Opioids and Cancer: Current Understanding and Clinical Considerations
Abstract
1. Introduction
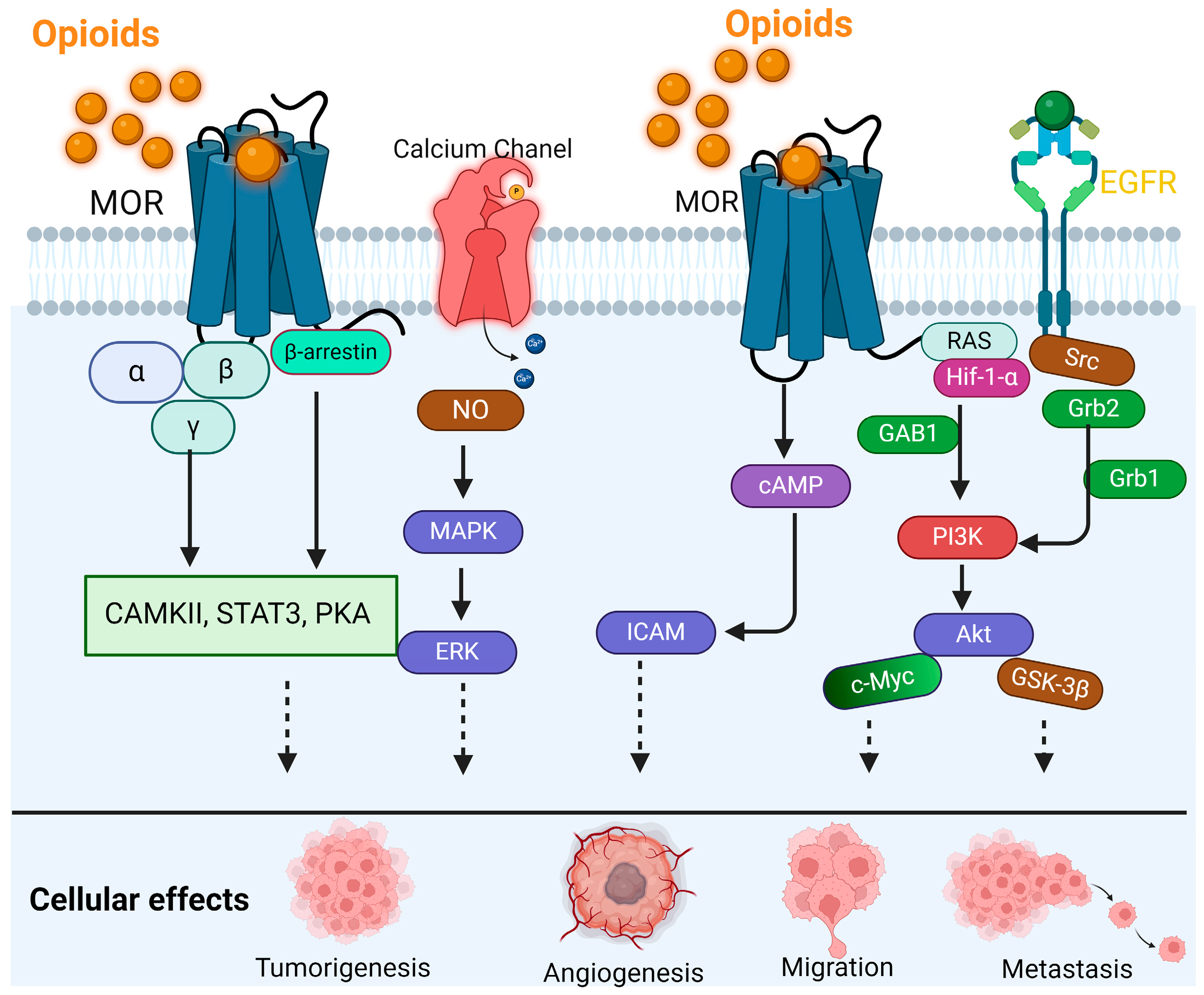
2. MOR Biology in Cancer
2.1. MOR and Cancer–Nerve Interaction
2.2. MOR Opioids and the Immune System
3. Prescription Opioids in Patients with Cancer
3.1. Epidemiological Studies on Prescription Opioids and Cancer Formation
3.2. Opioids in the Context of Cancer Surgery
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- van den Beuken-van Everdingen, M.H.J.; Hochstenbach, L.M.J.; Joosten, E.A.J.; Tjan-Heijnen, V.C.G.; Janssen, D.J.A. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090.e9. [Google Scholar] [CrossRef] [PubMed]
- Scharpf, J.; Karnell, L.H.; Christensen, A.J.; Funk, G.F. The Role of Pain in Head and Neck Cancer Recurrence and Survivorship. Arch. Otolaryngol.–Head Neck Surg. 2009, 135, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Zylla, D.; Kuskowski, M.A.; Gupta, K.; Gupta, P. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br. J. Anaesth. 2014, 113, i109–i116. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R. Friedrich Wilhelm Serturner and the discovery of morphine. Pharm. Hist. 1985, 27, 61–74. [Google Scholar] [PubMed]
- Stanley, T.H. The Fentanyl Story. J. Pain 2014, 15, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.M. Cicely Saunders: Selected Writings 1958–2004; Oxford University Press: Oxford, UK; New York, NY, USA, 2006; Volume xxviii, 300p. [Google Scholar]
- Paice, J.A.; Portenoy, R.; Lacchetti, C.; Campbell, T.; Cheville, A.; Citron, M.; Constine, L.S.; Cooper, A.; Glare, P.; Keefe, F.; et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3325–3345. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Fanciullo, G.J.; Fine, P.G.; Adler, J.A.; Ballantyne, J.C.; Davies, P.; Donovan, M.I.; Fishbain, D.A.; Foley, K.M.; Fudin, J.; et al. Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain. J. Pain 2009, 10, 113–130.e22. [Google Scholar] [CrossRef]
- Mercadante, S.; Arcuri, E. Opioids and renal function. J. Pain 2004, 5, 2–19. [Google Scholar] [CrossRef]
- Davis, M.P.; Pasternak, G.; Behm, B. Treating Chronic Pain: An Overview of Clinical Studies Centered on the Buprenorphine Option. Drugs 2018, 78, 1211–1228. [Google Scholar] [CrossRef]
- Tarkkila, P.; Tuominen, M.; Lindgren, L. Comparison of respiratory effects of tramadol and oxycodone. J. Clin. Anesth. 1997, 9, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Uhelski, M.L.; Gorur, A.; Bhoir, S.; Ilsin, N.; Dougherty, P.M. The µ-Opioid Receptor in Cancer and Its Role in Perineural Invasion: A Short Review and New Evidence. Adv. Biol. 2022, 6, 2200020. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.T.; Pan, J.H.; Chen, Y.H.; Xing, W.; Yan, Y.; Yuan, Y.F.; Zeng, W.A. The mu-opioid receptor is a molecular marker for poor prognosis in hepatocellular carcinoma and represents a potential therapeutic target. Br. J. Anaesth. 2019, 122, e157–e167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, Y.; Li, L.; Chen, J.; Zhang, X.; Xie, Y. Morphine Can Inhibit the Growth of Breast Cancer MCF-7 Cells by Arresting the Cell Cycle and Inducing Apoptosis. Biol. Pharm. Bull. 2017, 40, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Gach, K.; Piestrzeniewicz, M.; Fichna, J.; Stefanska, B.; Szemraj, J.; Janecka, A. Opioid-induced regulation of mu-opioid receptor gene expression in the MCF-7 breast cancer cell line. Biochem. Cell Biol. 2008, 86, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Gorur, A.; Patiño, M.; Takahashi, H.; Corrales, G.; Pickering, C.R.; Gleber-Netto, F.O.; Myers, J.N.; Cata, J.P. Mu-opioid receptor activation promotes in vitro and in vivo tumor growth in head and neck squamous cell carcinoma. Life Sci. 2021, 278, 119541. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, H.; Weng, M.l.; Zhang, J.; Jiang, N.; Cata, J.P.; Ma, D.; Chen, W.K.; Miao, C.H. Morphine promotes tumorigenesis and cetuximab resistance via EGFR signaling activation in human colorectal cancer. J. Cell. Physiol. 2020, 236, 4445–4454. [Google Scholar] [CrossRef]
- Grandhi, R.K.; Lee, S.; Abd-Elsayed, A. Does Opioid Use Cause Angiogenesis and Metastasis? Pain Med. 2017, 18, 140–151. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, M.; Zhou, D.; Gorur, A.; Sun, Z.; Zeng, W.; Cata, J.P.; Chen, W.; Miao, C. Increased mu-opioid receptor expression is associated with reduced disease-free and overall survival in laryngeal squamous cell carcinoma. Br. J. Anaesth. 2020, 125, 722–729. [Google Scholar] [CrossRef]
- Levi, L.; Hikri, E.; Popovtzer, A.; Dayan, A.; Levi, A.; Bachar, G.; Mizrachi, A.; Shoffel-Havakuk, H. Effect of Opioid Receptor Activation and Blockage on the Progression and Response to Treatment of Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2023, 12, 1277. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.K.; Song, K.Y.; Kim, C.S.; Choi, H.S.; Guo, X.-H.; Law, P.-Y.; Wei, L.-N.; Loh, H.H. Evidence of Endogenous Mu Opioid Receptor Regulation by Epigenetic Control of the Promoters. Mol. Cell. Biol. 2023, 27, 4720–4736. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Physician 2008, 11 (Suppl. S2), S133–S153. [Google Scholar] [CrossRef]
- Kiguchi, Y.; Aono, Y.; Watanabe, Y.; Yamamoto-Nemoto, S.; Shimizu, K.; Shimizu, T.; Kosuge, Y.; Waddington, J.L.; Ishige, K.; Ito, Y.; et al. In vivo neurochemical evidence that delta1-, delta2- and mu2-opioid receptors, but not mu1-opioid receptors, inhibit acetylcholine efflux in the nucleus accumbens of freely moving rats. Eur. J. Pharmacol. 2016, 789, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Opioids. Clinical and Research Information on Drug-Induced Liver Injury; LiverTox: Bethesda, MD, USA, 2012. [Google Scholar]
- Pasternak, G.W.; Pan, Y.-X.; Sibley, D.R. Mu Opioids and Their Receptors: Evolution of a Concept. Pharmacol. Rev. 2013, 65, 1257–1317. [Google Scholar] [CrossRef] [PubMed]
- Dumas, E.O.; Pollack, G.M. Opioid Tolerance Development: A Pharmacokinetic/Pharmacodynamic Perspective. AAPS J. 2008, 10, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, C.J.; Durieux, M.E. Differential Opioid Tolerance and Opioid-induced Hyperalgesia. Anesthesiology 2016, 124, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Dwivedi-Agnihotri, H.; Shukla, A.K.; Roth, B.L. Biased ligands at opioid receptors: Current status and future directions. Sci. Signal. 2021, 14, eaav0320. [Google Scholar] [CrossRef]
- Gillis, A.; Kliewer, A.; Kelly, E.; Henderson, G.; Christie, M.J.; Schulz, S.; Canals, M. Critical Assessment of G Protein-Biased Agonism at the mu-Opioid Receptor. Trends Pharmacol. Sci. 2020, 41, 947–959. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Amit, I.; Yarden, Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim. Biophys. Acta 2007, 1773, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Donnini, S.; Pellegrini, C.; Coppi, E.; Bocci, G. Opioid receptors beyond pain control: The role in cancer pathology and the debated importance of their pharmacological modulation. Pharmacol. Res. 2020, 159, 104938. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Lennon, F.E.; Siegler, J.; Mirzapoiazova, T.; Mambetsariev, N.; Sammani, S.; Gerhold, L.M.; LaRiviere, P.J.; Chen, C.T.; Garcia, J.G.; et al. The novel role of the mu opioid receptor in lung cancer progression: A laboratory investigation. Anesth. Analg. 2011, 112, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Lennon, F.E.; Mirzapoiazova, T.; Mambetsariev, B.; Salgia, R.; Moss, J.; Singleton, P.A. Overexpression of the mu-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology 2012, 116, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Singleton, P.A.; Moss, J.; Karp, D.D.; Atkins, J.T.; Janku, F. The mu opioid receptor: A new target for cancer therapy? Cancer 2015, 121, 2681–2688. [Google Scholar] [CrossRef]
- Lec, P.M.; Lenis, A.T.; Golla, V.; Brisbane, W.; Shuch, B.; Garraway, I.P.; Reiter, R.E.; Chamie, K. The Role of Opioids and Their Receptors in Urological Malignancy: A Review. J. Urol. 2020, 204, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Cambronero, O.; Mazzinari, G.; Giner, F.; Belltall, A.; Ruiz-Boluda, L.; Marques-Mari, A.; Sanchez-Guillen, L.; Eroles, P.; Cata, J.P.; Argente-Navarro, M.P. Mu Opioid Receptor 1 (MOR-1) Expression in Colorectal Cancer and Oncological Long-Term Outcomes: A Five-Year Retrospective Longitudinal Cohort Study. Cancers 2020, 12, 134. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, M.; Gorur, A.; Sun, Z.; Cata, J.P.; Chen, W.; Miao, C. Association of Mu-Opioid Receptor(MOR) Expression and Opioids Requirement With Survival in Patients With Stage I-III Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2021, 11, 686877. [Google Scholar] [CrossRef]
- Haque, M.R.; Barlass, U.; Armstrong, A.; Shaikh, M.; Bishehsari, F. Novel role of the Mu-opioid receptor in pancreatic cancer: Potential link between opioid use and cancer progression. Mol. Cell. Biochem. 2022, 477, 1339–1345. [Google Scholar] [CrossRef]
- Novy, D.M.; Nelson, D.V.; Koyyalagunta, D.; Cata, J.P.; Gupta, P.; Gupta, K. Pain, opioid therapy, and survival: A needed discussion. Pain 2020, 161, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qu, M.; Sun, C.; Wang, Y.; Li, T.; Xu, W.; Sun, Z.; Zhang, X.; Guo, K.; Chen, W.; et al. Association of Mu-Opioid Receptor Expression With Long-Term Survival and Perineural Nerve Invasion in Patients Undergoing Surgery for Ovarian Cancer. Front. Oncol. 2022, 12, 927262. [Google Scholar] [CrossRef] [PubMed]
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015, 75, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Santoni, A.; Santoni, M.; Arcuri, E. Chronic Cancer Pain: Opioids within Tumor Microenvironment Affect Neuroinflammation, Tumor and Pain Evolution. Cancers 2022, 14, 2253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, R.; Wang, Y.; Yu, M. Perineural invasion as a prognostic factor in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Acta Otolaryngol. 2019, 139, 1038–1043. [Google Scholar] [CrossRef]
- Eryılmaz, M.K.; Korkmaz, M.; Karaağaç, M.; Artaç, M. Perineural invasion is a better prognostic factor than extranodal extension in head and neck cancer. Egypt. J. Otolaryngol. 2022, 38, 9. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Li, Z.; Han, X.; Que, L. Prognostic Value of Perineural Invasion in Oral Tongue Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 683825. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Dai, H.; Li, R.; Wheeler, T.; Ozen, M.; Ittmann, M.; Anderson, M.; Wang, Y.; Rowley, D.; Younes, M.; Ayala, G.E. Enhanced survival in perineural invasion of pancreatic cancer: An in vitro approach. Hum. Pathol. 2007, 38, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Du, K.N.; Shepherd, A.J.; Ma, I.M.; Roldan, C.J.; Amit, M.; Fang, L.M.S.; Desai, S.; Cata, J.P. Lack of association between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and pain improvement in patients with oral cancer. Ecancermedicalscience 2020, 14, 1121. [Google Scholar] [CrossRef] [PubMed]
- Megat, S.; Ray, P.R.; Moy, J.K.; Lou, T.-F.; Barragán-Iglesias, P.; Li, Y.; Pradhan, G.; Wanghzou, A.; Ahmad, A.; Burton, M.D.; et al. Nociceptor Translational Profiling Reveals the Ragulator-Rag GTPase Complex as a Critical Generator of Neuropathic Pain. J. Neurosci. 2019, 39, 393–411. [Google Scholar] [CrossRef]
- Ma, J.; Kavelaars, A.; Dougherty, P.M.; Heijnen, C.J. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 2018, 124, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Asimomytis, A.; Karanikou, M.; Rodolakis, A.; Vaiopoulou, A.; Tsetsa, P.; Creatsas, G.; Stefos, T.; Antsaklis, A.; Patsouris, E.; Rassidakis, G.Z. mTOR downstream effectors, 4EBP1 and eIF4E, are overexpressed and associated with HPV status in precancerous lesions and carcinomas of the uterine cervix. Oncol. Lett. 2016, 12, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.F.; Gorur, A.; Cata, J.P. Opioids and cancer prognosis: A summary of the clinical evidence. Neurosci. Lett. 2021, 746, 135661. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Kawamata, T.; Niiyama, Y.; Omote, K.; Namiki, A. Down-regulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience 2008, 151, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, M.; Li, Y.; Rogers, T.; Poonawala, T.; Griffin, R.J.; Song, C.W.; Gupta, K. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. Br. J. Cancer 2007, 97, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Ding, Y.; Wang, Z.; Ma, J.; Hong, T.; Zhu, Y.; Li, H.; Pan, S. Impacts of anti-nerve growth factor antibody on pain-related behaviors and expressions of opioid receptor in spinal dorsal horn and dorsal root ganglia of rats with cancer-induced bone pain. Mol. Pain 2016, 12, 1744806916644928. [Google Scholar] [CrossRef]
- Sacerdote, P. Opioids and the immune system. Palliat. Med. 2006, 20 (Suppl. S1), s9–s15. [Google Scholar] [CrossRef]
- Sibinga, N.E.S.; Goldstein, A. Opioid Peptides and Opioid Receptors in Cells of the Immune System. Annu. Rev. Immunol. 1988, 6, 219–249. [Google Scholar] [CrossRef] [PubMed]
- Borner, C.; Stumm, R.; Hollt, V.; Kraus, J. Comparative analysis of mu-opioid receptor expression in immune and neuronal cells. J. Neuroimmunol. 2007, 188, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Koodie, L.; Krishnan, A.G.; Charboneau, R.; Barke, R.A.; Roy, S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am. J. Pathol. 2010, 176, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Börner, C.; Kraus, J.; Schröder, H.; Ammer, H.; Höllt, V. Transcriptional Regulation of the Human μ-Opioid Receptor Gene by Interleukin-6. Mol. Pharmacol. 2004, 66, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Borner, C.; Lanciotti, S.; Koch, T.; Hollt, V.; Kraus, J. mu opioid receptor agonist-selective regulation of interleukin-4 in T lymphocytes. J. Neuroimmunol. 2013, 263, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Franchi, S.; Moschetti, G.; Amodeo, G.; Sacerdote, P. Do All Opioid Drugs Share the Same Immunomodulatory Properties? A Review from Animal and Human Studies. Front. Immunol. 2019, 10, 2914. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.D.; Lewis, M.G.; Fan, X.; Rappaport, J.; Rogers, T.J. Effect of chronic morphine administration on circulating T cell population dynamics in rhesus macaques. J. Neuroimmunol. 2013, 265, 43–50. [Google Scholar] [CrossRef]
- Raffaeli, W.; Malafoglia, V.; Bonci, A.; Tenti, M.; Ilari, S.; Gremigni, P.; Iannuccelli, C.; Gioia, C.; Di Franco, M.; Mollace, V.; et al. Identification of MOR-Positive B Cell as Possible Innovative Biomarker (Mu Lympho-Marker) for Chronic Pain Diagnosis in Patients with Fibromyalgia and Osteoarthritis Diseases. Int. J. Mol. Sci. 2020, 21, 1499. [Google Scholar] [CrossRef] [PubMed]
- Borner, C.; Warnick, B.; Smida, M.; Hartig, R.; Lindquist, J.A.; Schraven, B.; Hollt, V.; Kraus, J. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J. Immunol. 2009, 183, 882–889. [Google Scholar] [CrossRef]
- Yeager, M.P.; Colacchio, T.A.; Yu, C.T.; Hildebrandt, L.; Howell, A.L.; Weiss, J.; Guyre, P.M. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology 1995, 83, 500–508. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Verschueren, M.V.; van der Welle, C.M.C.; Tonn, M.; Schramel, F.; Peters, B.J.M.; van de Garde, E.M.W. The association between gut microbiome affecting concomitant medication and the effectiveness of immunotherapy in patients with stage IV NSCLC. Sci. Rep. 2021, 11, 23331. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Sano, Y.; Niho, S.; Kawasumi, K.; Mochizuki, N.; Yoh, K.; Matsumoto, S.; Zenke, Y.; Ikeda, T.; Nosaki, K.; et al. Impact of concomitant medication on clinical outcomes in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors: A retrospective study. Thorac. Cancer 2021, 12, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Kostine, M.; Mauric, E.; Tison, A.; Barnetche, T.; Barre, A.; Nikolski, M.; Rouxel, L.; Dutriaux, C.; Dousset, L.; Prey, S.; et al. Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur. J. Cancer 2021, 157, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Cani, M.; Bironzo, P.; Garetto, F.; Buffoni, L.; Cotogni, P. Immune Checkpoint Inhibitors and Opioids in Patients with Solid Tumours: Is Their Association Safe? A Systematic Literature Review. Healthcare 2022, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Brook, K.; Bennett, J.; Desai, S.P. The Chemical History of Morphine: An 8000-year Journey, from Resin to de-novo Synthesis. J. Anesth. Hist. 2017, 3, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, C.I.; Santini, D.; Maranzano, E.; Berti, M.; Roila, F.; Group, E.G.W. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23 (Suppl. S7), vii139–vii154. [Google Scholar] [CrossRef] [PubMed]
- Florence, C.S.; Zhou, C.; Luo, F.; Xu, L. The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States 2013. Med. Care 2016, 54, 901–906. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef]
- Mudumbai, S.C.; He, H.; Chen, J.Q.; Kapoor, A.; Regala, S.; Mariano, E.R.; Stafford, R.S.; Abnet, C.C.; Pfeiffer, R.M.; Freedman, N.D.; et al. Opioid use in cancer patients compared with noncancer pain patients in a veteran population. JNCI Cancer Spectr. 2024, 8, pkae012. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Magnusson, P.; Christo, P.J.; Lequang, J.A.; Breve, F.; Mitchell, K.; Varrassi, G. Opioid Therapy in Cancer Patients and Survivors at Risk of Addiction, Misuse or Complex Dependency. Front. Pain Res. 2021, 2, 691720. [Google Scholar] [CrossRef]
- Lewis, C.E.S.; Schutzer-Weissmann, J.; Farquhar-Smith, P. Opioid use disorder in cancer patients. Curr. Opin. Support. Palliat. Care 2023, 17, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, H.; Zendehdel, K.; Kamangar, F.; Malekzadeh, R.; Haghdoost, A.A. An Ecological Study of the Association between Opiate Use and Incidence of Cancers. Addict. Health 2016, 8, 252–260. [Google Scholar] [PubMed]
- Mansouri, M.; Naghshi, S.; Parsaeian, M.; Sepanlou, S.G.; Poustchi, H.; Momayez Sanat, Z.; Sadeghi, O.; Pourshams, A. Opium Use and Cancer Risk: A Comprehensive Systematic Review and Meta-Analysis of Observational Studies. Int. J. Clin. Pract. 2022, 2022, 5397449. [Google Scholar] [CrossRef] [PubMed]
- Grinshpoon, A.; Barchana, M.; Lipshitz, I.; Rosca, P.; Weizman, A.; Ponizovsky, A.M. Methadone maintenance and cancer risk: An Israeli case registry study. Drug Alcohol Depend. 2011, 119, 88–92. [Google Scholar] [CrossRef]
- Boudreau, D.M.; Chen, L.; Yu, O.; Bowles, E.J.A.; Chubak, J. Risk of second breast cancer events with chronic opioid use in breast cancer survivors. Pharmacoepidemiol. Drug Saf. 2019, 28, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Song, I.A. Chronic Opioid Use and Risk of Cancer in Patients with Chronic Noncancer Pain: A Nationwide Historical Cohort Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lin, J.A.; Chang, C.L.; Wu, S.Y.; Zhang, J. Association between long-term opioid use and cancer risk in patients with chronic pain: A propensity score-matched cohort study. Br. J. Anaesth. 2022, 129, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Havidich, J.E.; Weiss, J.E.; Onega, T.L.; Low, Y.H.; Goodrich, M.E.; Davis, M.A.; Sites, B.D. The association of prescription opioid use with incident cancer: A Surveillance, Epidemiology, and End Results-Medicare population-based case-control study. Cancer 2021, 127, 1648–1657. [Google Scholar] [CrossRef]
- Zheng, J.; He, J.; Wang, W.; Zhou, H.; Cai, S.; Zhu, L.; Qian, X.; Wang, J.; Lu, Z.; Huang, C. The impact of pain and opioids use on survival in cancer patients. Medicine 2020, 99, e19306. [Google Scholar] [CrossRef]
- Nelson, D.B.; Cata, J.P.; Niu, J.; Mitchell, K.G.; Vaporciyan, A.A.; Antonoff, M.B.; Hofstetter, W.L.; Giordano, S.H.; Sepesi, B.; Mehran, R.J.; et al. Persistent opioid use is associated with worse survival after lobectomy for stage i non-small cell lung cancer. Pain 2019, 160, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Sng, D.D.D.; Uitenbosch, G.; de Boer, H.D.; Carvalho, H.N.; Cata, J.P.; Erdoes, G.; Heytens, L.; Lois, F.J.; Pelosi, P.; Rousseau, A.-F.; et al. Developing expert international consensus statements for opioid-sparing analgesia using the Delphi method. BMC Anesthesiol. 2023, 23, 62. [Google Scholar] [CrossRef]
- Smith, L.; Cata, J.P.; Forget, P. Immunological Insights into Opioid-Free Anaesthesia in Oncological Surgery: A Scoping Review. Curr. Oncol. Rep. 2022, 24, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Rangel, F.P.; Auler, J.O.C., Jr.; Carmona, M.J.C.; Cordeiro, M.D.; Nahas, W.C.; Coelho, R.F.; Simoes, C.M. Opioids and premature biochemical recurrence of prostate cancer: A randomised prospective clinical trial. Br. J. Anaesth. 2021, 126, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; Meyer-Treschan, T.A.; Grady, M.; Tan, E.Y.; et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-Z.; Li, H.-J.; Li, M.-H.; Huang, S.-M.; Li, X.; Liu, Q.-H.; Li, J.; Li, X.-Y.; Wang, D.-X.; Sessler, D.I. Epidural Anesthesia–Analgesia and Recurrence-free Survival after Lung Cancer Surgery: A Randomized Trial. Anesthesiology 2021, 135, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Tombal, B.; Scholtes, J.L.; Nzimbala, J.; Meulders, C.; Legrand, C.; Van Cangh, P.; Cosyns, J.P.; De Kock, M. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? Eur. J. Anaesthesiol. 2011, 28, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Keerty, V.; Keerty, D.; Feng, L.; Norman, P.H.; Gottumukkala, V.; Mehran, J.R.; Engle, M. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014, 3, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Jeon, J.H.; Lee, J.M.; Kim, M.S.; Kim, J.H.; Cho, H.; Kim, S.E.; Eom, W. Investigation of opioid use and long-term oncologic outcomes for non-small cell lung cancer patients treated with surgery. PLoS ONE 2017, 12, e0181672. [Google Scholar] [CrossRef]
- Oh, T.K.; Jeon, J.H.; Lee, J.M.; Kim, M.S.; Kim, J.H.; Lim, H.; Kim, S.E.; Eom, W. Association of high-dose postoperative opioids with recurrence risk in esophageal squamous cell carcinoma: Reinterpreting ERAS protocols for long-term oncologic surgery outcomes. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Maher, D.P.; Walia, D.; Heller, N.M. Suppression of Human Natural Killer Cells by Different Classes of Opioids. Anesth. Analg. 2019, 128, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Patino, M.A.; Ramirez, R.E.; Perez, C.A.; Feng, L.; Kataria, P.; Myers, J.; Cata, J.P. The impact of intraoperative opioid use on survival after oral cancer surgery. Oral Oncol. 2017, 74, 1–7. [Google Scholar] [CrossRef]
- Owusu-Agyemang, P.; Cata, J.P.; Meter, A.V.; Kapoor, R.; Zavala, A.M.; Williams, U.U.; Tsai, J.; Rebello, E.; Feng, L.; Hayes-Jordan, A. Perioperative factors associated with persistent opioid use after extensive abdominal surgery in children and adolescents: A retrospective cohort study. Paediatr. Anaesth. 2018, 28, 625–631. [Google Scholar] [CrossRef]
- Tai, Y.-H.; Wu, H.L.; Chang, W.K.; Tsou, M.Y.; Chen, H.H.; Chang, K.Y. Intraoperative Fentanyl Consumption Does Not Impact Cancer Recurrence or Overall Survival after Curative Colorectal Cancer Resection. Sci. Rep. 2017, 7, 10816. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Zafereo, M.; Villarreal, J.; Unruh, B.D.; Truong, A.; Truong, D.T.; Feng, L.; Gottumukkala, V. Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: A retrospective study. J. Clin. Anesth. 2015, 27, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Du, K.N.; Feng, L.; Newhouse, A.; Mehta, J.; Lasala, J.; Mena, G.E.; Hofstetter, W.L.; Cata, J.P. Effects of Intraoperative Opioid Use on Recurrence-Free and Overall Survival in Patients with Esophageal Adenocarcinoma and Squamous Cell Carcinoma. Anesth. Analg. 2018, 127, 210–216. [Google Scholar] [CrossRef]
- Sathornviriyapong, A.; Nagaviroj, K.; Anothaisintawee, T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat. Care 2016, 15, 95. [Google Scholar] [CrossRef]

| Author/Year/Ref # | Type of Cancer | Opioids | Dose | Findings |
|---|---|---|---|---|
| Forget et al, 2011 [99] | Prostate cancer | Sufentanil | Mean sufentanil dose: 23 μg | Increased risk of recurrence (HR: 7.78; 95% CI: 5.79–1.78) |
| Cata et al., 2014 [100] | Non–small cell Lung cancer | Morphine | Median morphine equivalents: 1358.6 mg | No impact on RFS (HR: 1.074 CI 95%:0.989–1.166) No impact on OS (HR: 1.06 CI 95%:0.964–1.165) |
| Oh et al., 2017 [101] | Non–small cell Lung cancer | Morphine | Median morphine equivalents: 819 mg | Increased risk of recurrence (aHR: 1.415; 95% CI: 1.123–1.781) Higher mortality (aHR: 1.514; 95% CI: 1.197–1.916) |
| Oh et al., 2017 [102] | Esophageal SCC | Remifentanil, Morphine, hydromorphone, fentanyl, oxycodone, | Morphine equivalent 10 mg | Increase risk of reoccurance. (aHR, 1.274; 95% CI: 0.922–1.761;) |
| Maher et al., 2019 [103] | Non–small cell Lung cancer | Morphine | Mean morphine equivalents: 124 vs. 232 mg | Increased risk of recurrence (OR: 1.003; 95% CI: 1.000–1.006) |
| Cata et al., 2014 [100] | Laryngeal SCC | Fentanyl | Median fentanyl equivalents: 526 μg | Shorter RFS (HR: 1.001; 95% CI: 1.00–1.001) Shorter OS (HR: 1.001; 95% CI: 1.00–1.001). |
| Patino et al., 2017 [104] | Oral cancer | Fentanyl | Median fentanyl equivalents: 1081 μg | No impact on RFS (HR: 1.27; CI 95%:0.838–1.924) Shorter OS (HR: 1.77; CI 95%: 0.995–3.149] |
| Owusu-Aygemang et al., 2018 [105] | Pediatric Abdominal Malignancies | Morphine | Median morphine equivalents: 18.9 mg | No impact on RFS (HR; 1.00; 95% CI: 0.99–1.02) No impact on OS (HR; 1.01; 95% CI: 0.99–1.03) |
| Tai et al.,2017 [106] | Colorectal cancer | Fentanyl | Mean fentanyl dose: 3 μg/kg | No impact on RFS (aHR: 0.93; 95% CI: 0.74–1.17) No impact on OS (aHR: 0.79; 95% CI: 0.52–1.19) |
| Cata et al., 2015 [107] | Non-small cell lung cancer | Fentanyl, sufentanil, remifentanil | Fentanyl equivalents >28.2 µg/kg | Impact survival (aHR: 0.779, 95% CI: 0.619–0.980 ) |
| Patino et al., 2017 [104] | Oral cancer | Fentanyl, sufentanil, remifentanil, morphine, hydromorphone | Fentanyl equivalent 1 µg/kg | Higher mortality risk (aHR: 1.27, 95% CI: 0.838–1.924) No impact on OS (aHR: 1.77, 95% CI: 0.995–3.149) |
| Du et al., 2018 [108] | Esophageal cancer | Fentanyl, sufentanil Remifentanil and hydromorphone | Fentanyl equivalent 1 μg of fentanyl were as follows: 0.1 μg of sufentanil, 1 μg of remifentanil, and 10 μg of hydromorphone | Better RFS (aHR: 0.376, CI 95%: 0.201–0.704) Improved OS (aHR: 0.346, CI 95%: 0.177–0.676) |
| Sathornviriyanpong et al.,2016 [109] | Advance Cancer | Morphine | Morphine equivalent 6.43 mg/day | No impact ≤30 vs >30 mg/day (aHR: 1.14, 95% CI: 0.77–1.69) |
| Zylla et al.,2014 [4] | Prostate | Oxycodone, hydrocodone, codeine, morphine, hydromorphone fentanyl and methadone | Morphine equivalent 5mg/day | Impact survival (aHR: 0.92, 95% CI: 0.68–1.25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sah, D.; Shoffel-Havakuk, H.; Tsur, N.; Uhelski, M.L.; Gottumukkala, V.; Cata, J.P. Opioids and Cancer: Current Understanding and Clinical Considerations. Curr. Oncol. 2024, 31, 3086-3098. https://doi.org/10.3390/curroncol31060235
Sah D, Shoffel-Havakuk H, Tsur N, Uhelski ML, Gottumukkala V, Cata JP. Opioids and Cancer: Current Understanding and Clinical Considerations. Current Oncology. 2024; 31(6):3086-3098. https://doi.org/10.3390/curroncol31060235
Chicago/Turabian StyleSah, Dhananjay, Hagit Shoffel-Havakuk, Nir Tsur, Megan L. Uhelski, Vijaya Gottumukkala, and Juan P. Cata. 2024. "Opioids and Cancer: Current Understanding and Clinical Considerations" Current Oncology 31, no. 6: 3086-3098. https://doi.org/10.3390/curroncol31060235
APA StyleSah, D., Shoffel-Havakuk, H., Tsur, N., Uhelski, M. L., Gottumukkala, V., & Cata, J. P. (2024). Opioids and Cancer: Current Understanding and Clinical Considerations. Current Oncology, 31(6), 3086-3098. https://doi.org/10.3390/curroncol31060235






