Price-Troska, T.; Villasboas, J.C.; Jalali, S.; Feldman, A.L.; Novak, A.J.; Yang, Z.Z.; Ansell, S.M. SIRPα expression delineates subsets of intratumoral monocyte/macrophages with different functional and prognostic impact in follicular lymphoma. Blood Cancer J. 2019, 9, 84. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Mitrakas, A.; Anestopoulos, I.; Kontosis, A.; Koukourakis, I.M.; Pappa, A.; Panayiotidis, M.I.; Koukourakis, M.I. Expression of CD47 and SIRPα Macrophage Immune-Checkpoint Pathway in Non-Small-Cell Lung Cancer. Cancers 2022, 14, 1801. [Google Scholar] [CrossRef]
- Koga, N.; Hu, Q.; Sakai, A.; Takada, K.; Nakanishi, R.; Hisamatsu, Y.; Ando, K.; Kimura, Y.; Oki, E.; Oda, Y.; et al. Clinical significance of signal regulatory protein alpha (SIRPα) expression in esophageal squamous cell carcinoma. Cancer Sci. 2021, 112, 3018–3028. [Google Scholar] [CrossRef]
- Timms, J.F.; Carlberg, K.; Gu, H.; Chen, H.; Kamatkar, S.; Nadler, M.J.; Rohrschneider, L.R.; Neel, B.G. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol. Cell Biol. 1998, 18, 3838–3850. [Google Scholar] [CrossRef]
- Foucher, E.D.; Blanchard, S.; Preisser, L.; Garo, E.; Ifrah, N.; Guardiola, P.; Delneste, Y.; Jeannin, P. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. antagonistic effects of GM-CSF and IFNγ. PLoS ONE 2013, 8, e56045. [Google Scholar] [CrossRef]
- Jones, C.V.; Ricardo, S.D. Macrophages and CSF-1: implications for development and beyond. Organogenesis 2013, 9, 249–260. [Google Scholar] [CrossRef]
- Hamilton, T.A.; Zhao, C.; Pavicic, P.G., Jr.; Datta, S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front. Immunol. 2014, 5, 554. [Google Scholar] [CrossRef]
- Boulakirba, S.; Pfeifer, A.; Mhaidly, R.; Obba, S.; Goulard, M.; Schmitt, T.; Chaintreuil, P.; Calleja, A.; Furstoss, N.; Orange, F.; et al. IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci. Rep. 2018, 8, 256. [Google Scholar] [CrossRef]
- Trus, E.; Basta, S.; Gee, K. Who’s in charge here? Macrophage colony stimulating factor and granulocyte macrophage colony stimulating factor: Competing factors in macrophage polarization. Cytokine 2020, 127, 154939. [Google Scholar] [CrossRef]
- Desgeorges, T.; Caratti, G.; Mounier, R.; Tuckermann, J.; Chazaud, B. Glucocorticoids Shape Macrophage Phenotype for Tissue Repair. Front. Immunol. 2019, 10, 1591. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Anderson, N.R.; Minutolo, N.G.; Gill, S.; Klichinsky, M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021, 81, 1201–1208. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab Plus Pomalidomide and Dexamethasone for Relapsed/Refractory Multiple Myeloma: Final Overall Survival Analysis From the Randomized Phase II ELOQUENT-3 Trial. J. Clin. Oncol. 2023, 41, 568–578. [Google Scholar] [CrossRef]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]
- Sait, S.; Modak, S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 889–904. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; Diccianni, M.B.; Gan, J.; Hank, J.A.; Batova, A.; London, W.B.; Tenney, S.C.; et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin. Cancer Res. 2021, 27, 2179–2189. [Google Scholar] [CrossRef]
- Mora, J.; Castañeda, A.; Gorostegui, M.; Santa-María, V.; Garraus, M.; Muñoz, J.P.; Varo, A.; Perez-Jaume, S.; Mañe, S. Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission. Pediatr. Blood Cancer 2021, 68, e29121. [Google Scholar] [CrossRef]
- Furman, W.L.; Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Davidoff, A.M.; Krasin, M.J.; Sahr, N.; Sykes, A.; Wu, J.; Brennan, R.C.; et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-Risk Neuroblastoma. Clin. Cancer Res. 2019, 25, 6320–6328. [Google Scholar] [CrossRef]
- Qu, T.; Li, B.; Wang, Y. Targeting CD47/SIRPα as a therapeutic strategy, where we are and where we are headed. Biomark. Res. 2022, 10, 20. [Google Scholar] [CrossRef]
- Wu, Z.H.; Li, N.; Mei, X.F.; Chen, J.; Wang, X.Z.; Guo, T.T.; Chen, G.; Nie, L.; Chen, Y.; Jiang, M.Z.; et al. Preclinical characterization of the novel anti-SIRPα antibody BR105 that targets the myeloid immune checkpoint. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Chan, H.; Trout, C.V.; Mikolon, D.; Adams, P.; Guzman, R.; Mavrommatis, K.; Abbasian, M.; Hadjivassiliou, H.; Dearth, L.; Fox, B.A.; et al. Discovery and Preclinical Activity of BMS-986351, an Antibody to SIRPα That Enhances Macrophage-mediated Tumor Phagocytosis When Combined with Opsonizing Antibodies. Cancer Res. Commun. 2024, 4, 505–515. [Google Scholar] [CrossRef]
- Voets, E.; Paradé, M.; Lutje Hulsik, D.; Spijkers, S.; Janssen, W.; Rens, J.; Reinieren-Beeren, I.; van den Tillaart, G.; van Duijnhoven, S.; Driessen, L.; et al. Functional characterization of the selective pan-allele anti-SIRPα antibody ADU-1805 that blocks the SIRPα-CD47 innate immune checkpoint. J. Immunother. Cancer 2019, 7, 340. [Google Scholar] [CrossRef]
- Gutierrez, M.; Jamal, R.; Yamamoto, N.; Doi, T.; Elgadi, M.; Ferrada, J.L.; Wojciekowski, S.M.; Patel, M.R. 697P Open-label, phase I, dose escalation/expansion trial of the anti-SIRPα monoclonal antibody BI 770371 in patients with advanced solid tumours, alone or in combination with the anti-PD-1 monoclonal antibody ezabenlimab. Ann. Oncol. 2023, 34, S485. [Google Scholar] [CrossRef]
- Wang, X.; Mathieu, M.; Brezski, R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018, 9, 63–73. [Google Scholar] [CrossRef]
- Stewart, R.; Hammond, S.A.; Oberst, M.; Wilkinson, R.W. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J. ImmunoTher. Cancer 2014, 2, 29. [Google Scholar] [CrossRef]
- Galvez-Cancino, F.; Simpson, A.P.; Costoya, C.; Matos, I.; Qian, D.; Peggs, K.S.; Litchfield, K.; Quezada, S.A. Fcγ receptors and immunomodulatory antibodies in cancer. Nat. Rev. Cancer 2024, 24, 51–71. [Google Scholar] [CrossRef]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daëron, M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef]
- Brooke, G.; Holbrook, J.D.; Brown, M.H.; Barclay, A.N. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J. Immunol. 2004, 173, 2562–2570. [Google Scholar] [CrossRef]
- Ho, C.C.; Guo, N.; Sockolosky, J.T.; Ring, A.M.; Weiskopf, K.; Özkan, E.; Mori, Y.; Weissman, I.L.; Garcia, K.C. “Velcro” engineering of high affinity CD47 ectodomain as signal regulatory protein α (SIRPα) antagonists that enhance antibody-dependent cellular phagocytosis. J. Biol. Chem. 2015, 290, 12650–12663. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Wissler, H.L.; Ehlerding, E.B.; Lyu, Z.; Zhao, Y.; Zhang, S.; Eshraghi, A.; Buuh, Z.Y.; McGuth, J.C.; Guan, Y.; Engle, J.W.; et al. Site-Specific Immuno-PET Tracer to Image PD-L1. Mol. Pharm. 2019, 16, 2028–2036. [Google Scholar] [CrossRef]
- Quintero-Hernández, V.; Juárez-González, V.R.; Ortíz-León, M.; Sánchez, R.; Possani, L.D.; Becerril, B. The change of the scFv into the Fab format improves the stability and in vivo toxin neutralization capacity of recombinant antibodies. Mol. Immunol. 2007, 44, 1307–1315. [Google Scholar] [CrossRef]
- Bahri, M.; Kailayangiri, S.; Vermeulen, S.; Galopin, N.; Rossig, C.; Paris, F.; Fougeray, S.; Birklé, S. SIRPα-specific monoclonal antibody enables antibody-dependent phagocytosis of neuroblastoma cells. Cancer Immunol. Immunother. 2022, 71, 71–83. [Google Scholar] [CrossRef]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef]
- Asare, P.F.; Roscioli, E.; Hurtado, P.R.; Tran, H.B.; Mah, C.Y.; Hodge, S. LC3-Associated Phagocytosis (LAP): A Potentially Influential Mediator of Efferocytosis-Related Tumor Progression and Aggressiveness. Front. Oncol. 2020, 10, 1298. [Google Scholar] [CrossRef]
- Werfel, T.A.; Cook, R.S. Efferocytosis in the tumor microenvironment. Semin. Immunopathol. 2018, 40, 545–554. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, M.C.; Guo, H.; Davidson, D.; Mishel, S.; Lu, Y.; Rhee, I.; Pérez-Quintero, L.A.; Zhang, S.; Cruz-Munoz, M.E.; et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature 2017, 544, 493–497. [Google Scholar] [CrossRef]
- Delord, J.-P.; Kotecki, N.; Marabelle, A.; Vinceneux, A.; Korakis, I.; Jungels, C.; Champiat, S.; Huhn, R.D.; Poirier, N.; Costantini, D.; et al. A Phase 1 Study Evaluating BI 765063, a First in Class Selective Myeloid Sirpa Inhibitor, As Stand-Alone and in Combination with BI 754091, a Programmed Death-1 (PD-1) Inhibitor, in Patients with Advanced Solid Tumours. Blood 2019, 134, 1040. [Google Scholar] [CrossRef]
- van Helden, M.J.; Zwarthoff, S.A.; Arends, R.J.; Reinieren-Beeren, I.M.J.; Paradé, M.; Driessen-Engels, L.; de Laat-Arts, K.; Damming, D.; Santegoeds-Lenssen, E.W.H.; van Kuppeveld, D.W.J.; et al. BYON4228 is a pan-allelic antagonistic SIRPα antibody that potentiates destruction of antibody-opsonized tumor cells and lacks binding to SIRPγ on T cells. J. Immunother. Cancer 2023, 11. [Google Scholar] [CrossRef]
- Mayumi, S.; Takuya, T.; Yoko, I.; Shinko, H.; Saori, I.; Yoshitaka, I.; Jun, I.; Reimi, K.; Toshiaki, O.; Teiji, W.; et al. 808 Blocking “don’t-eat-me” signal of CD47-SIRPα by anti-SIRPα antibody enhances anti-tumor efficacy of trastuzumab deruxtecan. J. ImmunoTher. Cancer 2022, 10, A844. [Google Scholar] [CrossRef]
- Xu, C.A.; Feng, A.Z.; Ramineni, C.K.; Wallace, M.R.; Culyba, E.K.; Guay, K.P.; Mehta, K.; Mabry, R.; Farrand, S.; Xu, J.; et al. L(445)P mutation on heavy chain stabilizes IgG(4) under acidic conditions. MAbs 2019, 11, 1289–1299. [Google Scholar] [CrossRef]
- Bloom, J.W.; Madanat, M.S.; Marriott, D.; Wong, T.; Chan, S.Y. Intrachain disulfide bond in the core hinge region of human IgG4. Protein Sci. 1997, 6, 407–415. [Google Scholar] [CrossRef]
- Hezareh, M.; Hessell, A.J.; Jensen, R.C.; van de Winkel, J.G.; Parren, P.W. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J. Virol. 2001, 75, 12161–12168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).