Vulnerability in Colorectal Cancer: Adjusted Gross Income and Geography as Factors in Determining Overall Survival in Colorectal Cancer: A Single-Center Study Across a Broad Income Inequality in an American Context
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
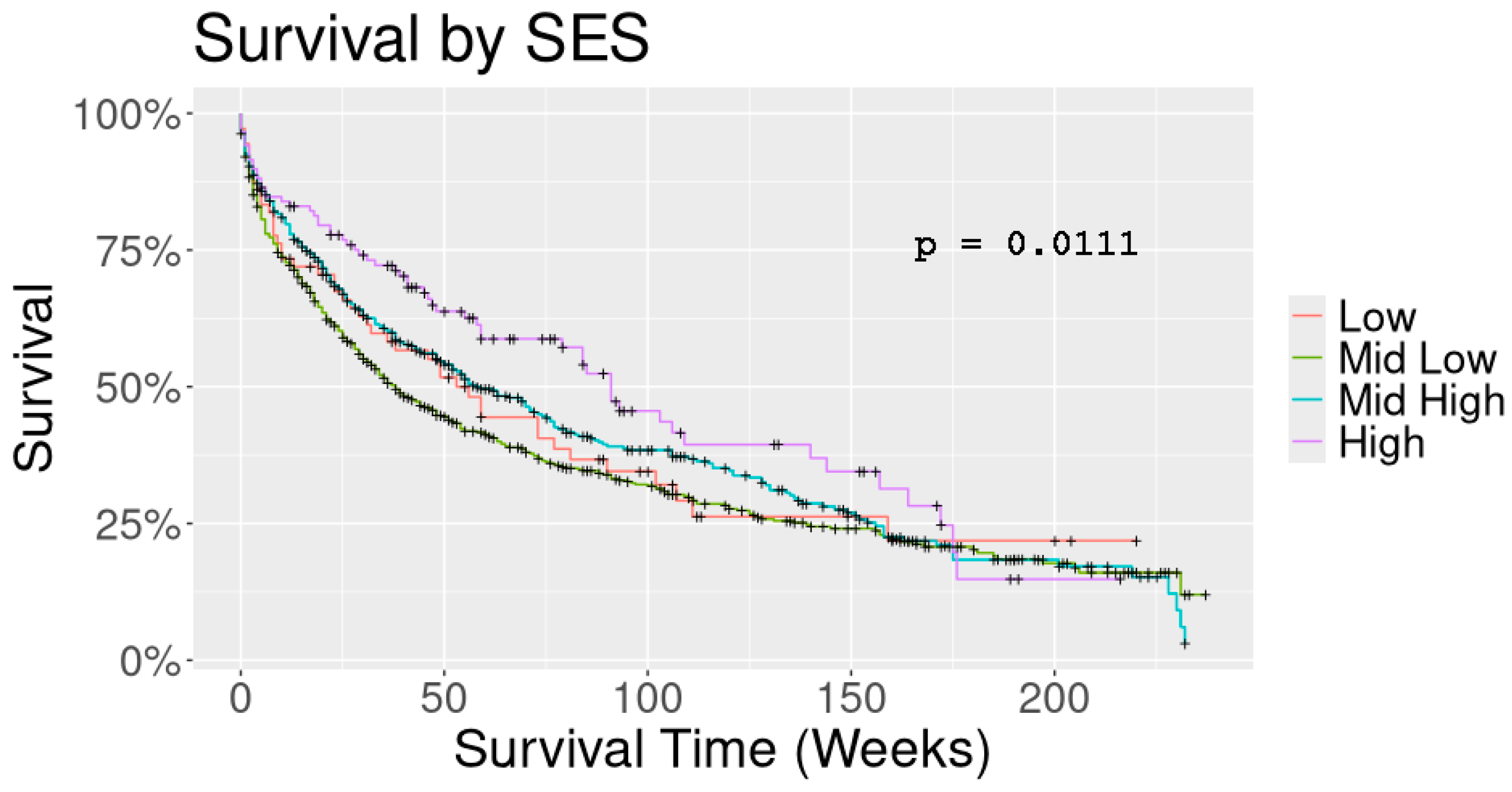
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | African Americans |
| CRC | colorectal cancer |
| PAOI | physical area of intervention |
| OS | overall survival |
| SES | socio-economic status |
| SEER | surveillance, epidemiology, and end results program |
References
- Boulware, L.E.; Corbie, G.; Aguilar-Gaxiola, S.; Wilkins, C.H.; Ruiz, R.; Vitale, A.; Leonard, E.; Egede, M.D. Combating Structural Inequities—Diversity, Equity, and Inclusion in Clinical and Translational Research. N. Engl. J. Med. 2022, 386, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Alese, O.B.; Yates, C.; Rivers, B.M.; Blackstock, W.; Newman, L.; Davis, M.; Byrd, G.; Harris, A.E. Cancer healthcare disparities among African Americans in the United States. J. Natl. Med. Assoc. 2022, 114, 236–250. [Google Scholar] [CrossRef] [PubMed]
- CDC. Colorectal Cancer, United States—2007–2016. Available online: https://www.cdc.gov/cancer/uscs/about/data-briefs/no16-colorectal-cancer-2007-2016.htm (accessed on 30 October 2023).
- Carethers, J.M.; Doubeni, C.A. Causes of Socioeconomic Disparities in Colorectal Cancer and Intervention Framework and Strategies. Gastroenterology 2020, 158, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Maque, J.; Galoosian, A.; Osuna-Garcia, A.; May, F.P. Optimal Strategies for Colorectal Cancer Screening. Curr. Treat. Options Oncol. 2022, 23, 474–493. [Google Scholar] [CrossRef]
- Joseph, D.A.; King, J.B.; Dowling, N.F.; Thomas, C.C.; Richardson, L.C. Vital Signs: Colorectal Cancer Screening Test Use—United States, 2018. MMWR Morb Mortal Wkly Rep 2020, 69, 253–259. [Google Scholar] [CrossRef]
- Godberg, D.W. A Geocoding Best Practice Guide; North American Association of Central Cancer Registries, Inc.: Springfield, IL, USA, 2008; pp. 1–287. Available online: https://www.naaccr.org/wp-content/uploads/2016/11/Geocoding_Best_Practices.pdf (accessed on 20 November 2024).
- Doria, C.; De Deyne, P.; Dolan, S.; Chung, J.; Yatcilla, K.; Zarifian, L.; Remstein, R.; Schwartz, E. Municipality and Adjusted Gross Income Influence Outcome of Patients Diagnosed with Pancreatic Cancer in a Newly Developed Cancer Center in Mercer County New Jersey, USA, a Single Center Study. Cancers 2021, 13, 1498. [Google Scholar] [CrossRef]
- Gawande, A. The hot spotters: Can we lower medical costs by giving the neediest patients better care? New Yorker 2011, 86, 40–51. [Google Scholar]
- Thatcher, E.J.; Camacho, F.; Anderson, R.T.; Li, L.; Cohn, W.F.; DeGuzman, P.B.; Porter, K.J.; Zoellner, J.M. Spatial analysis of colorectal cancer outcomes and socioeconomic factors in Virginia. BMC Public. Health 2021, 21, 1908. [Google Scholar] [CrossRef]
- Hassan, M.O.; Arthurs, Z.; Sohn, V.Y.; Steele, S.R. Race does not impact colorectal cancer treatment or outcomes with equal access. Am. J. Surg. 2009, 197, 485–490. [Google Scholar] [CrossRef]
- Aloysius, M.M.; Goyal, H.; Shah, N.J.; Pallav, K.; John, N.; Gajendran, M.; Perisetti, A.; Tharian, B. Impact of Race and Socioeconomics Disparities on Survival in Young-Onset Colorectal Adenocarcinoma-A SEER Registry Analysis. Cancers 2021, 13, 3262. [Google Scholar] [CrossRef]
- Robinson, J.R.M.; Phipps, A.I.; Barrington, W.E.; Hurvitz, P.M.; Sheppard, L.; Malen, R.C.; Newcomb, P.A. Associations of Household Income with Health-Related Quality of Life Following a Colorectal Cancer Diagnosis Varies with Neighborhood Socioeconomic Status. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S.A.; Traore, C.Y.; Singer, D.E.; Atlas, S.J. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: Results from a primary care network. Health Serv. Res. 2015, 50, 398–417. [Google Scholar] [CrossRef] [PubMed]
- Percac-Lima, S.; Ashburner, J.M.; Zai, A.H.; Chang, Y.; Oo, S.A.; Guimaraes, E.; Atlas, S.J. Patient Navigation for Comprehensive Cancer Screening in High-Risk Patients Using a Population-Based Health Information Technology System: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 930–967. [Google Scholar] [CrossRef] [PubMed]
- Ajeesh, S.; Luis, R. A Comprehensive Electronic Health Record Based Patient Navigation Module Including Technology Driven Colorectal Cancer Outreach and Education. J. Cancer Educ. 2018, 33, 627–633. [Google Scholar] [CrossRef]
- IRS. SOI Tax Stats County Data. 2017. Available online: https://www.irs.gov/statistics/soi-tax-stats-county-data-2017 (accessed on 20 October 2023).
- Liang, P.S.; Mayer, J.D.; Wakefield, J.; Ko, C.W. Temporal Trends in Geographic and Sociodemographic Disparities in Colorectal Cancer Among Medicare Patients, 1973–2010. J Rural. Health 2017, 33, 361–370. [Google Scholar] [CrossRef]
- Kurani, S.S.; McCoy, R.G.; Lampman, M.A.; Doubeni, C.A.; Finney Rutten, L.J.; Inselman, J.W.; Giblon, R.E.; Bunkers, K.S.; Stroebel, R.J.; Rushlow, D.; et al. Association of Neighborhood Measures of Social Determinants of Health with Breast, Cervical, and Colorectal Cancer Screening Rates in the US Midwest. JAMA Netw. Open 2020, 3, e200618. [Google Scholar] [CrossRef]
- Kozlovich, S.; Chen, G.; Watson, C.J.W.; Blot, W.J.; Lazarus, P. Role of l- and d-Menthol in the Glucuronidation and Detoxification of the Major Lung Carcinogen, NNAL. Drug Metab. Dispos. 2019, 47, 1388–1396. [Google Scholar] [CrossRef]
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef]
- Reeve, B.B.; Graves, K.D.; Lin, L.; Potosky, A.L.; Ahn, J.; Henke, D.M.; Pan, W.; Fall-Dickson, J.M. Health-Related Quality of Life by Race, Ethnicity, and Country of Origin Among Cancer Survivors. J. Natl. Cancer Inst. 2022, 115, 258–267. [Google Scholar] [CrossRef]
- Stromberg, U.; Bonander, C.; Westerberg, M.; Levin, L.Å.; CMetcalfe, C.; Steele, R.; Holmberg, L.; Forsberg, A.; Hultcrantz, R. Colorectal cancer screening with fecal immunochemical testing or primary colonoscopy: An analysis of health equity based on a randomised trial. EClinicalMedicine 2022, 47, 101398. [Google Scholar] [CrossRef]
- Powell, M.; Koenecke, A.; Byrd, J.B.; Nishimura, A.; Konig, M.F.; Xiong, R.; Mahmood, S.; Mucaj, V.; Bettegowda, C.; Rose, L.; et al. Ten Rules for Conducting Retrospective Pharmacoepidemiological Analyses: Example COVID-19 Study. Front. Pharm. 2021, 12, 700776. [Google Scholar] [CrossRef]
- Camm, A.J.; Fox, K.A.A. Strengths and weaknesses of ‘real-world’ studies involving non-vitamin K antagonist oral anticoagulants. Open Heart 2018, 5, e000788. [Google Scholar] [CrossRef]
| Variable | Value | Count (% of Total) |
|---|---|---|
| Race/Ethnicity | White | 786 (61.9) |
| African American | 388 (30.6) | |
| Asian | 32 (2.5) | |
| Hispanic | 63 (5.0) | |
| Sex | Male | 610 (48.1) |
| Female | 659 (51.9) | |
| Insurance | Private or Medicare | 1115 (87.9) |
| Medicaid | 58 (4.6) | |
| Not insured | 96 (7.6) | |
| SES * | Low | 73 (5.8) |
| Mid-Low | 621 (49.3) | |
| Mid-High | 446 (35.4) | |
| High | 119 (9.5) | |
| Histopathology | Adenocarcinoma | 1206 (95) |
| Carcinoma | 24 (1.9) | |
| Neuroendocrine carcinoma | 38 (3) | |
| Stage | Stage 0 | 17 (1.3) |
| Stage 1 | 234 (18.4) | |
| Stage 2 | 318 (25.1) | |
| Stage 3 | 285 (22.5) | |
| Stage 4 | 258 (20.3) | |
| Chemotherapy | Yes | 535 (42.2) |
| No | 727 (57.3) | |
| Surgery | Yes | 1061 (83.6) |
| No | 207 (16.3) | |
| Radiation therapy | Yes | 189 (14.9) |
| No | 1078 (84.9) | |
| Type Recurrence | Residual | 302 (23.8) |
| Local | 29 (2.3) | |
| Metastatic disease | 112 (8.8) | |
| None (disease free) | 421 (33.2) | |
| Status | Alive (based on latest visit to CHS) | 447 (35.2) |
| Dead | 822 (64.8) | |
| Average age at diagnosis | Mean ± st. dev. | 68.7 ± 14.3 |
| Overall Survival (months) | Median (IQR) | 35.0 (11.86) |
| Zip | No. Patients | Median Income (USD) | Median Cutoff SES |
|---|---|---|---|
| 8618 | 244 | 44,657 | Mid-Low |
| 8638 | 179 | 54,044 | Mid-Low |
| 8648 | 136 | 85,380 | Mid-High |
| 8611 | 81 | 42,358 | Mid-Low |
| 8628 | 75 | 76,676 | Mid-High |
| 8610 | 73 | 62,112 | Mid-Low |
| 8619 | 61 | 74,215 | Mid-High |
| 8609 | 57 | 31,119 | Low |
| 8534 | 35 | 146,532 | High |
| 8690 | 33 | 91,870 | Mid-High |
| 8629 | 27 | 55,083 | Mid-Low |
| 8505 | 25 | 80,391 | Mid-High |
| 8620 | 24 | 92,261 | Mid-High |
| 8560 | 21 | 126,500 | High |
| 8022 | 16 | 78,139 | Mid-High |
| 8540 | 15 | 122,921 | High |
| 8530 | 12 | 82,331 | Mid-High |
| 8520 | 11 | 90,268 | Mid-High |
| 8608 | 10 | 13,150 | Low |
| Race | |||||
|---|---|---|---|---|---|
| Variable | Caucasian | Black | Asian | Hispanic | p Value |
| n | 786 | 388 | 32 | 63 | |
| Stage (%) | 0.325 | ||||
| Stage 0 | 13 (1.9) | 2 (0.6) | 1 (3.3) | 1 (1.8) | |
| Stage 1 | 150 (21.6) | 64 (19.5) | 9 (30.0) | 11 (19.3) | |
| Stage 2 | 206 (29.6) | 91 (27.7) | 5 (16.7) | 16 (28.1) | |
| Stage 3 | 171 (24.6) | 84 (25.5) | 11 (36.7) | 19 (33.3) | |
| Stage 4 | 156 (22.4) | 88 (26.7) | 4 (13.3) | 10 (17.5) | |
| SES (%) | <0.001 | ||||
| Low | 20 (2.6) | 39 (10.2) | 3 (9.4) | 11 (17.5) | |
| Mid-Low | 273 (34.9) | 300 (78.7) | 4 (12.5) | 44 (69.8) | |
| Mid-High | 385 (49.2) | 39 (10.2) | 15 (46.9) | 7 (11.1) | |
| High | 105 (13.4) | 3 (0.8) | 10 (31.2) | 1 (1.6) | |
| Insurance (%) | <0.001 | ||||
| Private or Medicare | 734 (93.4) | 311 (80.2) | 28 (87.5) | 42 (66.7) | |
| Medicaid | 20 (2.5) | 34 (8.8) | 0 (0.0) | 4 (6.3) | |
| Not insured | 32 (4.1) | 43 (11.1) | 4 (12.5) | 17 (27.0) | |
| Surgery (%) | 125 (15.9) | 73 (18.8) | 2 (6.2) | 7 (11.1) | 0.140 |
| Chemotherapy (%) | 462 (59.2) | 225 (58.1) | 12 (37.5) | 28 (45.2) | 0.018 |
| Age at diagnosis (years) | 70.9 (14.1) | 66.5 (13.7) | 61.7 (10.9) | 58.5 (14.9) | <0.001 |
| Overall Survival (months) | 33 (11.85) | 35 (10.95) | 29 (15.65) | 46 (26.76) | 0.567 |
| Socio-Economic Status (Median Cutoff) | |||||
|---|---|---|---|---|---|
| Variable | Low | Mid-Low | Mid-High | High | p-Value |
| n | 73 | 621 | 446 | 119 | |
| Race | <0.001 | ||||
| Caucasian | 20 (27.4) | 273 (44.0) | 385 (86.3) | 105 (88.2) | |
| Black | 39 (53.4) | 300 (48.3) | 39 (8.7) | 3 (2.5) | |
| Asian | 3 (4.1) | 4 (0.6) | 15 (3.4) | 10 (8.4) | |
| Hispanic | 11 (15.1) | 44 (7.1) | 7 (1.6) | 1 (0.8) | |
| Stage | 0.066 | ||||
| Stage 0 | 0 (0.0) | 6 (1.1) | 5 (1.2) | 6 (5.7) | |
| Stage 1 | 9 (16.4) | 102 (19.1) | 99 (24.1) | 22 (21.0) | |
| Stage 2 | 17 (30.9) | 155 (29.0) | 115 (28.0) | 28 (26.7) | |
| Stage 3 | 13 (23.6) | 140 (26.2) | 101 (24.6) | 29 (27.6) | |
| Stage 4 | 16 (29.1) | 131 (24.5) | 90 (22.0) | 20 (19.0) | |
| Insurance (%) | <0.001 | ||||
| Private or Medicare | 60 (82.2) | 514 (82.8) | 418 (93.7) | 116 (97.5) | |
| Medicaid | 6 (8.2) | 37 (6.0) | 12 (2.7) | 1 (0.8) | |
| Not insured | 7 (9.6) | 70 (11.3) | 16 (3.6) | 2 (1.7) | |
| Surgery (%) | 11 (15.1) | 118 (19.0) | 61 (13.7) | 15 (12.6) | 0.075 |
| Chemotherapy (%) | 42 (58.3) | 354 (57.3) | 251 (56.7) | 73 (61.3) | 0.830 |
| Age at Diagnosis (years) | 65.1 (12.9) | 68.3 (14.2) | 70.1 (14.2) | 68.9 (15.9) | 0.024 |
| Overall Survival (months) | 40.5 (10.88) | 30.0 (8.79) | 38 (13.87) | 47 (22.91) | 0.020 |
| Parameter | Chi-Square | p Value | Hazard Ratio | 95% Hazard Ratio Confidence Limits | ||
|---|---|---|---|---|---|---|
| Stage * | Stage 1 | 1.98 | 0.1599 | 1.914 | 0.774 | 4.734 |
| Stage 2 | 7.69 | 0.0056 | 3.560 | 1.451 | 8.733 | |
| Stage 3 | 13.35 | 0.0003 | 5.418 | 2.188 | 13.413 | |
| Stage 4 | 44.28 | <0.0001 | 22.127 | 8.888 | 55.084 | |
| Race/Ethnicity * | Asian | 6.28 | 0.0122 | 2.016 | 1.165 | 3.489 |
| Black | 0.12 | 0.7323 | 0.968 | 0.802 | 1.168 | |
| Hispanic | 0.47 | 0.4931 | 0.861 | 0.561 | 1.321 | |
| SES * | High | 6.73 | 0.0095 | 0.547 | 0.347 | 0.863 |
| Mid-High | 1.16 | 0.2806 | 0.812 | 0.556 | 1.185 | |
| Mid-Low | 0.11 | 0.7425 | 1.062 | 0.743 | 1.516 | |
| Insurance * | Medicaid | 9.46 | 0.0021 | 1.742 | 1.223 | 2.481 |
| Not Insured | 0.14 | 0.7089 | 1.063 | 0.772 | 1.462 | |
| Surgery | 50.07 | <0.0001 | 0.420 | 0.330 | 0.534 | |
| Chemotherapy | 43.68 | <0.0001 | 0.520 | 0.428 | 0.631 | |
| Age at Diagnosis | 100.23 | <0.0001 | 1.038 | 1.030 | 1.045 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doria, C.; De Deyne, P.G.; Charalampos, P. Vulnerability in Colorectal Cancer: Adjusted Gross Income and Geography as Factors in Determining Overall Survival in Colorectal Cancer: A Single-Center Study Across a Broad Income Inequality in an American Context. Curr. Oncol. 2024, 31, 7754-7764. https://doi.org/10.3390/curroncol31120570
Doria C, De Deyne PG, Charalampos P. Vulnerability in Colorectal Cancer: Adjusted Gross Income and Geography as Factors in Determining Overall Survival in Colorectal Cancer: A Single-Center Study Across a Broad Income Inequality in an American Context. Current Oncology. 2024; 31(12):7754-7764. https://doi.org/10.3390/curroncol31120570
Chicago/Turabian StyleDoria, Cataldo, Patrick G. De Deyne, and Papachristou Charalampos. 2024. "Vulnerability in Colorectal Cancer: Adjusted Gross Income and Geography as Factors in Determining Overall Survival in Colorectal Cancer: A Single-Center Study Across a Broad Income Inequality in an American Context" Current Oncology 31, no. 12: 7754-7764. https://doi.org/10.3390/curroncol31120570
APA StyleDoria, C., De Deyne, P. G., & Charalampos, P. (2024). Vulnerability in Colorectal Cancer: Adjusted Gross Income and Geography as Factors in Determining Overall Survival in Colorectal Cancer: A Single-Center Study Across a Broad Income Inequality in an American Context. Current Oncology, 31(12), 7754-7764. https://doi.org/10.3390/curroncol31120570





