The Impact of Chemotherapy on Cardiovascular Mortality across Breast Cancer Subtypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. Relationship among Breast Cancer Subtype, Stage, Patient Age, and Cause of Death
3.3. Cumulative Crude Probability of Death by Cause of Death
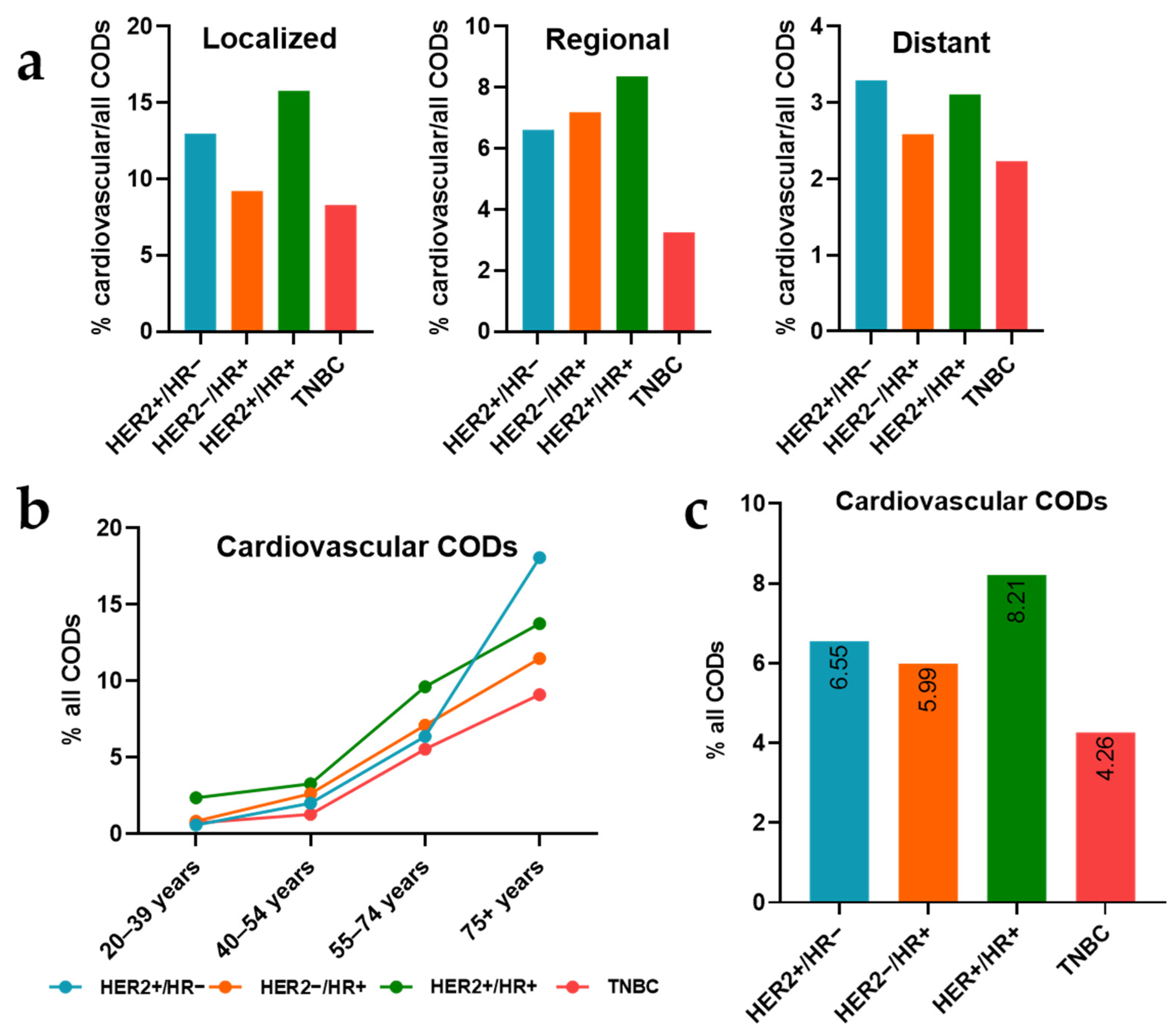
3.4. Subtype-Specific Patterns of Cardiovascular Mortality across Stages and Ages
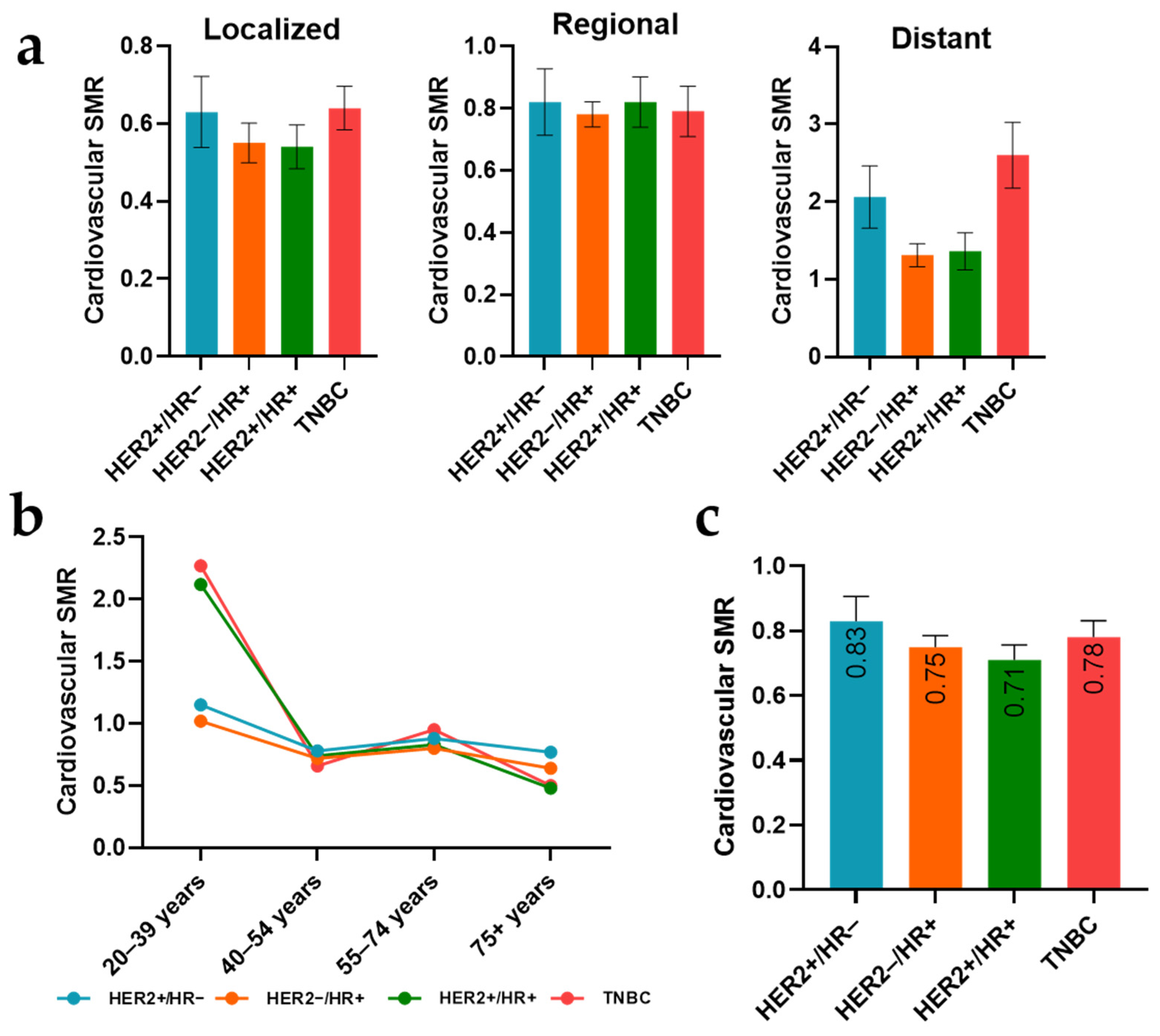
3.5. Subtype-Specific Cardiovascular SMRs across Stages and Age Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; DiGuiseppi, C.; Dabelea, D.; Denberg, T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011, 13, R64. [Google Scholar] [CrossRef]
- Cherukuri, S.P.; Chikatimalla, R.; Dasaradhan, T.; Koneti, J.; Gadde, S.; Kalluru, R. Breast Cancer and the Cardiovascular Disease: A Narrative Review. Cureus 2022, 14, e27917. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Thomas, A.; Rhoads, A.; Suhl, J.; Conway, K.M.; Hundley, W.G.; McNally, L.R.; Oleson, J.; Melin, S.A.; Lynch, C.F.; Romitti, P.A. Incidence and Survival by Human Epidermal Growth Factor Receptor 2 Status in Young Women with Stage I–III Breast Cancer: SEER, 2010–2016. Clin. Breast Cancer 2020, 20, e410–e422. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Florescu, D.R.; Nistor, D.E. Therapy-induced cardiotoxicity in breast cancer patients: A well-known yet unresolved problem. Discoveries 2019, 7, e89. [Google Scholar] [CrossRef]
- Pandit, P.; Patil, R.; Palwe, V.; Gandhe, S.; Patil, R.; Nagarkar, R. Prevalence of Molecular Subtypes of Breast Cancer: A Single Institutional Experience of 2062 Patients. Eur. J. Breast Health 2020, 16, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.; Moore, J.X.; Altekruse, S.F. Breast cancer survival in African-American women by hormone receptor subtypes. Breast Cancer Res. Treat. 2015, 153, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Fallahpour, S.; Navaneelan, T.; De, P.; Borgo, A. Breast cancer survival by molecular subtype: A population-based analysis of cancer registry data. CMAJ Open 2017, 5, E734–E739. [Google Scholar] [CrossRef]
- Press, D.J.; Miller, M.E.; Liederbach, E.; Yao, K.; Huo, D. De novo metastasis in breast cancer: Occurrence and overall survival stratified by molecular subtype. Clin. Exp. Metastasis 2017, 34, 457–465. [Google Scholar] [CrossRef]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef]
- Jenkins, E.O.; Deal, A.M.; Anders, C.K.; Prat, A.; Perou, C.M.; Carey, L.A.; Muss, H.B. Age-specific changes in intrinsic breast cancer subtypes: A focus on older women. Oncologist 2014, 19, 1076–1083. [Google Scholar] [CrossRef]
- Agha, A.; Wang, X.; Wang, M.; Lehrer, E.J.; Horn, S.R.; Rosenberg, J.C.; Trifiletti, D.M.; Diaz, R.; Louie, A.V.; Zaorsky, N.G. Long-Term Risk of Death From Heart Disease Among Breast Cancer Patients. Front. Cardiovasc. Med. 2022, 9, 784409. [Google Scholar] [CrossRef]
- Vo, J.B.; Ramin, C.; Barac, A.; Berrington de Gonzalez, A.; Veiga, L. Trends in heart disease mortality among breast cancer survivors in the US, 1975–2017. Breast Cancer Res. Treat. 2022, 192, 611–622. [Google Scholar] [CrossRef]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- Anwar, S.L.; Cahyono, R.; Prabowo, D.; Avanti, W.S.; Choridah, L.; Dwianingsih, E.K.; Harahap, W.A.; Aryandono, T. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer 2021, 21, 590. [Google Scholar] [CrossRef]
- Acheampong, T.; Kehm, R.D.; Terry, M.B.; Argov, E.L.; Tehranifar, P. Incidence Trends of Breast Cancer Molecular Subtypes by Age and Race/Ethnicity in the US from 2010 to 2016. JAMA Netw. Open 2020, 3, e2013226. [Google Scholar] [CrossRef]
- Connor, A.E.; Schmaltz, C.L.; Jackson-Thompson, J.; Visvanathan, K. Comorbidities and the risk of cardiovascular disease mortality among racially diverse patients with breast cancer. Cancer 2021, 127, 2614–2622. [Google Scholar] [CrossRef]
- Padegimas, A.; Clasen, S.; Ky, B. Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends Cardiovasc. Med. 2020, 30, 22–28. [Google Scholar] [CrossRef]
- Gulati, M.; Mulvagh, S.L. The connection between the breast and heart in a woman: Breast cancer and cardiovascular disease. Clin. Cardiol. 2018, 41, 253–257. [Google Scholar] [CrossRef]
- Barish, R.; Lynce, F.; Unger, K.; Barac, A. Management of Cardiovascular Disease in Women with Breast Cancer. Circulation 2019, 139, 1110–1120. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Ohtani, K.; Fujino, T.; Ide, T.; Funakoshi, K.; Sakamoto, I.; Hiasa, K.I.; Higo, T.; Kamezaki, K.; Akashi, K.; Tsutsui, H. Recovery from left ventricular dysfunction was associated with the early introduction of heart failure medical treatment in cancer patients with anthracycline-induced cardiotoxicity. Clin. Res. Cardiol. 2019, 108, 600–611. [Google Scholar] [CrossRef]
- Suto, H.; Suto, M.; Inui, Y.; Okamura, A. Late-onset doxorubicin-induced congestive heart failure in an elderly cancer survivor: A case report. Front. Cardiovasc. Med. 2023, 10, 1124276. [Google Scholar] [CrossRef]
- Henry, M.L.; Niu, J.; Zhang, N.; Giordano, S.H.; Chavez-MacGregor, M. Cardiotoxicity and Cardiac Monitoring among Chemotherapy-Treated Breast Cancer Patients. JACC Cardiovasc. Imaging 2018, 11, 1084–1093. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Cooper, J.; McCracken, C.; Crosbie, E.J.; Walter, F.M.; Manisty, C.H.; Robson, J.; Mamas, M.A.; Harvey, N.C.; Neubauer, S.; et al. Incident cardiovascular events and imaging phenotypes in UK Biobank participants with past cancer. Heart 2023, 109, 1007–1015. [Google Scholar] [CrossRef]

| Total | Breast Cancer CODs | Cardiovascular CODs | Other CODs | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Subtype | ||||||||
| HER2+/HR− | 2075 | 100 | 1522 | 73.35 | 136 | 6.55 | 417 | 20.10 |
| HER2−/HR+ | 11,010 | 100 | 8053 | 73.14 | 660 | 5.99 | 2297 | 20.86 |
| HER2+/HR+ | 3045 | 100 | 2055 | 67.49 | 250 | 8.21 | 740 | 24.30 |
| TNBC | 7133 | 100 | 5593 | 78.41 | 304 | 4.26 | 1236 | 17.33 |
| Stage | ||||||||
| Localized | 4835 | 100 | 2758 | 57.04 | 490 | 10.13 | 1587 | 32.82 |
| Regional | 10,961 | 100 | 7992 | 72.91 | 662 | 6.04 | 2307 | 21.05 |
| Distant | 7467 | 100 | 6473 | 86.69 | 198 | 2.65 | 796 | 10.66 |
| Age | ||||||||
| 20–39 years | 1941 | 100 | 1670 | 86.04 | 18 | 0.93 | 253 | 13.03 |
| 40–54 years | 6180 | 100 | 5169 | 83.64 | 136 | 2.20 | 875 | 14.16 |
| 55–74 years | 12,161 | 100 | 8508 | 69.96 | 843 | 6.93 | 2810 | 23.11 |
| 75+ years | 2981 | 100 | 1876 | 62.93 | 353 | 11.84 | 752 | 25.23 |
| Total | 23,263 | 100 | 17,223 | 74.04 | 1350 | 5.80 | 4690 | 20.16 |
| Breast Cancer CODs | Cardiovascular CODs | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Subtype | ||||||
| HER2+/HR− | 0.97 | 0.87–1.07 | 0.463 | 1.15 | 0.96–1.39 | 0.125 |
| HER2−/HR+ | 0.92 | 0.86–0.97 | 0.003 | 1.07 | 0.96–1.19 | 0.237 |
| HER2+/HR+ | 0.69 | 0.64–0.75 | <0.001 | 1.56 | 1.35–1.79 | <0.001 |
| TNBC | 1.40 | 1.32–1.50 | <0.001 | 0.64 | 0.56–0.73 | <0.001 |
| Receptor positivity | ||||||
| HER2-positive | 0.76 | 0.71–0.82 | <0.001 | 1.45 | 1.29–1.64 | <0.001 |
| HER2-negative | 1.31 | 1.22–1.40 | <0.001 | 0.69 | 0.61–0.78 | <0.001 |
| HR-positive | 0.75 | 0.71–0.80 | <0.001 | 1.37 | 1.23–1.55 | <0.001 |
| HR-negative | 1.33 | 1.25–1.41 | <0.001 | 0.73 | 0.65–0.82 | <0.001 |
| Stage | ||||||
| Localized | 0.36 | 0.34–0.39 | <0.001 | 2.3 | 2.05–2.59 | <0.001 |
| Regional | 0.90 | 0.84–0.95 | <0.001 | 1.08 | 0.97–1.21 | 0.146 |
| Distant | 3.06 | 2.84–3.29 | <0.001 | 0.35 | 0.30–0.40 | <0.001 |
| Age | ||||||
| 20–39 years | 2.29 | 2.00–2.61 | <0.001 | 0.14 | 0.09–0.22 | <0.001 |
| 40–54 years | 2.13 | 1.98–2.30 | <0.001 | 0.29 | 0.25–0.35 | <0.001 |
| 55–74 years | 0.64 | 0.60–0.68 | <0.001 | 1.56 | 1.39–1.74 | <0.001 |
| 75+ years | 0.55 | 0.50–0.59 | <0.001 | 2.6 | 2.29–2.95 | <0.001 |
| Breast Cancer CODs | Cardiovascular CODs | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Subtype | ||||||
| HER2+/HR− | 0.71 | 0.62–0.81 | <0.001 | 1.27 | 1.01–1.61 | 0.041 |
| HER2−/HR+ | 0.69 | 0.64–0.75 | <0.001 | 1.17 | 1.01–1.37 | 0.042 |
| HER2+/HR+ | 0.57 | 0.51–0.63 | <0.001 | 1.32 | 1.09–1.60 | 0.005 |
| TNBC | 1 | 1 | ||||
| Stage | ||||||
| Localized | 3.99 | 3.43–4.64 | <0.001 | 0.07 | 0.04–0.11 | <0.001 |
| Regional | 7.84 | 6.76–9.08 | <0.001 | 0.06 | 0.04–0.10 | <0.001 |
| Distant | 19.78 | 16.84–23.23 | <0.001 | 0.05 | 0.03–0.09 | <0.001 |
| Age | ||||||
| 20–39 years | 1 | 1 | ||||
| 40–54 years | 0.93 | 0.79–1.08 | 0.317 | 2.16 | 1.30–3.60 | 0.003 |
| 55–74 years | 0.48 | 0.42–0.55 | <0.001 | 4.14 | 2.55–6.72 | <0.001 |
| 75+ years | 0.38 | 0.32–0.45 | <0.001 | 6.47 | 3.95–10.61 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngô, T.M.; Lê, Á.N.; Đinh, D.P.H. The Impact of Chemotherapy on Cardiovascular Mortality across Breast Cancer Subtypes. Curr. Oncol. 2024, 31, 649-659. https://doi.org/10.3390/curroncol31020047
Ngô TM, Lê ÁN, Đinh DPH. The Impact of Chemotherapy on Cardiovascular Mortality across Breast Cancer Subtypes. Current Oncology. 2024; 31(2):649-659. https://doi.org/10.3390/curroncol31020047
Chicago/Turabian StyleNgô, Toàn Minh, Ánh Ngọc Lê, and Dương Phạm Hoàng Đinh. 2024. "The Impact of Chemotherapy on Cardiovascular Mortality across Breast Cancer Subtypes" Current Oncology 31, no. 2: 649-659. https://doi.org/10.3390/curroncol31020047
APA StyleNgô, T. M., Lê, Á. N., & Đinh, D. P. H. (2024). The Impact of Chemotherapy on Cardiovascular Mortality across Breast Cancer Subtypes. Current Oncology, 31(2), 649-659. https://doi.org/10.3390/curroncol31020047





