Radiological and Not Clinical Variables Guide the Surgical Plan in Patients with Glioblastoma
Abstract
1. Introduction
2. Methods
2.1. Type of Study
2.2. Subjects
2.3. Clinical Variable Evaluation
2.4. Pathological Variable Evaluation
2.5. Radiological Variable Evaluation
2.6. Statistics
3. Results
3.1. Comparison between B and CR Patients
3.2. Factors Associated with the Selection of Biopsy or Resection
3.3. Factors Associated with Prognosis in the B and CR Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-ALA | 5-Aminolevulinic acid |
| B | Biopsy |
| CNS | Central nervous system |
| CR | Complete resection |
| EOR | Extent of resection |
| HR | Hazard ratio |
| KPS | Karnofsky Performance Status |
| MGMT | O(6)-methylguanine-DNA methyltransferase |
| MRI | Magnetic resonance imaging |
| NCR | Necrosis-to-contrast enhancement ratio |
| OR | Odds ratio |
| OS | Overall survival |
| PFS | Progression-free survival |
| RANO | Response Assessment in Neuro-Oncology |
| RV | Residual volume |
| SD | Standard deviation |
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro. Oncol. 2021, 23, III1–III105. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours of the Central Nervous System, 5th ed.; WHO: Lyon, France, 2021. [Google Scholar]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Van Den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet. Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Nabors, L.B.; Portnow, J.; Ahluwalia, M.; Baehring, J.; Brem, H.; Brem, S.; Butowski, N.; Campian, J.L.; Clark, S.W.; Fabiano, A.J.; et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1537–1570. [Google Scholar] [CrossRef]
- Rogers, C.M.; Jones, P.S.; Weinberg, J.S. Intraoperative MRI for Brain Tumors. J. Neuro-Oncol. 2021, 151, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.T.; Lee, H.; Shui, C.; Lamba, N.; Korde, R.; Devi, S.; Chawla, S.; Nam, Y.; Patel, R.; Doucette, J.; et al. Intraoperative Magnetic Resonance Imaging for Low-Grade and High-Grade Gliomas: What Is the Evidence? A Meta-Analysis. World Neurosurg. 2021, 149, 232–243. [Google Scholar] [CrossRef]
- Incekara, F.; Smits, M.; Dirven, L.; Bos, E.M.; Balvers, R.K.; Haitsma, I.K.; Schouten, J.W.; Vincent, A.J.P.E. Intraoperative B-Mode Ultrasound Guided Surgery and the Extent of Glioblastoma Resection: A Randomized Controlled Trial. Front. Oncol. 2021, 11, 649797. [Google Scholar] [CrossRef]
- Saß, B.; Zivkovic, D.; Pojskic, M.; Nimsky, C.; Bopp, M.H.A. Navigated Intraoperative 3D Ultrasound in Glioblastoma Surgery: Analysis of Imaging Features and Impact on Extent of Resection. Front. Neurosci. 2022, 16, 883584. [Google Scholar] [CrossRef]
- Li, Y.-C.; Chiu, H.-Y.; Lin, Y.-J.; Chen, K.-T.; Hsu, P.-W.; Huang, Y.-C.; Chen, P.-Y.; Wei, K.-C. The Merits of Awake Craniotomy for Glioblastoma in the Left Hemispheric Eloquent Area: One Institution Experience. Clin. Neurol. Neurosurg. 2021, 200, 106343. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Muragaki, Y.; Saito, T.; Maruyama, T.; Nitta, M.; Tsuzuki, S.; Iseki, H.; Okada, Y. Strategy of surgical resection for glioma based on intraoperative functional mapping and monitoring. Neurol. Med.-Chir. 2015, 55, 383–398. [Google Scholar] [CrossRef]
- Hardesty, D.A.; Sanai, N. The value of glioma extent of resection in the modern neurosurgical era. Front. Neurol. 2012, 3, 140. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Cofano, F.; Salvati, L.F.; Monticelli, M.; Zeppa, P.; Di Perna, G.; Melcarne, A.; Altieri, R.; La Rocca, G.; Sabatino, G.; et al. Fluorescence-Guided Surgery for High-Grade Gliomas: State of the Art and New Perspectives. Technol. Cancer Res. Treat. 2021, 20, 15330338211021605. [Google Scholar] [CrossRef]
- Wykes, V.; Zisakis, A.; Irimia, M.; Ughratdar, I.; Sawlani, V.; Watts, C. Importance and Evidence of Extent of Resection in Glioblastoma. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2021, 82, 075–086. [Google Scholar] [CrossRef] [PubMed]
- Revilla-Pacheco, F.; Rodríguez-Salgado, P.; Barrera-Ramírez, M.; Morales-Ruiz, M.P.; Loyo-Varela, M.; Rubalcava-Ortega, J.; Herrada-Pineda, T. Extent of resection and survival in patients with glioblastoma multiforme: Systematic review and meta-analysis. Medicine 2021, 100, e26432. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Kuhnt, D.; Becker, A.; Ganslandt, O.; Bauer, M.; Buchfelder, M.; Nimsky, C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro-Oncology 2011, 13, 1339–1348. [Google Scholar] [CrossRef]
- Orringer, D.; Lau, D.; Khatri, S.; Zamora-Berridi, G.J.; Zhang, K.; Wu, C.; Chaudhary, N.; Sagher, O. Extent of resection in patients with glioblastoma: Limiting factors, perception of resectability, and effect on survival. J. Neurosurg. 2012, 117, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-Oncology 2014, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Segovia, J.; Ganslandt, O.; Ringel, F.; Wirtz, C.R.; Renovanz, M. Counseling Patients with a Glioblastoma Amenable Only for Subtotal Resection: Results of a Multicenter Retrospective Assessment of Survival and Neurologic Outcome. World Neurosurg. 2018, 114, e1180–e1185. [Google Scholar] [CrossRef]
- Yong, R.L.; Lonser, R.R. Surgery for glioblastoma multiforme: Striking a balance. World Neurosurg. 2011, 76, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.; Price, S.; Santarius, T. Current concepts in the surgical management of Glioma patients. Clin. Oncol. 2014, 26, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Leiss, L.; Yang, N.; Rygh, C.B.; Mitra, S.S.; Cheshier, S.H.; Weissman, I.L.; Huang, B.; Miletic, H.; Bjerkvig, R.; et al. Surgical debulking promotes recruitment of macrophages and triggers glioblastoma phagocytosis in combination with CD47 blocking immunotherapy. Oncotarget 2017, 8, 12145–12157. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Heath, R.N.; Shah, A.H.; Sanjurjo, A.D.; Eichberg, D.G.; Luther, E.M.; de la Fuente, M.I.; Komotar, R.J.; Ivan, M.E. Resection versus biopsy in the treatment of multifocal glioblastoma: A weighted survival analysis. J. Neuro-Oncol. 2020, 148, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Tunthanathip, T.; Madteng, S. Factors associated with the extent of resection of glioblastoma. Precis. Cancer Med. 2020, 3, 12. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Broekman, M.L.D.; De Vleeschouwer, S.; Schucht, P.; Jungk, C.; Krieg, S.M.; Nahed, B.V.; Berger, M.S.; Vincent, A.J.P.E. Decision making and surgical modality selection in glioblastoma patients: An international multicenter survey. J. Neuro-Oncol. 2022, 156, 465–482. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Ewelt, C.; Goeppert, M.; Rapp, M.; Steiger, H.-J.; Stummer, W.; Sabel, M. Glioblastoma multiforme of the elderly: The prognostic effect of resection on survival. J. Neuro-Oncol. 2010, 103, 611–618. [Google Scholar] [CrossRef]
- Bloch, O.; Han, S.J.; Cha, S.; Sun, M.Z.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Impact of extent of resection for recurrent glioblastoma on overall survival: Clinical article. J. Neurosurg. 2012, 117, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Gallego Pérez-Larraya, J.; Delattre, J.-Y. Management of Elderly Patients with Gliomas. Oncologist 2014, 19, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Almenawer, S.A.; Badhiwala, J.H.; Alhazzani, W.; Greenspoon, J.; Farrokhyar, F.; Yarascavitch, B.; Algird, A.; Kachur, E.; Cenic, A.; Sharieff, W.; et al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: A systematic review and meta-analysis. Neuro-Oncology 2015, 17, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipour, Y.; Kaur, M.; Pierscianek, D.; Gembruch, O.; Oppong, M.D.; Mueller, O.; Jabbarli, R.; Glas, M.; Sure, U.; El Hindy, N. Association of Surgical Resection, Disability, and Survival in Patients with Glioblastoma. J. Neurol. Surgery Part A Cent. Eur. Neurosurg. 2019, 80, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Satoer, D.; Visch-Brink, E.; Dirven, C.; Vincent, A. Glioma surgery in eloquent areas: Can we preserve cognition? Acta Neurochir. 2016, 158, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Is non-awake surgery for supratentorial adult low-grade glioma treatment still feasible? Neurosurg. Rev. Neurosurg. Rev. 2018, 41, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Aabedi, A.A.; Young, J.S.; Zhang, Y.; Ammanuel, S.; Morshed, R.A.; Ore, C.D.; Brown, D.; Phillips, J.J.; Bush, N.A.O.; Taylor, J.W.; et al. Association of Neurological Impairment on the Relative Benefit of Maximal Extent of Resection in Chemoradiation-Treated Newly Diagnosed Isocitrate Dehydrogenase Wild-Type Glioblastoma. Neurosurgery 2022, 90, 124–130. [Google Scholar] [CrossRef]
- Leu, S.; von Felten, S.; Frank, S.; Vassella, E.; Vajtai, I.; Taylor, E.; Schulz, M.; Hutter, G.; Hench, J.; Schucht, P.; et al. IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro-Oncology 2013, 15, 469–479. [Google Scholar] [CrossRef]
- Incekara, F.; Smits, M.; Van der Voort, S.R.; Dubbink, H.J.; Atmodimedjo, P.N.; Kros, J.M.; Vincent, A.J.; van den Bent, M. The Association Between the Extent of Glioblastoma Resection and Survival in Light of MGMT Promoter Methylation in 326 Patients with Newly Diagnosed IDH-Wildtype Glioblastoma. Front. Oncol. 2020, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Gessler, F.; Bernstock, J.D.; Braczynski, A.; Lescher, S.; Baumgarten, P.; Harter, P.N.; Mittelbronn, M.; Wu, T.; Seifert, V.; Senft, C. Surgery for glioblastoma in light of molecular markers: Impact of resection and MGMT promoter methylation in newly diagnosed IDH-1 wild-type glioblastomas. Neurosurgery 2019, 84, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor with Survival Within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Binabaj, M.M.; Bahrami, A.; ShahidSales, S.; Joodi, M.; Joudi Mashhad, M.; Hassanian, S.M.; Anvari, K.; Avan, A. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J. Cell. Physiol. 2018, 233, 378–386. [Google Scholar] [CrossRef]
- Chojak, R.; Koźba-Gosztyła, M.; Słychan, K.; Gajos, D.; Kotas, M.; Tyliszczak, M.; Czapiga, B. Impact of surgical resection of butterfly glioblastoma on survival: A meta-analysis based on comparative studies. Sci. Rep. 2021, 11, 13934. [Google Scholar] [CrossRef]
- Kommers, I.O.; Eijgelaar, R.S.; Barkhof, F.; Bouget, D.; Pedersen, A.; Ardon, H.; Bello, L.; Berger, M.S.; Bouwknegt, W.; Nibali, M.C.; et al. P11.37.B When to resect or biopsy for patients with supratentorial glioblastoma: A multivariable prediction model. Neuro-Oncology 2022, 24, ii65–ii66. [Google Scholar] [CrossRef]
| Variable | Mean (SD) Count (%) | |
|---|---|---|
| Age | 61.18 (SD = 11.45) | |
| Gender (female:male) | 41:58 | |
| Karnofsky Performance Status (KPS) ≥ 70 | 91 (91.9%) | |
| Neurological deficit | 74 (74.7%) | |
| Epileptic seizures | 16 (16.2%) | |
| Headache | 23 (23.2%) | |
| Brain hemisphere | Left | 57 (57.6%) |
| Right | 40 (40.4% | |
| Bilateral | 2 (2.0%) | |
| Contrast-enhancement type | Ring | 50 (51.0%) |
| Heterogeneous | 47 (48.0%) | |
| No enhancement | 1 (1.0%) | |
| >2 lobes affected | 27 (27.3%) | |
| Subventricular zone involvement | 74 (74.7%) | |
| Corpus callosum involvement | 39 (39.4%) | |
| Internal capsule < 1 cm | 41 (41.4%) | |
| Contrast-enhancement volume (cc) | 21.77 (SD = 15.22) | |
| Edema volumen (cc) | 55.32 (SD = 34.78) | |
| Necrosis volumen (cc) | 9.39 (SD = 9.92) | |
| Necrosis–tumor enhancement ratio (NTR) | 0.41 (SD = 0.41) | |
| ASA | 1–2 | 76 (76.8%) |
| 3–4 | 23 (23.2%) | |
| Risk of serious complication (%) | 7.22 (SD = 3.44) | |
| Risk of any complication (%) | 8.64 (SD = 3.41) | |
| Surgical intention | Biopsy | 31 (31.3%) |
| Complete | 68 (68.7%) | |
| IDH mutation | 1 (1.0%) | |
| Ki-67 > 20% | 47 (52.2%) | |
| MGMT methylation | 57 (58.2%) | |
| Variable | Surgical Intention | p-Value | |||
|---|---|---|---|---|---|
| Biopsy (n = 31) | Complete Resection (n = 68) | ||||
| Age | 60.9 (SD = 12.98) | 61.31 (SD = 10.78) | 0.824 | ||
| Gender (female:male) | 14:17 | 27:41 | 0.663 | ||
| Karnofsky Performance Status (KPS) | <70 | 2 (6.5%) | 6 (8.8%) | 0.688 | |
| ≥70 | 29 (93.5%) | 62 (91.2%) | |||
| Neurological deficit | 24 (77.4%) | 50 (73.5%) | 0.805 | ||
| Epileptic seizures | 5 (16.1%) | 11 (16.2%) | 1 | ||
| Headache | 11 (35.5%) | 12 (17.6%) | 0.072 | ||
| Brain hemisphere | Left | 20 (64.5%) | 37 (54.4%) | 0.045 | |
| Right | 9 (29.0%) | 31 (45.6%) | |||
| Bilateral | 2 (6.5%) | 0 | |||
| >2 lobes affected | 5 (17.9% | 22 (32.4%) | 0.213 | ||
| Subventricular zone involvement | 27 (87.1%) | 47 (69.1%) | 0.080 | ||
| Corpus callosum involvement | 17 (54.8%) | 22 (32.4%) | 0.046 | ||
| Internal capsule < 1 cm | 21 (67.7%) | 20 (29.4%) | 0.000 | ||
| Contrast-enhancement type | Ring | 11 (36.7%) | 39 (57.4%) | 0.070 | |
| Heterogeneous | 18 (60.0%) | 29 (42.6%) | |||
| No enhancement | 1 (3.3%) | 0 | |||
| Contrast-enhancement volume (cc) | 21.79 (SD = 17.92) | 21.76 (SD = 13.96) | 0.597 | ||
| Edema volume (cc) | 42.19 (SD = 29.15) | 61.30 (SD = 35.67) | 0.025 | ||
| Necrosis volume (cc) | 7.45 (SD = 10.42) | 10.27 (SD = 9.64) | 0.073 | ||
| Necrosis–tumor enhancement ratio (NTR) | 0.35 (SD = 0.52) | 0.44 (SD = 0.35) | 0.035 | ||
| ASA | 1–2 | 26 (83.9%) | 50 (73.5%) | 0.313 | |
| 3–4 | 5 (16.1%) | 18 (26.5%) | |||
| Risk of serious complication | 6.86 (SD = 2.57) | 7.38 (SD = 3.79) | 0.765 | ||
| Risk of any complication | 8.29 (SD = 2.55) | 8.80 (SD = 3.74) | 0.955 | ||
| IDH mutation | 1 (3.2%) | - | 0.313 | ||
| Ki-67 > 20% | 19 (67.9%) | 28 (45.2%) | 0.068 | ||
| MGMT methylation | 16 (53.3%) | 41 (60.3%) | 0.657 | ||
| Progression-free survival (months) | 6.2 [3.0–9.4] | 9.4 [7.9–10.8] | 0.068 | ||
| Overall survival (months) | 10.1 [8.3–12.0] | 15.6 [13.1–18.2] | 0.002 | ||
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Factor | Odds Ratio (95% C.I.) | p-Value | Odds Ratio (95% C.I.) | p-Value | |
| Age | 1.003 (0.967–1.041) | 0.870 | |||
| Gender | Male | 1.251 (0.530–2.950) | 0.610 | ||
| Female | 0.800 (0.339–1.886) | ||||
| Karnofsky Performance Status (KPS) < 70 | 1.403 (0.267–7.380) | 0.689 | |||
| Neurological deficit | 0.810 (0.298–2.201) | 0.680 | |||
| Hemiparesis | 5.789 (0.713–47.009) | 0.100 | |||
| Language disorder | 1.092 (0.431–2.770) | 0.853 | |||
| Cognitive impairment | 0.985 (0.336–2.890) | 0.978 | |||
| Sensory deficit | 0.909 (0.079–10.419) | 0.939 | |||
| Asthenia/apathy | 1.609 (0.410–6.312) | 0.495 | |||
| Behavioral disorder | 0.793 (0.242–2.598) | 0.702 | |||
| Gait disturbance | 0.804 (0.268–2.416) | 0.698 | |||
| Epileptic seizures | 1.004 (0.316–3.183) | 0.995 | |||
| Headache | 0.390 (0.149–1.022) | 0.055 | |||
| Brain hemisphere | Left | 0.656 (0.273–1.578) | 0.347 | ||
| Right | 2.048 (0.824–5.091) | 0.123 | |||
| Bilateral | - | - | |||
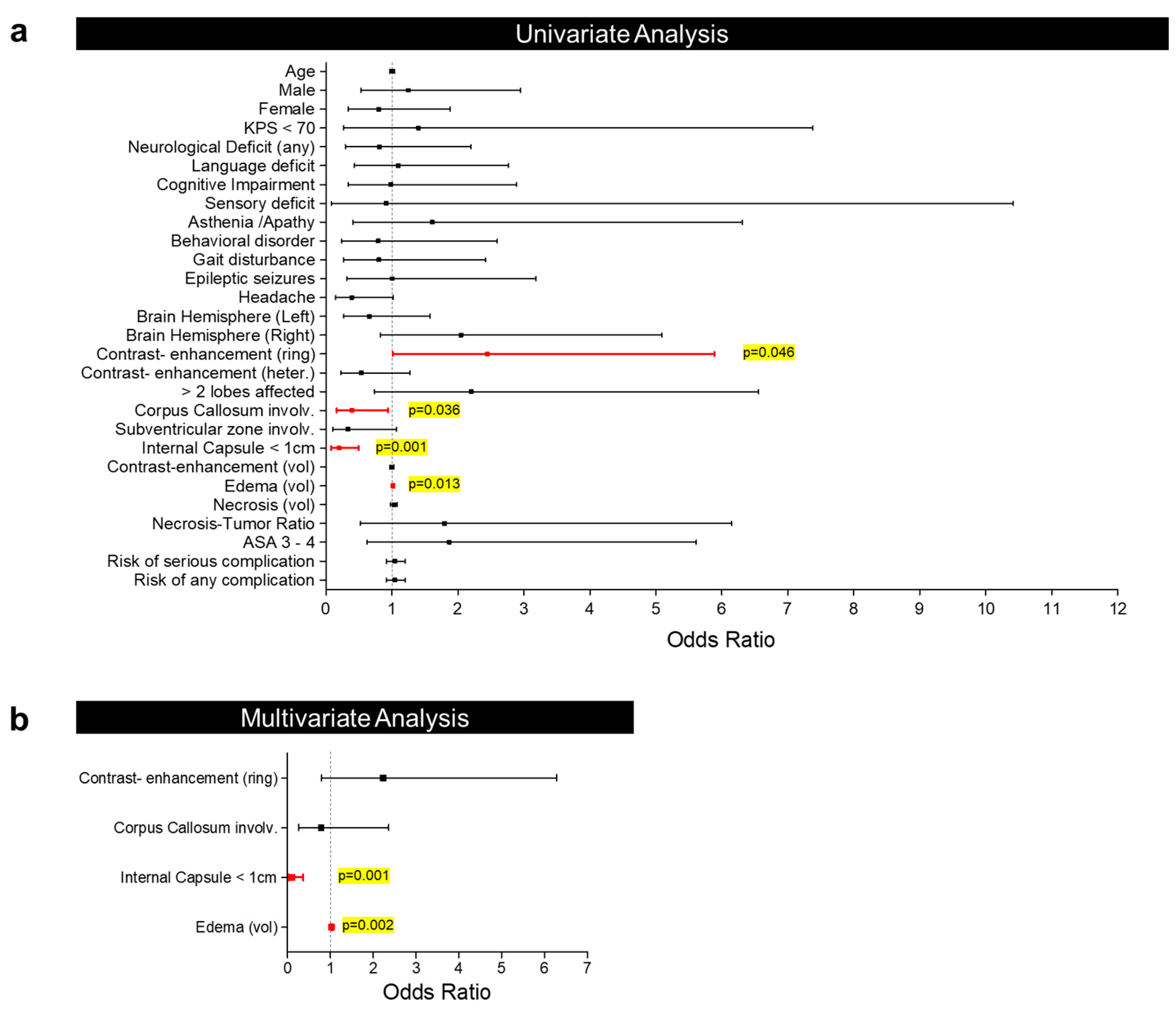
| Contrast-enhancement type | Ring | 2.445 (1.015–5.888) | 0.046 | 2.239 (0.798–6.282) | 0.126 |
| Heterogeneous | 0.537 (0.227–1.269) | 0.157 | |||
| No enhancement | - | - | |||
| >2 lobes affected | 2.200 (0.738–6.559) | 0.157 | |||
| Subventricular zone involvement | 0.332 (0.103–1.068) | 0.064 | |||
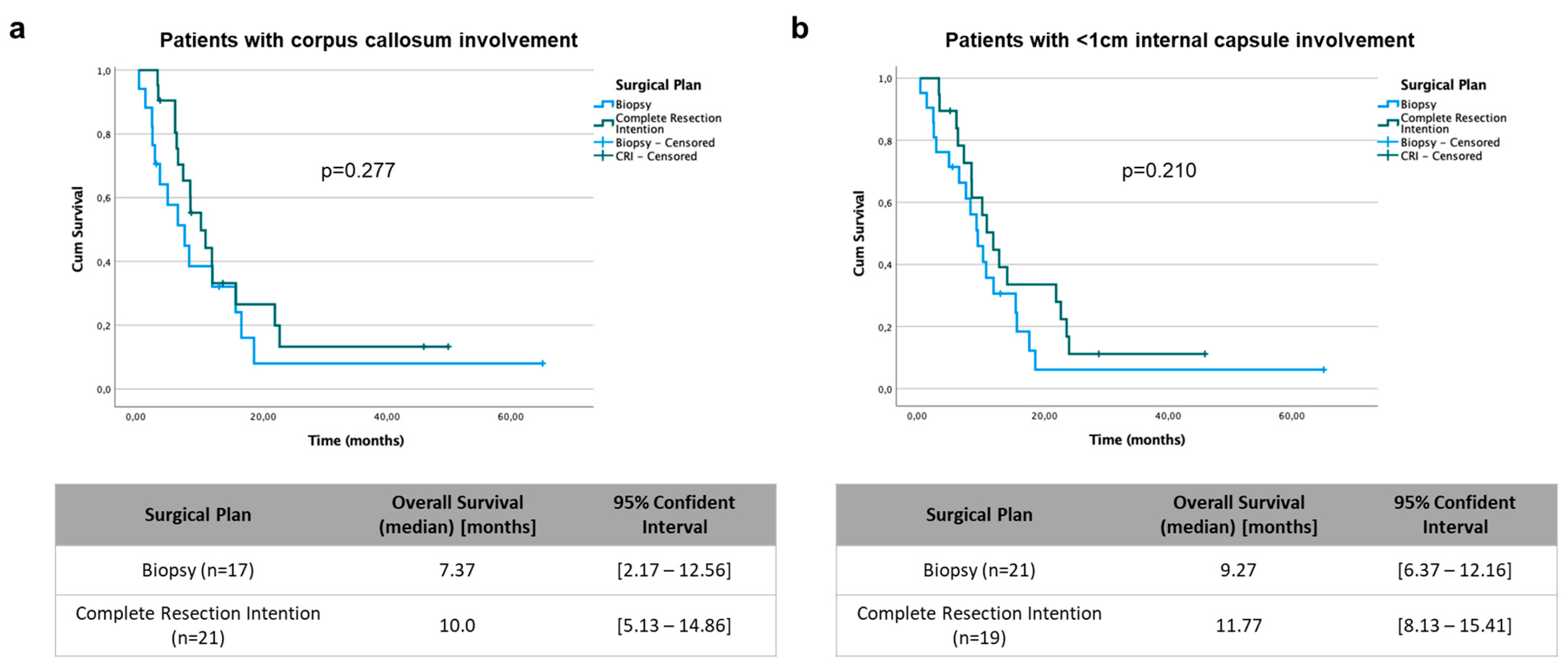
| Corpus callosum involvement | 0.394 (0.165–0.941) | 0.036 | 0.786 (0.261–2.365) | 0.669 | |
| Internal capsule < 1 cm | 0.198 (0.079–0.496) | 0.001 | 0.104 (0.029–0.372) | 0.001 | |
| Contrast-enhancement volume (cc) | 1.000 (0.972–1.028) | 0.993 | |||
| Edema volume (cc) | 1.019 (1.004–1.034) | 0.013 | 1.031 (1.011–1.051) | 0.002 | |
| Necrosis volume (cc) | 1.033 (0.984–1.084) | 0.194 | |||
| Necrosis–tumor enhancement ratio (NTR) | 1.793 (0.523–6.148) | 0.353 | |||
| ASA 3–4 | 1.872 (0.624–5.614) | 0.263 | |||
| Risk of serious complication | 1.048 (0.917–1.198) | 0.489 | |||
| Risk of any complication | 1.048 (0.916–1.198) | 0.494 | |||
| Biopsy | Complete Resection | ||||
|---|---|---|---|---|---|
| Factor | Hazard Ratio (95% C.I.) | p-Value | Hazard Ratio (95% C.I.) | p-Value | |
| Age | 1.017 (0.981–1.054) | 0.364 | 1.007 (0.976–1.040) | 0.653 | |
| Gender | Male | 1.397 (0.638–3.061) | 0.403 | 1.145 (0.635–2.067) | 0.652 |
| Female | 0.716 (0.327–1.568) | 0.873 (0.484–1.575) | |||
| Karnofsky Performance Status (KPS) < 70 | 10.319 (1.702–62.563) | 0.011 | 0.548 (0.170–1.769) | 0.314 | |
| Neurological deficit | 1.077 (0.428–2.714) | 0.875 | 0.705 (0.388–1.279) | 0.250 | |
| Epileptic seizures | 0.672 (0.200–2.257) | 0.520 | 2.123 (1.040–4.330) | 0.039 | |
| Headache | 0.540 (0.230–1.272) | 0.159 | 1.678 (0.821–3.430) | 0.156 | |
| Brain hemisphere | Left | 1.197 (0.511–2.804) | 0.679 | 0.591 (0.332–1.052) | 0.074 |
| Right | 0.716 (0.295–1.737) | 0.461 | 1.693 (0.950–3.016) | 0.074 | |
| Bilateral | 5.073 (0.557–46.223) | 0.150 | - | - | |
| Contrast-enhancement type | Ring | 1.357 (0.585–3.150) | 0.477 | 0.837 (0.467–1.500) | 0.550 |
| Heterogeneous | 0.649 (0.285–1.479) | 0.304 | 1.195 (0.667–2.141) | 0.550 | |
| No enhancement | 2.069 (0.266–16.071) | 0.487 | - | - | |
| >2 lobes affected | 1.520 (0.545–4.239) | 0.424 | 0.946 (0.509–1.756) | 0.860 | |
| Subventricular zone involvement | 1.512 (0.442–5.170) | 0.510 | 1.300 (0.702–2.407) | 0.404 | |
| Corpus callosum involvement | 1.117 (0.498–2.505) | 0.788 | 1.722 (0.934–3.174) | 0.082 | |
| Internal capsule < 1 cm | 1.065 (0.452–2.505) | 0.886 | 1.596 (0.869–2.933) | 0.132 | |
| Contrast-enhancement volume (cc) | 1.031 (1.006–1.058) | 0.017 | 0.993 (0.972–1.015) | 0.522 | |
| Edema volume (cc) | 1.013 (0.998–1.028) | 0.081 | 1.001 (0.992–1.010) | 0.792 | |
| Necrosis volume (cc) | 1.025 (0.987–1.065) | 0.200 | 0.984 (0.953–1.017) | 0.342 | |
| Necrosis–tumor enhancement ratio (NTR) | 0.496 (0.135–1.822) | 0.291 | 0.570 (0.247–1.312) | 0.186 | |
| ASA 3–4 | 1.716 (0.634–4.644) | 0.288 | 1.149 (0.595–2.219) | 0.679 | |
| Risk of serious complication | 1.093 (0.935–1.278) | 0.263 | 1.072 (0.988–1.163) | 0.096 | |
| Risk of any complication | 1.093 (0.930–1.284) | 0.282 | 1.068 (0.983–1.161) | 0.121 | |
| IDH mutation | 0.045 (0.000–324.863) | 0.495 | - | - | |
| Ki-67 > 20% | 1.619 (0.653–4.012) | 0.298 | 1.019 (0.548–1.892) | 0.953 | |
| MGMT methylation | 0.823 (0.374–1.811) | 0.628 | 0.448 (0.246–0.816) | 0.009 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Abreu, C.; Fariña-Jerónimo, H.; Plata-Bello, J. Radiological and Not Clinical Variables Guide the Surgical Plan in Patients with Glioblastoma. Curr. Oncol. 2024, 31, 1899-1912. https://doi.org/10.3390/curroncol31040142
Martín-Abreu C, Fariña-Jerónimo H, Plata-Bello J. Radiological and Not Clinical Variables Guide the Surgical Plan in Patients with Glioblastoma. Current Oncology. 2024; 31(4):1899-1912. https://doi.org/10.3390/curroncol31040142
Chicago/Turabian StyleMartín-Abreu, Carla, Helga Fariña-Jerónimo, and Julio Plata-Bello. 2024. "Radiological and Not Clinical Variables Guide the Surgical Plan in Patients with Glioblastoma" Current Oncology 31, no. 4: 1899-1912. https://doi.org/10.3390/curroncol31040142
APA StyleMartín-Abreu, C., Fariña-Jerónimo, H., & Plata-Bello, J. (2024). Radiological and Not Clinical Variables Guide the Surgical Plan in Patients with Glioblastoma. Current Oncology, 31(4), 1899-1912. https://doi.org/10.3390/curroncol31040142




