Exploiting Integrin-αVβ3 to Enhance Radiotherapy Efficacy in Medulloblastoma via Ferroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines and Culture Conditions
2.3. Genetic Disruption of β3-Integrin and Lentiviral Transductions
2.4. RT-qPCR
2.5. Immunoblotting
2.6. Irradiation Procedure
2.7. Colony Formation Assay
2.8. Flow Cytometry
2.9. Statistics
3. Results
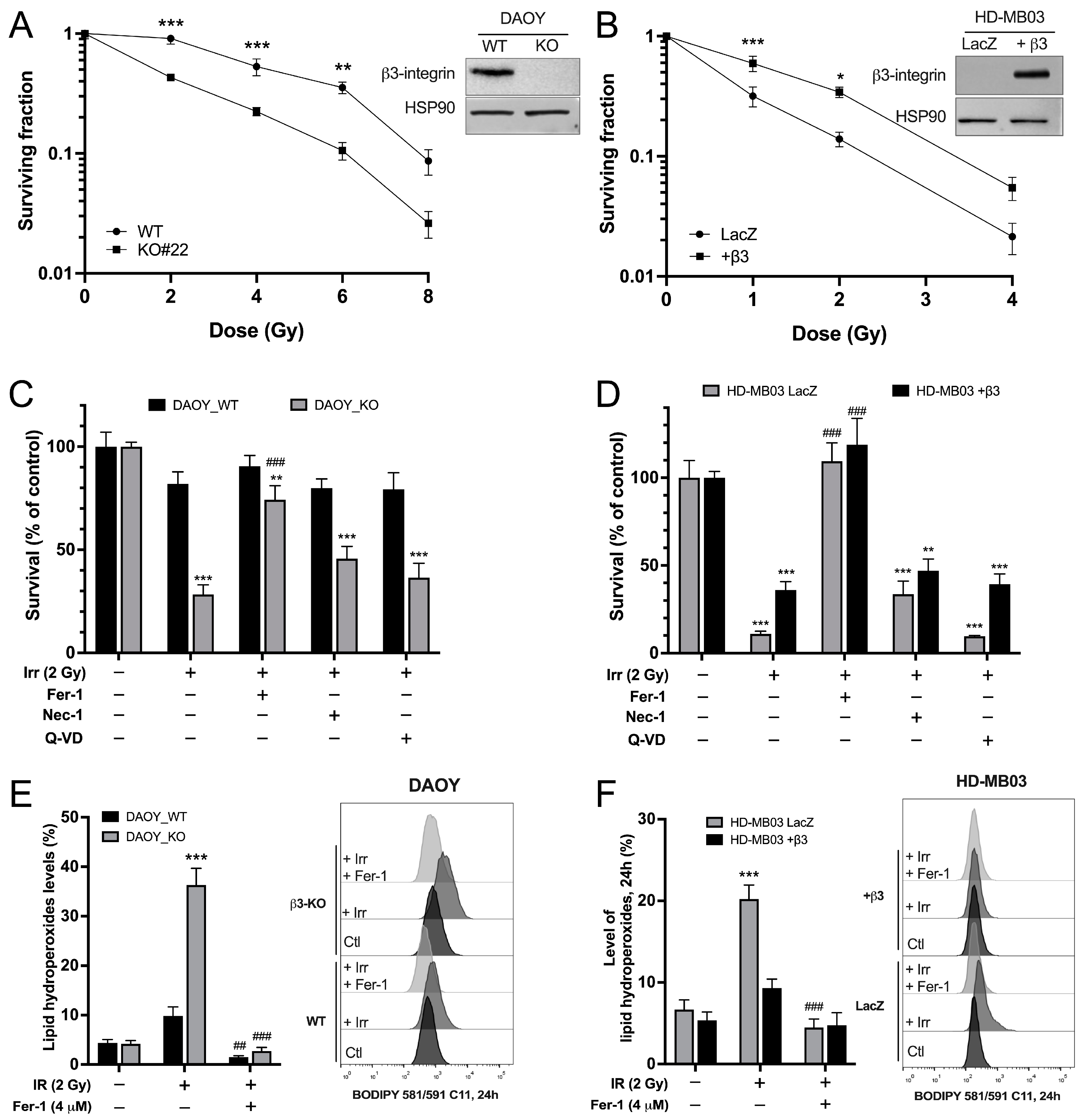
3.1. Integrin-αvβ3 Promotes Resistance to IR-Induced Ferroptosis
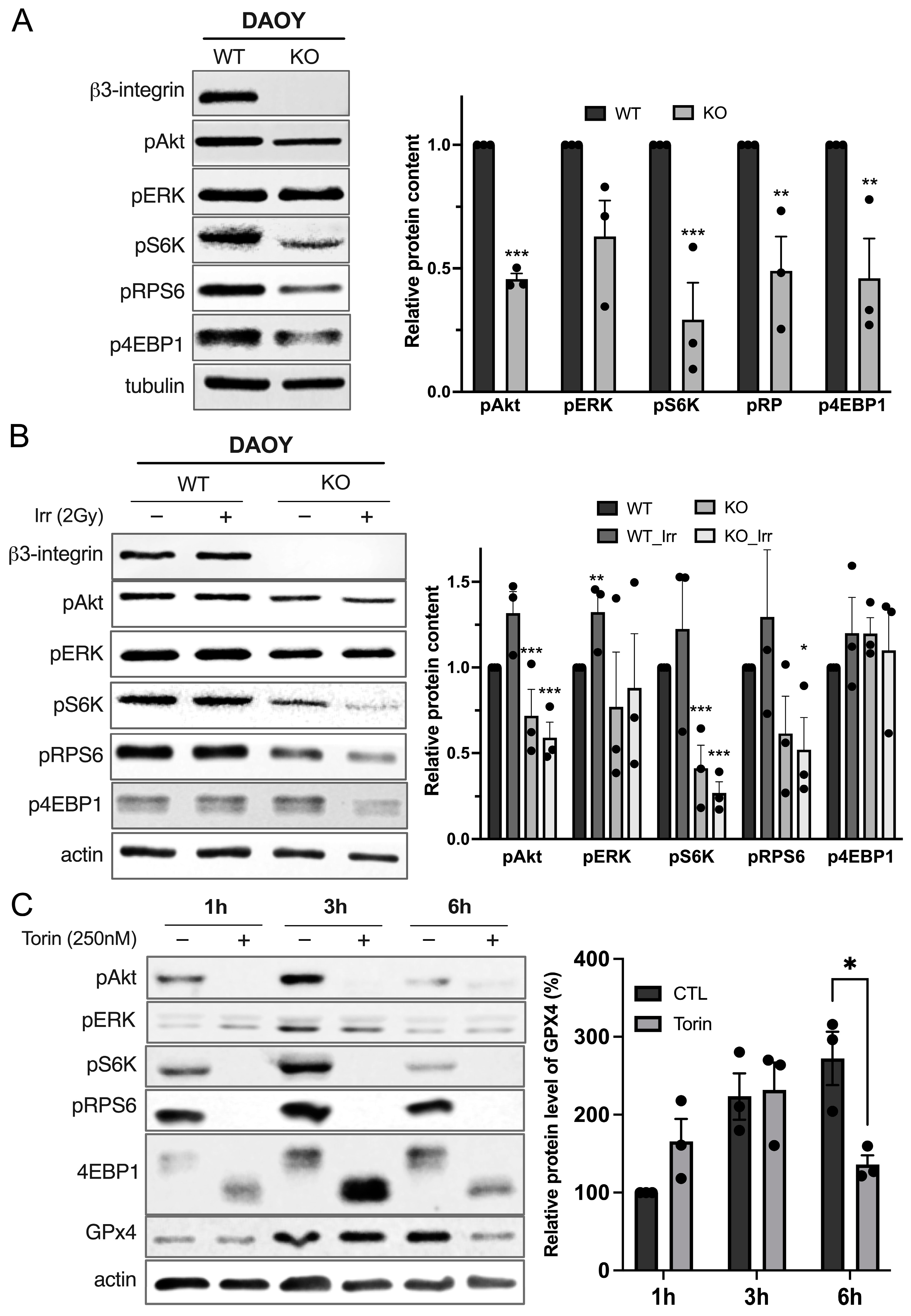
3.2. Integrin-αvβ3 Regulates Antioxidant Protein Expression
3.3. Integrin-αvβ3 Regulates GPX4 Expression by Modulating mTORC1 Axis
3.4. Cilengitide Mimics Radiosensitivity Induced by β3-Depletion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, K.J.; Cullen, J.; Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Langer, C.E.; Turner, M.C.; McKean-Cowdin, R.; Fisher, J.L.; Lupo, P.J.; Partap, S.; et al. Childhood Brain Tumor Epidemiology: A Brain Tumor Epidemiology Consortium Review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2716–2736. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Padovani, L.; Horan, G.; Ajithkumar, T. Radiotherapy Advances in Paediatric Medulloblastoma Treatment. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, 171–181. [Google Scholar] [CrossRef]
- Seidel, C.; Heider, S.; Hau, P.; Glasow, A.; Dietzsch, S.; Kortmann, R.-D. Radiotherapy in Medulloblastoma-Evolution of Treatment, Current Concepts and Future Perspectives. Cancers 2021, 13, 5945. [Google Scholar] [CrossRef]
- Chevignard, M.; Câmara-Costa, H.; Doz, F.; Dellatolas, G. Core Deficits and Quality of Survival after Childhood Medulloblastoma: A Review. Neurooncol. Pract. 2017, 4, 82–97. [Google Scholar] [CrossRef]
- Pizer, B.L.; Clifford, S.C. The Potential Impact of Tumour Biology on Improved Clinical Practice for Medulloblastoma: Progress towards Biologically Driven Clinical Trials. Br. J. Neurosurg. 2009, 23, 364–375. [Google Scholar] [CrossRef]
- Jakacki, R.I.; Burger, P.C.; Zhou, T.; Holmes, E.J.; Kocak, M.; Onar, A.; Goldwein, J.; Mehta, M.; Packer, R.J.; Tarbell, N.; et al. Outcome of Children With Metastatic Medulloblastoma Treated With Carboplatin During Craniospinal Radiotherapy: A Children’s Oncology Group Phase I/II Study. J. Clin. Oncol. 2012, 30, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.M.; Richardson, S.; Schwalbe, E.C.; Hicks, D.; Lindsey, J.C.; Crosier, S.; Rafiee, G.; Grabovska, Y.; Wharton, S.B.; Jacques, T.S.; et al. Time, Pattern, and Outcome of Medulloblastoma Relapse and Their Association with Tumour Biology at Diagnosis and Therapy: A Multicentre Cohort Study. Lancet Child Adolesc. Health 2020, 4, 865–874. [Google Scholar] [CrossRef]
- Ferreira, S.; Foray, C.; Gatto, A.; Larcher, M.; Heinrich, S.; Lupu, M.; Mispelter, J.; Boussin, F.D.; Pouponnot, C.; Dutreix, M. AsiDNA Is a Radiosensitizer with No Added Toxicity in Medulloblastoma Pediatric Models. Clin. Cancer Res. 2020, 26, 5735–5746. [Google Scholar] [CrossRef]
- Douyère, M.; Gong, C.; Richard, M.; Pellegrini-Moïse, N.; Daouk, J.; Pierson, J.; Chastagner, P.; Boura, C. NRP1 Inhibition Modulates Radiosensitivity of Medulloblastoma by Targeting Cancer Stem Cells. Cancer Cell Int. 2022, 22, 377. [Google Scholar] [CrossRef] [PubMed]
- Buck, J.; Dyer, P.J.C.; Hii, H.; Carline, B.; Kuchibhotla, M.; Byrne, J.; Howlett, M.; Whitehouse, J.; Ebert, M.A.; McDonald, K.L.; et al. Veliparib Is an Effective Radiosensitizing Agent in a Preclinical Model of Medulloblastoma. Front. Mol. Biosci. 2021, 8, 633344. [Google Scholar] [CrossRef] [PubMed]
- Echavidre, W.; Durivault, J.; Gotorbe, C.; Blanchard, T.; Pagnuzzi, M.; Vial, V.; Raes, F.; Broisat, A.; Villeneuve, R.; Amblard, R.; et al. Integrin-Avβ3 Is a Therapeutically Targetable Fundamental Factor in Medulloblastoma Tumorigenicity and Radioresistance. Cancer Res. Commun. 2023, 3, 2483–2496. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The Integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Ellert-Miklaszewska, A.; Poleszak, K.; Pasierbinska, M.; Kaminska, B. Integrin Signaling in Glioma Pathogenesis: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 888. [Google Scholar] [CrossRef]
- Echavidre, W.; Picco, V.; Faraggi, M.; Montemagno, C. Integrin-Avβ3 as a Therapeutic Target in Glioblastoma: Back to the Future? Pharmaceutics 2022, 14, 1053. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Gregolin, C. The Selenoenzyme Phospholipid Hydroperoxide Glutathione Peroxidase. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 1985, 839, 62–70. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Daher, B.; Vučetić, M.; Pouysségur, J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front. Oncol. 2020, 10, 723. [Google Scholar] [CrossRef]
- Endersby, R.; Whitehouse, J.; Pribnow, A.; Kuchibhotla, M.; Hii, H.; Carline, B.; Gande, S.; Stripay, J.; Ancliffe, M.; Howlett, M.; et al. Small-Molecule Screen Reveals Synergy of Cell Cycle Checkpoint Kinase Inhibitors with DNA-Damaging Chemotherapies in Medulloblastoma. Sci. Transl. Med. 2021, 13, eaba7401. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Masutomi, K.; Kyo, S.; Hashimoto, M.; Maida, Y.; Kanaya, T.; Tanaka, M.; Hahn, W.C.; Inoue, M. Efficient Inhibition of Human Telomerase Reverse Transcriptase Expression by RNA Interference Sensitizes Cancer Cells to Ionizing Radiation and Chemotherapy. Hum. Gene Ther. 2005, 16, 859–868. [Google Scholar] [CrossRef]
- Patties, I.; Kortmann, R.-D.; Menzel, F.; Glasow, A. Enhanced Inhibition of Clonogenic Survival of Human Medulloblastoma Cells by Multimodal Treatment with Ionizing Irradiation, Epigenetic Modifiers, and Differentiation-Inducing Drugs. J. Exp. Clin. Cancer Res. 2016, 35, 94. [Google Scholar] [CrossRef]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef]
- Pachane, B.C.; Selistre-de-Araujo, H.S. The Role of Avβ3 Integrin in Cancer Therapy Resistance. Biomedicines 2024, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Penet, M.-F.; Kakkad, S.; Wildes, F.; Bhujwalla, Z.M. Water and Collagen Content Are High in Pancreatic Cancer: Implications for Quantitative Metabolic Imaging. Front. Oncol. 2020, 10, 599204. [Google Scholar] [CrossRef]
- Lu, Z.; Zheng, X.; Ding, C.; Zou, Z.; Liang, Y.; Zhou, Y.; Li, X. Deciphering the Biological Effects of Radiotherapy in Cancer Cells. Biomolecules 2022, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Bian, Y.; Liu, L.; Liu, L.; Liu, X.; Ma, S. Molecular Pathways Associated with Oxidative Stress and Their Potential Applications in Radiotherapy (Review). Int. J. Mol. Med. 2022, 49, 65. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing Radiation-Induced Metabolic Oxidative Stress and Prolonged Cell Injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Adjemian, S.; Oltean, T.; Martens, S.; Wiernicki, B.; Goossens, V.; Vanden Berghe, T.; Cappe, B.; Ladik, M.; Riquet, F.B.; Heyndrickx, L.; et al. Ionizing Radiation Results in a Mixture of Cellular Outcomes Including Mitotic Catastrophe, Senescence, Methuosis, and Iron-Dependent Cell Death. Cell Death Dis. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Haimovitz-Friedman, A.; Kan, C.C.; Ehleiter, D.; Persaud, R.S.; McLoughlin, M.; Fuks, Z.; Kolesnick, R.N. Ionizing Radiation Acts on Cellular Membranes to Generate Ceramide and Initiate Apoptosis. J. Exp. Med. 1994, 180, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Green, M.D.; Wang, W.; Yu, J.; Choi, J.E.; Jiang, L.; Liao, P.; Zhou, J.; Zhang, Q.; Dow, A.; et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019, 9, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.F.; Chaudhary, K.R.; Zandkarimi, F.; Harken, A.D.; Kinslow, C.J.; Upadhyayula, P.S.; Dovas, A.; Higgins, D.M.; Tan, H.; Zhang, Y.; et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem. Biol. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-Lactoferrin: The Link between Ferroptosis and Radiotherapy in Triple-Negative Breast Cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, T.; Chen, P.; Sun, R.; Li, C.; Zhao, X.; Ou, J.; Li, J.; Liu, T.; Zeng, M.; et al. Platelet-Derived Extracellular Vesicles Inhibit Ferroptosis and Promote Distant Metastasis of Nasopharyngeal Carcinoma by Upregulating ITGB3. Int. J. Biol. Sci. 2022, 18, 5858–5872. [Google Scholar] [CrossRef]
- Brown, C.W.; Amante, J.J.; Goel, H.L.; Mercurio, A.M. The A6β4 Integrin Promotes Resistance to Ferroptosis. J. Cell Biol. 2017, 216, 4287–4297. [Google Scholar] [CrossRef]
- Soung, Y.H.; Korneeva, N.; Kim, T.H.; Chung, J. The Role of C-Src in Integrin (A6β4) Dependent Translational Control. BMC Cell Biol. 2013, 14, 49. [Google Scholar] [CrossRef]
- Mousavizadeh, R.; Hojabrpour, P.; Eltit, F.; McDonald, P.C.; Dedhar, S.; McCormack, R.G.; Duronio, V.; Jafarnejad, S.M.; Scott, A. Β1 Integrin, ILK and mTOR Regulate Collagen Synthesis in Mechanically Loaded Tendon Cells. Sci. Rep. 2020, 10, 12644. [Google Scholar] [CrossRef]
- Osburn, W.O.; Wakabayashi, N.; Misra, V.; Nilles, T.; Biswal, S.; Trush, M.A.; Kensler, T.W. Nrf2 Regulates an Adaptive Response Protecting against Oxidative Damage Following Diquat-Mediated Formation of Superoxide Anion. Arch. Biochem. Biophys. 2006, 454, 7–15. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, P.; Zhou, Z.; Xu, G.; Li, Q.; Li, K.; Zheng, Y. Nrf2 Signaling Activation by a Small Molecule Activator Compound 16 Inhibits Hydrogen Peroxide-Induced Oxidative Injury and Death in Osteoblasts. Cell Death Discov. 2022, 8, 353. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 Plays a Critical Role in Mitigating Lipid Peroxidation and Ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Daher, B.; Parks, S.K.; Durivault, J.; Cormerais, Y.; Baidarjad, H.; Tambutte, E.; Pouysségur, J.; Vučetić, M. Genetic Ablation of the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. Cancer Res. 2019, 79, 3877–3890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Swanda, R.V.; Nie, L.; Liu, X.; Wang, C.; Lee, H.; Lei, G.; Mao, C.; Koppula, P.; Cheng, W.; et al. mTORC1 Couples Cyst(e)Ine Availability with GPX4 Protein Synthesis and Ferroptosis Regulation. Nat. Commun. 2021, 12, 1589. [Google Scholar] [CrossRef]
- Cai, J.; Ye, Z.; Hu, Y.; Ye, L.; Gao, L.; Wang, Y.; Sun, Q.; Tong, S.; Zhang, S.; Wu, L.; et al. Fatostatin Induces Ferroptosis through Inhibition of the AKT/mTORC1/GPX4 Signaling Pathway in Glioblastoma. Cell Death Dis. 2023, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Neyns, B.; Goldbrunner, R.; Schlegel, U.; Clement, P.M.J.; Grabenbauer, G.G.; Ochsenbein, A.F.; Simon, M.; Dietrich, P.-Y. Phase I/IIa Study of Cilengitide and Temozolomide with Concomitant Radiotherapy Followed by Cilengitide and Temozolomide Maintenance Therapy in Patients with Newly Diagnosed Glioblastoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2712–2718. [Google Scholar] [CrossRef]
- Weller, M.; Nabors, L.B.; Gorlia, T.; Leske, H.; Rushing, E.; Bady, P.; Hicking, C.; Perry, J.; Hong, Y.-K.; Roth, P. Cilengitide in Newly Diagnosed Glioblastoma: Biomarker Expression and Outcome. Oncotarget 2016, 7, 15018–15032. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, J.K.; Kim, B.; DeWitt, J.P.; Lee, J.E.; Han, J.H.; Kim, S.-K.; Oh, C.W.; Kim, C.-Y. Combination Therapy of Cilengitide with Belotecan against Experimental Glioblastoma. Int. J. Cancer 2013, 133, 749–756. [Google Scholar] [CrossRef]
- Mikkelsen, T.; Brodie, C.; Finniss, S.; Berens, M.E.; Rennert, J.L.; Nelson, K.; Lemke, N.; Brown, S.L.; Hahn, D.; Neuteboom, B.; et al. Radiation Sensitization of Glioblastoma by Cilengitide Has Unanticipated Schedule-Dependency. Int. J. Cancer 2009, 124, 2719–2727. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotorbe, C.; Segui, F.; Echavidre, W.; Durivault, J.; Blanchard, T.; Vial, V.; Pagnuzzi-Boncompagni, M.; Villeneuve, R.; Amblard, R.; Garnier, N.; et al. Exploiting Integrin-αVβ3 to Enhance Radiotherapy Efficacy in Medulloblastoma via Ferroptosis. Curr. Oncol. 2024, 31, 7390-7402. https://doi.org/10.3390/curroncol31110545
Gotorbe C, Segui F, Echavidre W, Durivault J, Blanchard T, Vial V, Pagnuzzi-Boncompagni M, Villeneuve R, Amblard R, Garnier N, et al. Exploiting Integrin-αVβ3 to Enhance Radiotherapy Efficacy in Medulloblastoma via Ferroptosis. Current Oncology. 2024; 31(11):7390-7402. https://doi.org/10.3390/curroncol31110545
Chicago/Turabian StyleGotorbe, Célia, Fabien Segui, William Echavidre, Jérôme Durivault, Thays Blanchard, Valérie Vial, Marina Pagnuzzi-Boncompagni, Rémy Villeneuve, Régis Amblard, Nicolas Garnier, and et al. 2024. "Exploiting Integrin-αVβ3 to Enhance Radiotherapy Efficacy in Medulloblastoma via Ferroptosis" Current Oncology 31, no. 11: 7390-7402. https://doi.org/10.3390/curroncol31110545
APA StyleGotorbe, C., Segui, F., Echavidre, W., Durivault, J., Blanchard, T., Vial, V., Pagnuzzi-Boncompagni, M., Villeneuve, R., Amblard, R., Garnier, N., Ortholan, C., Serrano, B., Picco, V., Pouysségur, J., Vucetic, M., & Montemagno, C. (2024). Exploiting Integrin-αVβ3 to Enhance Radiotherapy Efficacy in Medulloblastoma via Ferroptosis. Current Oncology, 31(11), 7390-7402. https://doi.org/10.3390/curroncol31110545





