Ductal Adenocarcinoma of the Prostate with Novel Genetic Alterations Characterized by Next-Generation Sequencing
Abstract
1. Introduction
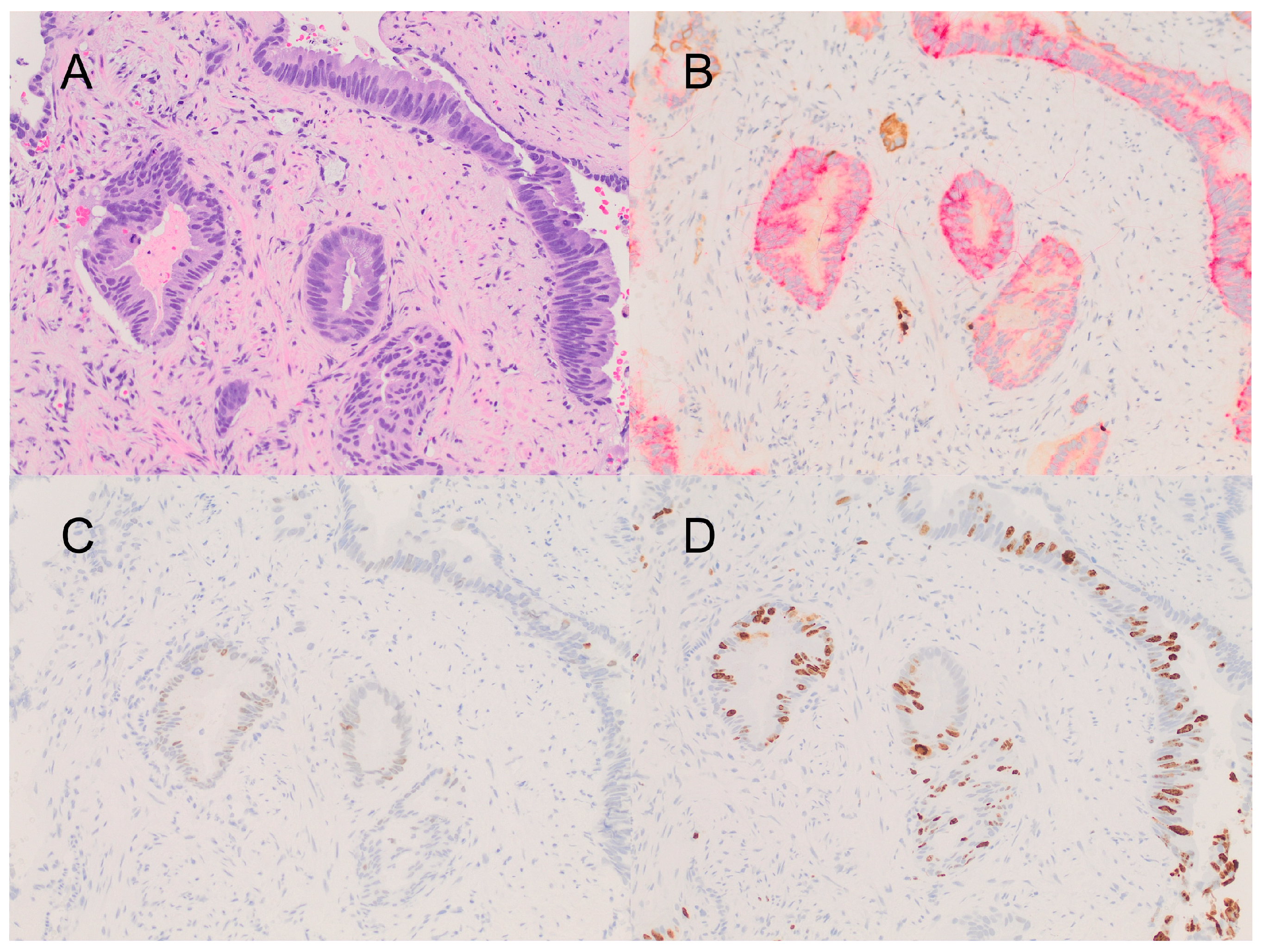
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Leenders, G.J.L.H.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N.; et al. ISUP Grading Workshop Panel Members. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87–e99. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V.E. (Eds.) WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; IARC: Lyon, France, 2016. [Google Scholar]
- Kumar, D.; Lobo, J. Prostatic Ductal Adenocarcinoma: An Unusual Subtype of Prostate Cancer. Int. J. Oncol. Res. 2019, 2, 013. [Google Scholar] [CrossRef]
- Inamura, K. Prostatic Cancers: Understanding Their Molecular Pathology and the 2016 WHO Classification. Oncotarget 2018, 9, 1472337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M. High-Grade Prostatic Intraepithelial Neoplasia, PIN-Like Carcinoma, Ductal Carcinoma, and Intraductal Carcinoma of the Prostate. Mod. Pathol. 2018, 31, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ranasinha, N.; Omer, A.; Philippou, Y.; Harriss, E.; Davies, L.; Chow, K.; Chetta, P.M.; Erickson, A.; Rajakumar, T.; Mills, I.G. Ductal adenocarcinoma of the prostate: A systematic review and meta-analysis of incidence, presentation, prognosis, and management. BJUI Compass 2021, 2, 13–23. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Antonarakis, E.S.; Bismar, T.A.; Guedes, L.B.; Cheng, H.H.; Tretiakova, M.S.; Vakar-Lopez, F.; Klemfuss, N.; Konnick, E.Q.; Mostaghel, E.A.; et al. Genomic Characterization of Prostatic Ductal Adenocarcinoma Identifies a High Prevalence of DNA Repair Gene Mutations. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Sanati, S.; Watson, M.A.; Salavaggione, A.L.; Humphrey, P.A. Gene expression profiles of ductal versus acinar adenocarcinoma of the prostate. Mod. Pathol. 2009, 22, 1273–1279. [Google Scholar] [CrossRef]
- Lotan, T.L.; Toubaji, A.; Albadine, R.; Latour, M.; Herawi, M.; Meeker, A.K.; DeMarzo, A.M.; Platz, E.A.; Epstein, J.I.; Netto, G.J. TMPRSS2-ERG gene fusions are infrequent in prostatic ductal adenocarcinomas. Mod. Pathol. 2009, 22, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Gillard, M.; Lack, J.; Pontier, A.; Gandla, D.; Hatcher, D.; Sowalsky, A.G.; Rodriguez-Nieves, J.; Vander Griend, D.; Paner, G.; VanderWeele, D. Integrative Genomic Analysis of Coincident Cancer Foci Implicates CTNNB1 and PTEN Alterations in Ductal Prostate Cancer. Eur. Urol. Focus 2019, 5, 433–442. [Google Scholar] [CrossRef]
- Khani, F.; Wobker, S.E.; Hicks, J.L.; Robinson, B.D.; Barbieri, C.E.; De Marzo, A.M.; Epstein, J.I.; Pritchard, C.C.; Lotan, T.L. Intraductal carcinoma of the prostate in the absence of high-grade invasive carcinoma represents a molecularly distinct type of in situ carcinoma enriched with oncogenic driver mutations. J. Pathol. 2019, 249, 79–89. [Google Scholar] [CrossRef]
- Boormans, J.L.; Korsten, H.; Ziel-van der Made, A.C.; van Leenders, G.J.; Verhagen, P.C.; Trapman, J. E17K substitution in AKT1 in prostate cancer. Br. J. Cancer 2010, 102, 1491–1494. [Google Scholar] [CrossRef]
- Ateeq, B.; Kunju, L.P.; Carskadon, S.L.; Pandey, S.K.; Singh, G.; Pradeep, I.; Tandon, V.; Singhai, A.; Goel, A.; Amit, S.; et al. Molecular profiling of ETS and non-ETS aberrations in prostate cancer patients from northern India. Prostate 2015, 75, 1051–1062. [Google Scholar] [CrossRef]
- The AACR Project GENIE Consortium. AACR Project GENIE: Powering precision medicine through an international consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Chmielecki, J.; Gay, L.; Johnson, A.; Chudnovsky, J.; Yelensky, R.; Lipson, D.; Ali, S.M.; Elvin, J.A.; et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int. J. Cancer 2016, 138, 881–890. [Google Scholar] [CrossRef]
- Robinson, D.; Chinnaiyan, A.M. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228, Erratum in Cell 2015, 162, 454. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hu, L.; Wu, Z.; Chen, Z.; Liu, S.; Xu, X.; Qian, A. CDK12: A potent target and biomarker for human cancer therapy. Cells 2020, 9, 1483. [Google Scholar] [CrossRef] [PubMed]
- Aldera, A.P.; Govender, D. Gene of the month: BCOR. J. Clin. Pathol. 2020, 73, 314–317. [Google Scholar] [CrossRef]
- Grossmann, V.; Tiacci, E.; Holmes, A.B.; Kohlmann, A.; Martelli, M.P.; Kern, W.; Spanhol-Rosseto, A.; Klein, H.; Dugas, M.; Schindela, S.; et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood 2011, 118, 6153–6163. [Google Scholar] [CrossRef]
- Lempiäinen, J.K.; Manjur, A.B.M.K.; Malinen, M.; Ketola, K.; Niskanen, E.A.; Palvimo, J.J. BCOR-coupled H2A monoubiquitination represses a subset of androgen receptor target genes regulating prostate cancer proliferation. Oncogene 2020, 39, 2391–2407. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Laxman, B.; Varambally, S.; Cao, X.; Yu, J.; Helgeson, B.E.; Cao, Q.; Prensner, J.R.; Rubin, M.A.; Shah, R.B.; et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 2008, 10, 177–188. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhang, J.; Hu, Q.; Zhi, F.; Zhang, S.; Mao, D.; Zhang, Y.; Liang, H. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol. Med. Rep. 2017, 16, 5450–5458. [Google Scholar] [CrossRef]
- Taris, M.; Irani, J.; Blanchet, P.; Multigner, L.; Cathelineau, X.; Fromont, G. ERG expression in prostate cancer: The prognostic paradox. Prostate 2014, 74, 1481–1487. [Google Scholar] [CrossRef]
- Lu, R.M.; Chiu, C.Y.; Liu, I.J.; Chang, Y.L.; Liu, Y.J.; Wu, H.C. Novel human Ab against vascular endothelial growth factor receptor 2 shows therapeutic potential for leukemia and prostate cancer. Cancer Sci. 2019, 110, 3773–3787. [Google Scholar] [CrossRef]
- Henninger, E.E.; Pursell, Z.F. DNA polymerase ε and its roles in genome stability. IUBMB Life 2014, 66, 339–351. [Google Scholar] [CrossRef]
- Lee, L.; Ali, S.; Genega, E.; Reed, D.; Sokol, E.; Mathew, P. Aggressive-Variant Microsatellite-Stable POLE Mutant Prostate Cancer with High Mutation Burden and Durable Response to Immune Checkpoint Inhibitor Therapy. JCO Precis. Oncol. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- cBioPortal for Cancer Genomics. Prostate Adenocarcinoma TCGA PanCancer Data. Available online: https://www.cbioportal.org/study/summary?id=prad_tcga_pan_can_atlas_2018 (accessed on 10 April 2023).
- Cheng, H.; Zhang, N.; Pati, D. Cohesin subunit RAD21: From biology to disease. Gene 2020, 758, 144966. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Huiling, X.; Thorne, H.; Willems-Jones, A.; Clouston, D.; Bolton, D.; Ramsay, R.; Fox, S.B. RAD21 overexpression is frequently observed in BRCA-X Prostate Cancers. Hered. Cancer Clin. Pract. 2012, 10, As59. [Google Scholar] [CrossRef][Green Version]
- Xu, H.; Yan, M.; Patra, J.; Natrajan, R.; Yan, Y.; Swagemakers, S.; Tomaszewski, J.M.; Verschoor, S.; Millar, E.K.; van der Spek, P.; et al. Enhanced RAD21 cohesin expression confers poor prognosis and resistance to chemotherapy in high grade luminal, basal and HER2 breast cancers. Breast Cancer Res. 2011, 13, R9. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Men’s Health 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, R.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Kırkali, Z.; Montironi, R. Rare and unusual histological variants of prostatic carcinoma: Clinical significance. BJUI 2008, 102, 1369–1374. [Google Scholar] [CrossRef]
- Iǧdem, Ş.; Spiegel, D.Y.; Efstathiou, J.; Miller, R.C.; Poortmans, P.M.P.; Koca, S.; Kiliç-Ünsa, D.; Okkan, S.; Zietman, A. Prostatic duct adenocarcinoma: Clinical characteristics, treatment options, and outcomes—A rare cancer network study. Onkologie 2010, 33, 169–173. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zara Rozalen, A.; Martin, J.M.; Rajendran, R.; Jain, M.; Nava, V.E. Ductal Adenocarcinoma of the Prostate with Novel Genetic Alterations Characterized by Next-Generation Sequencing. Curr. Oncol. 2024, 31, 1556-1561. https://doi.org/10.3390/curroncol31030118
Zara Rozalen A, Martin JM, Rajendran R, Jain M, Nava VE. Ductal Adenocarcinoma of the Prostate with Novel Genetic Alterations Characterized by Next-Generation Sequencing. Current Oncology. 2024; 31(3):1556-1561. https://doi.org/10.3390/curroncol31030118
Chicago/Turabian StyleZara Rozalen, Alexandra, Jose Manuel Martin, Rithika Rajendran, Maneesh Jain, and Victor E. Nava. 2024. "Ductal Adenocarcinoma of the Prostate with Novel Genetic Alterations Characterized by Next-Generation Sequencing" Current Oncology 31, no. 3: 1556-1561. https://doi.org/10.3390/curroncol31030118
APA StyleZara Rozalen, A., Martin, J. M., Rajendran, R., Jain, M., & Nava, V. E. (2024). Ductal Adenocarcinoma of the Prostate with Novel Genetic Alterations Characterized by Next-Generation Sequencing. Current Oncology, 31(3), 1556-1561. https://doi.org/10.3390/curroncol31030118





