Scheduled and Breakthrough Opioid Use for Cancer Pain in an Inpatient Setting at a Tertiary Cancer Hospital
Abstract
1. Introduction
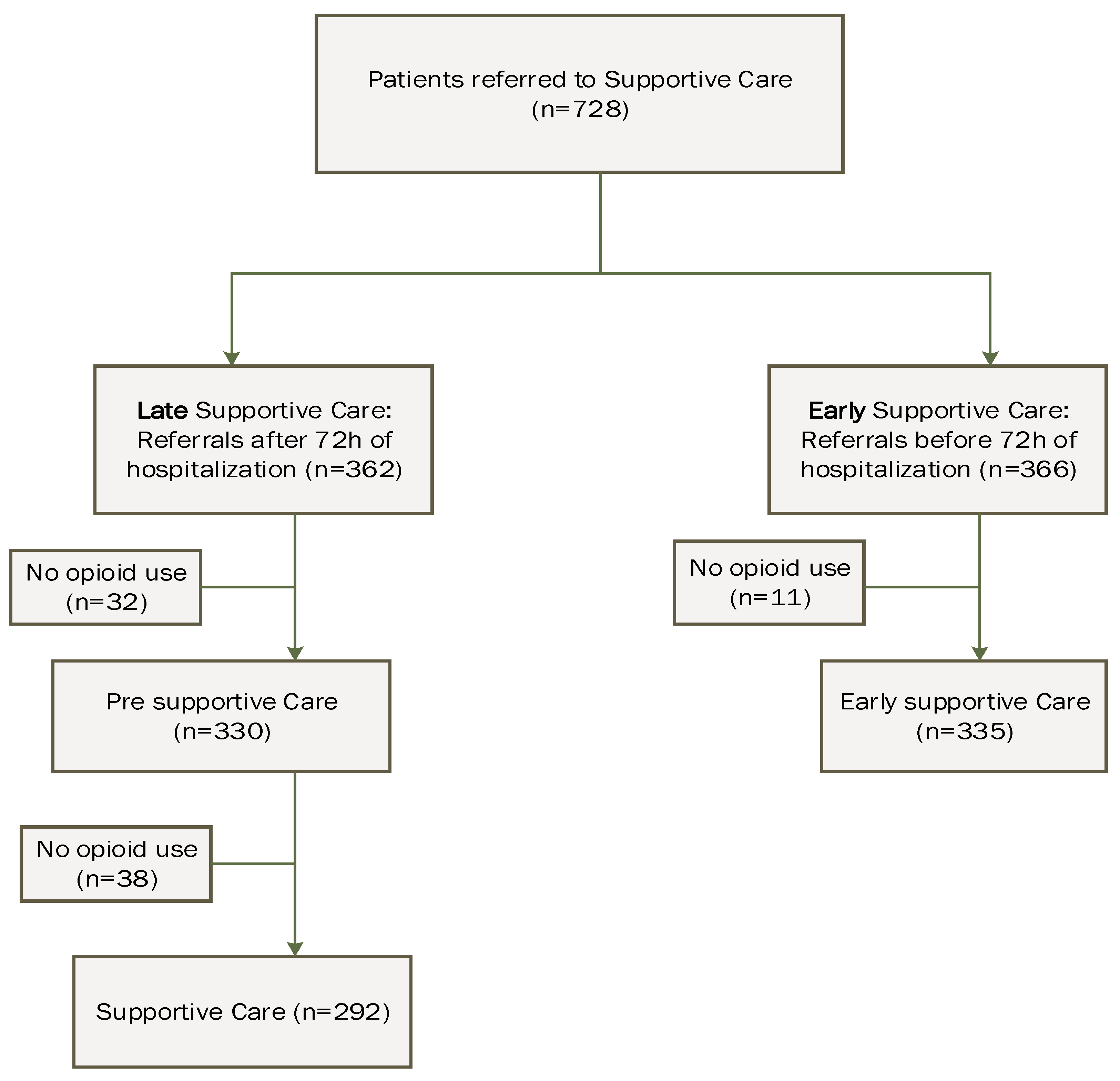
2. Materials and Methods
2.1. Process of the Supportive Care Service and Decision-Making Process for Opioid Prescription Adjustments
2.2. Assessments
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haenen, V.; Evenepoel, M.; De Baerdemaecker, T.; Meeus, M.; Devoogdt, N.; Morlion, B.; Dams, L.; Van Dijck, S.; Van der Gucht, E.; De Vrieze, T.; et al. Pain prevalence and characteristics in survivors of solid cancers: A systematic review and meta-analysis. Support. Care Cancer 2022, 31, 85. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Wang, Q.; Luo, Y.; Sidlow, R.; Han, X. Prevalence of Chronic Pain and High-Impact Chronic Pain in Cancer Survivors in the United States. JAMA Oncol. 2019, 5, 1224–1226. [Google Scholar] [CrossRef]
- Bennett, M.; Paice, J.A.; Wallace, M. Pain and Opioids in Cancer Care: Benefits, Risks, and Alternatives. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 705–713. [Google Scholar] [CrossRef]
- Dalal, S.; Bruera, E. Pain Management for Patients with Advanced Cancer in the Opioid Epidemic Era. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 24–35. [Google Scholar] [CrossRef]
- Portenoy, R.K. Treatment of cancer pain. Lancet 2011, 377, 2236–2247. [Google Scholar] [CrossRef]
- Deandrea, S.; Montanari, M.; Moja, L.; Apolone, G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann. Oncol. 2008, 19, 1985–1991. [Google Scholar] [CrossRef]
- Brinkman-Stoppelenburg, A.; Witkamp, F.E.; van Zuylen, L.; van der Rijt, C.C.D.; van der Heide, A. Palliative care team consultation and quality of death and dying in a university hospital: A secondary analysis of a prospective study. PLoS ONE 2018, 13, e0201191. [Google Scholar] [CrossRef]
- Liu, A.Y.; O’Riordan, D.L.; Marks, A.K.; Bischoff, K.E.; Pantilat, S.Z. A Comparison of Hospitalized Patients with Heart Failure and Cancer Referred to Palliative Care. JAMA Netw. Open 2020, 3, e200020. [Google Scholar] [CrossRef] [PubMed]
- Løhre, E.T.; Jakobsen, G.; Solheim, T.S.; Klepstad, P.; Thronæs, M. Breakthrough and Episodic Cancer Pain from a Palliative Care Perspective. Curr. Oncol. 2023, 30, 10249–10259. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Hagen, N.A. Breakthrough pain: Definition, prevalence and characteristics. Pain 1990, 41, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Bertetto, O.; Labianca, R.; Maltoni, M.; Mercadante, S.; Varrassi, G.; Zaninetta, G.; Zucco, F.; Bagnasco, M.; Lanata, L.; et al. Breakthrough/Episodic Pain Italian Study Group. Episodic (breakthrough) pain prevalence in a population of cancer pain patients. Comparison of clinical diagnoses with the QUDEI--Italian questionnaire for intense episodic pain. J. Pain Symptom Manag. 2012, 43, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Li, X.; Yang, S.; Bu, X.; Chen, Y.; Wang, Y.; Qiu, C. Retrospective Observational Study on the Characteristics of Pain and Associated Factors of Breakthrough Pain in Advanced Cancer Patients. Pain. Res. Manag. 2022, 2022, 8943292. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Davies, A.; Poulain, P.; Cortés-Funes, H.; Panchal, S.J.; Fanelli, G. Guidelines for the management of breakthrough pain in patients with cancer. J. Natl. Compr. Cancer Netw. 2013, 11 (Suppl. S1), S29–S36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, J.H.; Koh, S.J.; Oh, S.Y.; Kim, R.B.; Shin, S.H.; Lee, Y.G.; Kim, B.S.; Ryoo, H.M.; Yoon, S.Y.; Jang, J.S.; et al. Interference with daily functioning by breakthrough pain in patients with cancer. Support. Care Cancer 2020, 28, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- Tagami, K.; Okizaki, A.; Miura, T.; Watanabe, Y.S.; Matsumoto, Y.; Morita, T.; Fujimori, M.; Kinoshita, H. Breakthrough Cancer Pain Influences General Activities and Pain Management: A Comparison of Patients with and without Breakthrough Cancer Pain. J. Palliat. Med. 2018, 21, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Paice, J.A.; Bohlke, K.; Barton, D.; Craig, D.S.; El-Jawahri, A.; Hershman, D.L.; Kong, L.R.; Kurita, G.P.; LeBlanc, T.W.; Mercadante, S.; et al. Use of Opioids for Adults with Pain from Cancer or Cancer Treatment: ASCO Guideline. J. Clin. Oncol. 2023, 41, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Fournier, V.; Duprez, C.; Grynberg, D.; Antoine, P.; Lamore, K. Are digital health interventions valuable to support patients with cancer and caregivers? An umbrella review of web-based and app-based supportive care interventions. Cancer Med. 2023, 12, 21436–21451. [Google Scholar] [CrossRef]
- Lu, D.J.; Girgis, M.; David, J.M.; Chung, E.M.; Atkins, K.M.; Kamrava, M. Evaluation of Mobile Health Applications to Track Patient-Reported Outcomes for Oncology Patients: A Systematic Review. Adv. Radiat. Oncol. 2021, 6, 100576. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Payne, D.; Jacobsen, P. Breakthrough pain: Characteristics and impact in patients with cancer pain. Pain 1999, 81, 129–134. [Google Scholar] [CrossRef]
- Mercadante, S.; Masedu, F.; Valenti, M.; Aielli, F. Breakthrough Pain in Patients with Lung Cancer. A Secondary Analysis of IOPS MS Study. J. Clin. Med. 2020, 9, 1337. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Kaasa, S.; Caraceni, A.; Loge, J.H.; Pedersen, T.; Haugen, D.F.; Aass, N. European Palliative Care Research Collaborative (EPCRC). Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support. Palliat. Care 2016, 6, 344–352. [Google Scholar] [CrossRef]
- Pérez-Hernández, C.; Jiménez-López, A.J.; Sanz-Yagüe, A.; Mar-Medina, J.; Larrañaga, I.; Soler-López, B. Observational Study Evaluating the Economic Impact of Breakthrough Pain in Cancer Patients in Clinical Practice in Spain: The IMDI Study. Pain Ther. 2018, 7, 227–240. [Google Scholar] [CrossRef]
- Bedard, G.; Davies, A.; McDonald, R.; Hawley, P.; Buchanan, A.; Popovic, M.; Wong, E.; Chow, E. Breakthrough cancer pain: A comparison of surveys with European and Canadian patients. Support. Care Cancer 2015, 23, 791–796. [Google Scholar] [CrossRef]
- Mercadante, S. Once again… breakthrough cancer pain: An updated overview. J. Anesth. Analg. Crit. Care 2023, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Adile, C.; Masedu, F.; Marchetti, P.; Costanzi, A.; Aielli, F. Factors influencing the use of opioids for breakthrough cancer pain: A secondary analysis of the IOPS-MS study. Eur. J. Pain 2019, 23, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Daeninck, P.; Gagnon, B.; Gallagher, R.; Henderson, J.D.; Shir, Y.; Zimmermann, C.; Lapointe, B. Canadian recommendations for the management of breakthrough cancer pain. Curr. Oncol. 2016, 23, 96–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bossi, P.; Escobar, Y.; Pea, F. Rapid-Onset Opioids for Management of Breakthrough Cancer Pain: Considerations for Daily Practice. Front. Pain Res. 2022, 3, 893530. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S. The use of rapid onset opioids for breakthrough cancer pain: The challenge of its dosing. Crit. Rev. Oncol. 2011, 80, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.; Kim, Y.J.; Haider, A.; Hui, D.; Balankari, V.R.; Epner, M.C.; Park, M.; Liu, D.D.; Williams, J.; Frisbee-Hume, S.E.; et al. Response to Oral Immediate-Release Opioids for Breakthrough Pain in Patients with Advanced Cancer with Adequately Controlled Background Pain. Oncologist 2019, 24, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I.; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv166–iv191. [Google Scholar] [CrossRef]
- Pergolizzi, J.; Böger, R.H.; Budd, K.; Dahan, A.; Erdine, S.; Hans, G.; Kress, H.G.; Langford, R.; Likar, R.; Raffa, R.B.; et al. Opioids and the management of chronic severe pain in the elderly: Consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008, 8, 287–313. [Google Scholar] [CrossRef]
- Hanks, G.W.; Conno, F.; Cherny, N.; Hanna, M.; Kalso, E.; McQuay, H.J.; Mercadante, S.; Meynadier, J.; Poulain, P.; Ripamonti, C.; et al. Morphine and alternative opioids in cancer pain: The EAPC recommendations. Br. J. Cancer 2001, 84, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.G.; Portenoy, R.K. Establishing "best practices" for opioid rotation: Conclusions of an expert panel. J. Pain Symptom Manag. 2009, 38, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Talari, K.; Goyal, M. Retrospective Studies—Utility and Caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef]
- Ogrinc, G.; Davies, L.; Goodman, D.; Balataden, P.; Davidoff, F.; Stevens, D. SQUIRE 2.0 (Standards for Quality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process. BMJ Qual. Saf. 2016, 25, 986–992. [Google Scholar] [CrossRef]
- Madden, K.; Bruera, E. The M.D. Anderson Supportive and Palliative Care Handbook, 7th ed.; University of Health Science Center at Houston: Houston, TX, USA, 2023. [Google Scholar]
- National Consensus Project for Quality Palliative Care (NCP). Clinical Practice Guidelines for Quality Palliative Care, Second Edition. 2018. Available online: www. nationalconsensusproject.org (accessed on 6 February 2024).
- Dans, M.; Kutner, J.S.; Agarwal, R.; Baker, J.N.; Bauman, J.R.; Beck, A.C.; Campbell, T.C.; Carey, E.C.; Case, A.A.; Dalal, S.; et al. NCCN Guidelines® Insights: Palliative Care, Version 2. J. Natl. Compr. Cancer Netw. 2021, 19, 780–788. [Google Scholar] [CrossRef]
- Bruera, E.; Kuehn, N.; Miller, M.J.; Selmser, P.; Macmillan, K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J. Palliat. Care 1991, 7, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, W.; Rosenfeld, B.; Roth, A.; Smith, M.J.; Cohen, K.; Passik, S. The Memorial Delirium Assessment Scale. J. Pain Symptom Manag. 1997, 13, 128–137. [Google Scholar] [CrossRef]
- Hui, D.; Shamieh, O.; Paiva, C.E.; Perez-Cruz, P.E.; Kwon, J.H.; Muckaden, M.A.; Park, M.; Yennu, S.; Kang, J.H.; Bruera, E. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer 2015, 121, 3027–3035. [Google Scholar] [CrossRef]
- Ewing, J.A. Detecting alcoholism. The CAGE questionnaire. JAMA 1984, 252, 1905–1907. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; RTaylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Villari, P.; Ferrera, P.; Mangione, S.; Casuccio, A. The use of opioids for breakthrough pain in acute palliative care unit by using doses proportional to opioid basal regimen. Clin. J. Pain 2010, 26, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Haider, A.; Lu, Z.; Naqvi, S.; Zhuang, A.; Nguyen, K.; Reddy, A.; Arthur, J.; Tanco, K.; Williams, J.; et al. Factors Associated with Improvement in Uncontrolled Cancer Pain without Increasing the Opioid Daily Dose among Patients Seen by an Inpatient Palliative Care Team. J. Palliat. Med. 2020, 23, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Adile, C.; Cuomo, A.; Aielli, F.; Marinangeli, F.; Casuccio, A. The use of low doses of a sublingual fentanyl formulation for breakthrough pain in patients receiving low doses of opioids. Support. Care Cancer 2017, 25, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Caraceni, A.; Masedu, F.; Scipioni, T.; Aielli, F. Breakthrough Cancer Pain in Patients Receiving Low Doses of Opioids for Background Pain. Oncologist 2020, 25, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.C.; Clark, K.; Louw, S.; Fazekas, B.; Greene, A.; Sanderson, C.R. A randomized, double-blind, crossover, dose ranging study to determine the optimal dose of oral opioid to treat breakthrough pain for patients with advanced cancer already established on regular opioids. Eur. J. Pain 2020, 24, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Spillane, S.; Shiels, M.S.; Young, L.; Quach, D.; Berrington de González, A.; Freedman, N.D. Trends in Opioid Use Among Cancer Patients in the United States: 2013. JNCI Cancer Spectr. 2021, 6, pkab095. [Google Scholar] [CrossRef] [PubMed]
- Goldstick, J.E.; Guy, G.P.; Losby, J.L.; Baldwin, G.; Myers, M.; Bohnert, A.S.B. Changes in Initial Opioid Prescribing Practices After the 2016 Release of the CDC Guideline for Prescribing Opioids for Chronic Pain. JAMA Netw. Open 2021, 4, e2116860. [Google Scholar] [CrossRef]
- Harsanyi, H.; Yang, L.; Harper, A.; Jarada, T.N.; Quan, M.L.; Cheung, W.Y.; Lupichuk, S.; Cuthbert, C.; Xu, Y. Improvement in patient-reported pain among patients with metastatic cancer and its association with opioid prescribing. Support. Care Cancer 2023, 31, 427. [Google Scholar] [CrossRef]
- Yennurajalingam, S.; Astolfi, A.; Indio, V.; Beccaro, M.; Schipani, A.; Yu, R.; Shete, S.; Reyes-Gibby, C.; Lu, Z.; Williams, J.L.; et al. Genetic Factors Associated with Pain Severity, Daily Opioid Dose Requirement, and Pain Response Among Advanced Cancer Patients Receiving Supportive Care. J. Pain Symptom Manag. 2021, 62, 785–795. [Google Scholar] [CrossRef]
- Raad, M.; López, W.O.C.; Sharafshah, A.; Assefi, M.; Lewandrowski, K.-U. Personalized Medicine in Cancer Pain Manage ment. J. Pers. Med. 2023, 13, 1201. [Google Scholar] [CrossRef]
- Nagireddi, J.N.; Vyas, A.K.; Sanapati, M.R.; Soin, A.; Manchikanti, L. The Analysis of Pain Research through the Lens of Artificial Intelligence and Machine Learning. Pain Physician 2022, 25, E211. [Google Scholar]
- Zhang, M.; Zhu, L.; Lin, S.-Y.; Herr, K.; Chi, C.-L.; Demir, I.; Lopez, K.D.; Chi, N.-C. Using artificial intelligence to improve pain assessment and pain management: A scoping review. J. Am. Med. Inform. Assoc. 2023, 30, 570–587. [Google Scholar] [CrossRef] [PubMed]
- Piette, J.D.; Newman, S.; Krein, S.L.; Marinec, N.; Chen, J.; Williams, D.A.; Edmond, S.N.; Driscoll, M.; LaChappelle, K.M.; Kerns, R.D.; et al. Patient-Centered Pain Care Using Artificial Intelligence and Mobile Health Tools: A Randomized Comparative Effectiveness Trial. JAMA Intern. Med. 2022, 182, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Paice, J.; Portenoy, R.; Bruera, E.; Reid, M.C.; Bao, Y. Prescription Opioids Dispensed to Patients with Cancer with Bone Metastasis: 2011–2017. Oncologist 2021, 26, e1890–e1892. [Google Scholar] [CrossRef] [PubMed]

| Pre-Supportive Care (n = 330) | Supportive Care (n = 292) | p * | |
|---|---|---|---|
| Age in years, (median IQR) | 60 (52, 70) | 60 (52, 70) | 0.99 |
| Female, n (%) | 160 (48.5%) | 146 (49.8%) | 0.59 |
| Cancer diagnosis, n (%) | 0.09 | ||
| Gastrointestinal | 96 (29.1%) | 90 (30.6%) | |
| Genitourinary | 30 (10.3%) | 32 (10.9%) | |
| Hematologic | 60 (18.2%) | 50 (17.1%) | |
| Lung | 41 (12.4%) | 37 (12.6%) | |
| Gynecological | 33 (10%) | 30 (10.2%) | |
| Head and neck | 22 (6.7%) | 18 (6.1%) | |
| Breast | 23 (7%) | 19 (6.5%) | |
| Marital Status, n (%) | 0.21 | ||
| Divorced/legally separated | 32 (9.7) | 30 (10.2%) | |
| Married/significant other | 225 (68.4%) | 194 (66.2%) | |
| Single | 44 (13.4%) | 40 (13.7%) | |
| Widowed | 23 (7%) | 23 (7.9%) | |
| Race, n (%) | 0.998 | ||
| White or Caucasian | 223 (67.6%) | 199 (68.2%) | |
| Black or African American | 43 (13.1%) | 39 (13.4%) | |
| Asian | 20 (6.1%) | 16 (5.5%) | |
| Smoking Status, n (%) | 0.853 | ||
| Current | 19 (5.8%) | 17 (5.8%) | |
| Former | 109 (33%) | 101 (34.5%) | |
| Never | 170 (51.5%) | 149 (50.8) | |
| Illicit Drug Use | 0.59 | ||
| Yes | 18 (5.9%) | 17 (6.3%) | |
| Number of follow up visits | 2 (1, 5) | 2 (1, 5) | 1.0 |
| ECOG, n (%) | <0.001 | ||
| ≤2 | 75 (56.8%) | 68 (58.2%) | |
| 3 or 4 | 57 (43.2%) | 49 (41.8%) | |
| CAGE-AID, n (%) | 1.0 | ||
| Positive (≥2) | 13 (5.5%) | 13 (6%) | |
| MDAS **, median (IQR) | 1 (0, 4) | 1 (0, 4) | 1.0 |
| Pain score (0–10), median (IQR) at baseline | 4 (1, 7) | 6 (3, 6) | <0.001 |
| Follow up pain, median (IQR) | 6 (4, 8) | 5 (3, 7) | <0.001 |
| Difference (follow up-baseline), median (IQR) | 1 (−1, 4) | −1 (−3, 0) | <0.001 |
| Number of doses of BTO, median (IQR) | 4 (2, 8) | 2 (1, 4) | <0.001 |
| Number of Schedule doses median (IQR) | 4 (2, 9) | 2 (1, 6.5) | 0.07 |
| BTO MEDD ***, median (IQR) | 11.46 (4.5, 26.25) | 20 (10, 50) | <0.0001 |
| Scheduled MEDD ***, median (IQR) | 0 (0, 12.5) | 9.38 (0, 50) | <0.0001 |
| Total MEDD ***, median (IQR) | 18.5 (6.67, 45.26) | 44.38 (17.5, 90) | <0.0001 |
| Pre-Supportive Care (n = 330) | Supportive Care (n = 292) | p * | |
|---|---|---|---|
| Ratio of MEDD BTO/MEDD Schedule Opioids, median IQR | 0.10 (0.04, 0.21) | 0.17 (0.10, 0.30) | <0.001 * |
| Under, n (%) | 121 (49%) | 51 (19.2%) | 0.69 |
| Normal, n (%) | 57 (23.1%) | 91 (34.3%) | |
| Over, n (%) | 69 (27.9%) | 123 (46.4%) |
| Breakthrough | Pre-Supportive Care | Supportive Care | ||||
|---|---|---|---|---|---|---|
| Hydromorphone (N = 76) | Morphine (N = 94) | Hydromorphone (N = 103) | Morphine (N = 102) | |||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | p * | ||
| Scheduled | Fentanyl | 0.06 (0.04, 0.10) | 0.10 (0.07, 0.21) | 0.13 (0.10, 0.21) | 0.21(0.10, 0.42) | 0.043 |
| Hydromorphone | 0.63 (0.03, 1.25) | 0.09 (0.05, 1.28) | 0.12 (0.09, 0.18) | NA | 0.33 | |
| Methadone | 0.04 (0.03, 0.09) | 0.03 (0.01, 0.22) | 0.20 (0.10, 0.40) | 0.30 (0.10, 0.45) | <0.001 | |
| Morphine | 0.11 (0.04, 0.17) | 0.07 (0.04, 0.13) | 0.17 (0.08, 0.67) | 0.25 (0.15, 0.27) | <0.001 | |
| Oxycodone ER | 0.05 (0.04, 0.11) | 0.06 (0.02, 0.08) | 0.17 (0.11, 0.33) | 0.08 (0.04, 0.21) | 0.036 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozman de Moraes, A.; Erdogan, E.; Azhar, A.; Reddy, S.K.; Lu, Z.; Geller, J.A.; Graves, D.M.; Kubiak, M.J.; Williams, J.L.; Wu, J.; et al. Scheduled and Breakthrough Opioid Use for Cancer Pain in an Inpatient Setting at a Tertiary Cancer Hospital. Curr. Oncol. 2024, 31, 1335-1347. https://doi.org/10.3390/curroncol31030101
Rozman de Moraes A, Erdogan E, Azhar A, Reddy SK, Lu Z, Geller JA, Graves DM, Kubiak MJ, Williams JL, Wu J, et al. Scheduled and Breakthrough Opioid Use for Cancer Pain in an Inpatient Setting at a Tertiary Cancer Hospital. Current Oncology. 2024; 31(3):1335-1347. https://doi.org/10.3390/curroncol31030101
Chicago/Turabian StyleRozman de Moraes, Aline, Elif Erdogan, Ahsan Azhar, Suresh K. Reddy, Zhanni Lu, Joshua A. Geller, David Mill Graves, Michal J. Kubiak, Janet L. Williams, Jimin Wu, and et al. 2024. "Scheduled and Breakthrough Opioid Use for Cancer Pain in an Inpatient Setting at a Tertiary Cancer Hospital" Current Oncology 31, no. 3: 1335-1347. https://doi.org/10.3390/curroncol31030101
APA StyleRozman de Moraes, A., Erdogan, E., Azhar, A., Reddy, S. K., Lu, Z., Geller, J. A., Graves, D. M., Kubiak, M. J., Williams, J. L., Wu, J., Bruera, E., & Yennurajalingam, S. (2024). Scheduled and Breakthrough Opioid Use for Cancer Pain in an Inpatient Setting at a Tertiary Cancer Hospital. Current Oncology, 31(3), 1335-1347. https://doi.org/10.3390/curroncol31030101







