Comparative Efficacy and Safety of Neoadjuvant Immunotherapy with Nivolumab vs. Pembrolizumab in Resectable Non-Small Cell Lung Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
Inclusion and Exclusion Criteria
- (a)
- Types of publications: Reviews, meta-analyses, abstracts, letters, editorials, conference proceedings, laboratory studies, and animal studies were excluded.
- (b)
- Language restrictions: Non-English studies were excluded.
- (c)
- Cancer type: Studies focusing on cancers other than NSCLC were excluded.
- (d)
- Therapy use: Studies not evaluating immunotherapy as a neoadjuvant treatment were excluded.
- (e)
- Specificity of treatment: Studies that did not assess nivolumab or pembrolizumab as neoadjuvant therapies were excluded.
2.3. Information Sources and Search Strategy
2.4. Definitions, Interventions, and Outcome Measures
2.5. Data Management, Study Selection, and Data Extraction
2.6. Risk of Bias Assessment
2.7. Quality of Evidence across Studies
3. Results
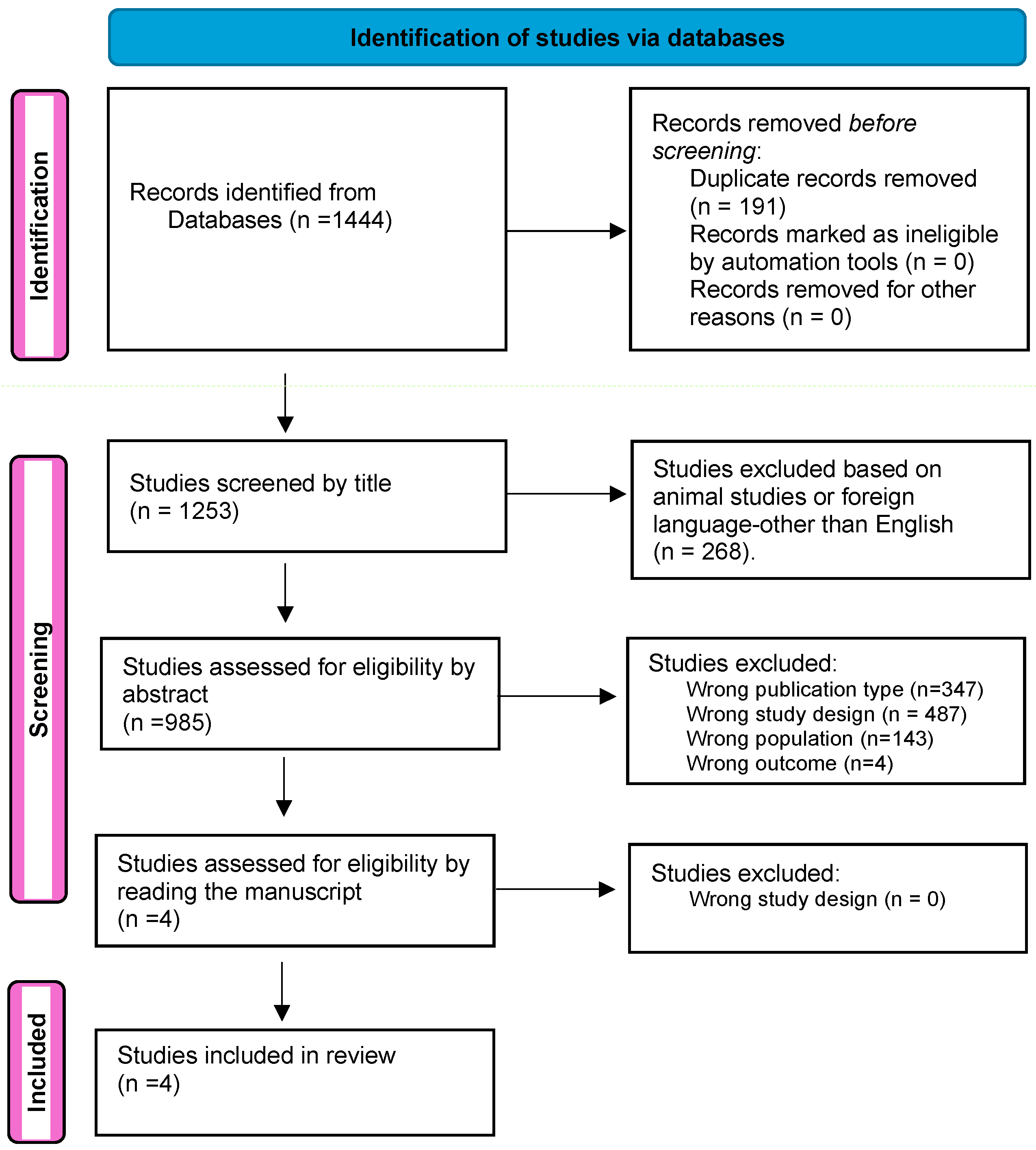
3.1. Study Selection and Characteristics
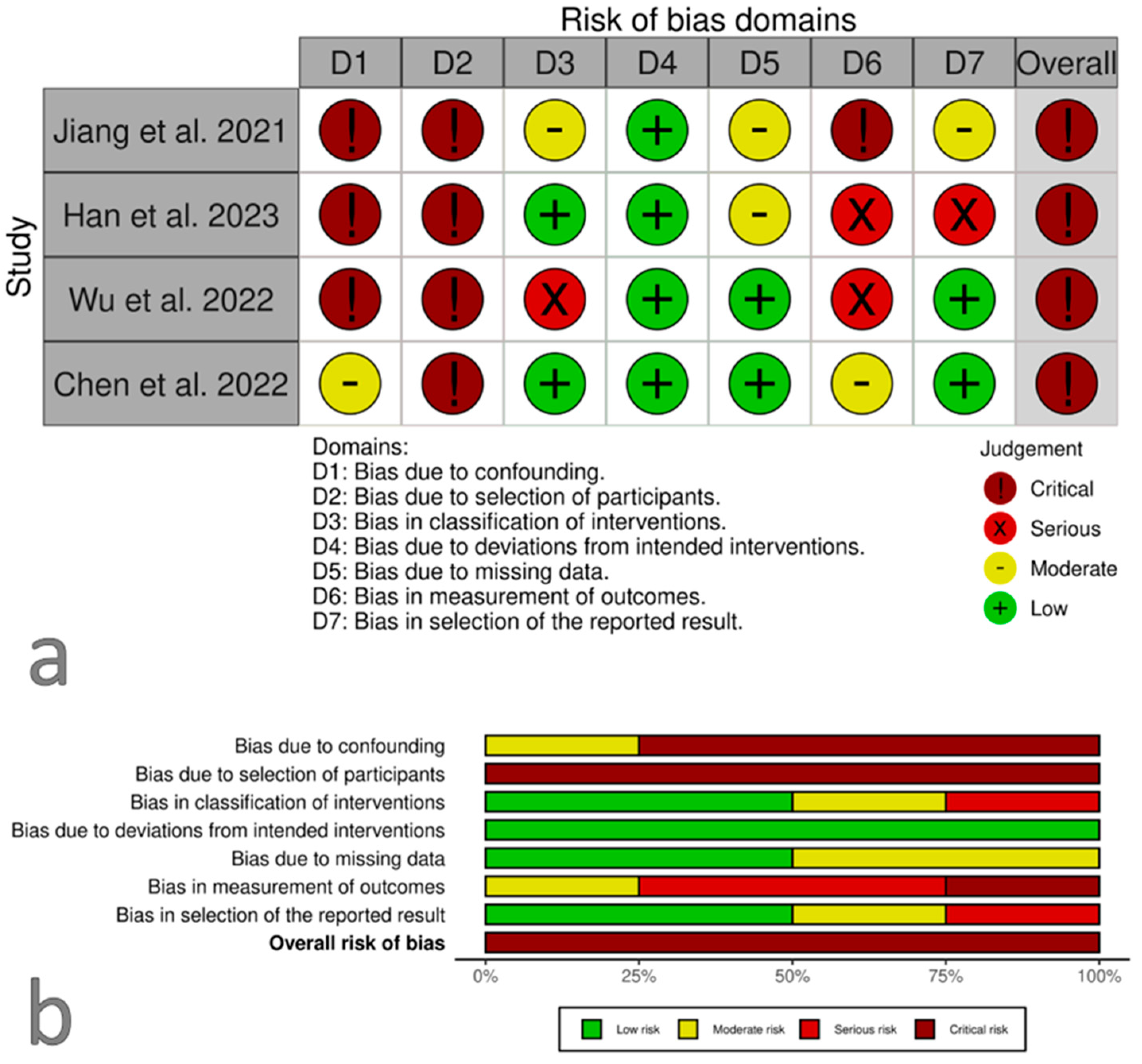
3.2. Risk of Bias within Studies
3.3. Risk of Bias across Studies
3.4. Results of Data Synthesis
3.5. Studies Focusing on Surgery
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S. Results of pulmonary resection following neoadjuvant therapy for locally advanced (IIIA-IIIB) lung cancer. Eur. J. Cardio-Thorac. Surg. 2006, 30, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Sun, L.; Hu, J.; Duan, L.; Xia, H.; Zhu, X.; Sun, F.; Zhang, L.; Yu, H.; Xiong, Y.; et al. Neoadjuvant Afatinib for stage III EGFR-mutant non-small cell lung cancer: A phase II study. Nat. Commun. 2023, 14, 4655. [Google Scholar] [CrossRef] [PubMed]
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Calles, A.; Aguado, G.; Sandoval, C.; Álvarez, R. The role of immunotherapy in small cell lung cancer. Clin. Transl. Oncol. 2019, 21, 961–976. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef]
- Mencoboni, M.; Ceppi, M.; Bruzzone, M.; Taveggia, P.; Cavo, A.; Scordamaglia, F.; Gualco, M.; Filiberti, R.A. Effectiveness and Safety of Immune Checkpoint Inhibitors for Patients with Advanced Non Small-Cell Lung Cancer in Real-World: Review and Meta-Analysis. Cancers 2021, 13, 1388. [Google Scholar] [CrossRef]
- Cascone, T.; William, W.N., Jr.; Weissferdt, A.; Leung, C.H.; Lin, H.Y.; Pataer, A.; Godoy, M.C.B.; Carter, B.W.; Federico, L.; Reuben, A.; et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nat. Med. 2021, 27, 504–514. [Google Scholar] [CrossRef]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, F.; Klotz, L.V.; Kriegsmann, M.; Bischoff, H.; Schneider, M.A.; Muley, T.; Kriegsmann, K.; Haberkorn, U.; Heussel, C.P.; Savai, R.; et al. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: First clinical experience. Lung Cancer. 2021, 153, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhao, Y.; Cai, X.; Chen, H.; Cheng, B.; Zhong, R.; Li, F.; Xiong, S.; Li, J.; Liu, J.; et al. PD-L1 expression and Tumor mutation burden as Pathological response biomarkers of Neoadjuvant immunotherapy for Early-stage Non-small cell lung cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2022, 170, 103582. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, C.; Zhong, W.Z. Neoadjuvant immunotherapy for non-small cell lung cancer: State of the art. Cancer Commun. 2021, 41, 287–302. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Mei, T.; Zhou, Q.; Gong, Y. Comparison of the Efficacy and Safety of Perioperative Immunochemotherapeutic Strategies for Resectable Non-small Cell Lung Cancer: A Systematic Review and Network Meta-analysis. Clin. Oncol. 2024, 36, 107–118. [Google Scholar] [CrossRef]
- You, W.; Liu, M.; Miao, J.D.; Liao, Y.Q.; Song, Y.B.; Cai, D.K.; Gao, Y.; Peng, H. A Network Meta-analysis Comparing the Efficacy and Safety of Anti-PD-1 with Anti-PD-L1 in Non-small Cell Lung Cancer. J. Cancer 2018, 9, 1200–1206. [Google Scholar] [CrossRef]
- Duan, J.; Cui, L.; Zhao, X.; Bai, H.; Cai, S.; Wang, G.; Zhao, Z.; Zhao, J.; Chen, S.; Song, J.; et al. Use of Immunotherapy with Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients with Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 375–384. [Google Scholar] [CrossRef]
- Ren, S.; Xu, A.; Lin, Y.; Camidge, D.R.; Di Maio, M.; Califano, R.; Hida, T.; Rossi, A.; Guibert, N.; Zhu, C.; et al. A narrative review of primary research endpoints of neoadjuvant therapy for lung cancer: Past, present and future. Transl. Lung Cancer Res. 2021, 10, 3264–3275. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016, 355, 4919. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef] [PubMed]
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. 2024. Available online: https://www.gradepro.org/ (accessed on 30 June 2024).
- Jiang, L.; Huang, J.; Jiang, S.; Rong, W.; Shen, Y.; Li, C.; Tian, Y.; Ning, J.; Chen, X.; Yang, Y.; et al. The surgical perspective in neoadjuvant immunotherapy for resectable non-small cell lung cancer. Cancer. Immunol. Immunother. 2021, 70, 2313–2321. [Google Scholar] [CrossRef]
- Han, R.; Zhang, Y.; Wang, T.; Xiao, H.; Luo, Z.; Shen, C.; Li, J.; Zhao, C.; Li, L.; Zhu, M.; et al. Tumor immune microenvironment predicts the pathologic response of neoadjuvant chemoimmunotherapy in non-small-cell lung cancer. Cancer Sci. 2023, 114, 2569–2583. [Google Scholar] [CrossRef]
- Wu, J.; Hou, L.; Haoran, E.; Zhao, Y.; Yu, X.; Xu, L.; Ning, Y.; Deng, J.; Sun, K.; Zhang, J.; et al. Real-world clinical outcomes of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Lung Cancer. 2022, 165, 115–123. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Fu, R.; Tan, X.Y.; Yan, L.X.; Tang, W.F.; Qiu, Z.B.; Qi, Y.F.; Li, Y.F.; Hou, Q.Y.; Wu, Y.L.; et al. Dynamic 18 F-FDG PET/CT can predict the major pathological response to neoadjuvant immunotherapy in non-small cell lung cancer. Thorac. Cancer 2022, 13, 2524–2531. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Chaft, J.E.; William, W.N., Jr.; Rusch, V.; Pisters, K.M.; Kalhor, N.; Pataer, A.; Travis, W.D.; Swisher, S.G.; Kris, M.G. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014, 15, e42–e50. [Google Scholar] [CrossRef]
- Peng, T.R.; Lin, H.H.; Tsai, F.P.; Wu, T.W. Immune checkpoint inhibitors for first-line treatment of advanced non-small-cell lung cancer: A systematic review and network meta-analysis. Thorac. Cancer. 2021, 12, 2873–2885. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. IMpower010 Investigators. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Feng, B.; Chen, J.; You, L. Efficacy, safety, and survival of neoadjuvant immunochemotherapy in operable non-small cell lung cancer: A systematic review and meta-analysis. Front Immunol. 2023, 14, 1273220. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, S.; Yu, Y.; Han, Y. Efficacy and safety of neoadjuvant immunotherapy protocols and cycles for non-small cell lung cancer: A systematic review and meta-analysis. Front Oncol. 2024, 14, 1276549. [Google Scholar] [CrossRef] [PubMed]

| Authors, Ref. | Methods | Participants | Interventions | Primary Outcomes | Outcomes for the Efficacy of Nivolumab vs. Pembrolizumab | Secondary Outcomes - PD-L1 Staining |
|---|---|---|---|---|---|---|
| Long Jiang, et al, 2021 [27] | RCS. pCR was defined as 0% viable tumor cells in residual tumor, while major pathological response as 10% remaining. | 31 NSCLC Patients stage IIA (1), IIB (4), IIIA (16), IIIB (10) | Neoadjuvant chemoimmunotherapy (27) or immunotherapy alone (4) with Pembrolizumab (15) or Nivolumab (16). | Analysis of surgical perspective outcome data after neoadjuvant immunotherapy followed by surgery for resectable NSCLC. | Pembrolizumab MPR (5/15 = 33%). Nivolumab MPR (7/16 = 43%). | PD-L1 < 1%: 10/31. PD-L1 > 1%: 12/31. unknown PD-L1 status: 9/31. MPR in PD-L1 < 1%: 5/22. MPR in PD-L1 > 1%: 3/22. |
| Rui Han, et al., 2023 [28] | RCS. Pathological assessment according to the multidisciplinary recommendations of the IASLC (MPR < 10% viable tumor cells, pCR: 0%). | 29 NSCLC Patients stage III (23), stage IV (6) | Neoadjuvant Chemoimmunotherapy with Pembrolizumab (6), Nivolumab (5), Sentilimab and Tislelizumab (18). | Clinical features of patients with good pathological response to neoadjuvant chemoimmunotherapy and potential biomarkers that discriminate population sensitivity to this therapy. | Pembrolizumab cPR (3/6 = 50%) &MPR (2/6 = 33%). Nivolumab cPR (5/5 = 100%). | Treated patients had either > 1% (7/11) or unknown (4/11) PD-L1 status. |
| Junqi Wu, et al., 2022 [29] | RCS. MPR: no more than 10% viable tumor cells, pCR: the absence of viable tumor cells in all slides. In addition, tumor bed without any characteristic of treatment related response was classified as NR. The presence of treatment-associated necrosis or fibrotic tissue while vital tumors cells > 10% was labeled PR. | 76 Patients with NSCLC: Stage IB:1, Stage IIB:5, Stage IIIA: 41, Stage IIIB: 29. | Chemoimmunotherapy with Nivolumab: 34. Chemoimmunotherapy with Pembrolizumab: 42. | The feasibility, safety, and antitumor activity of pembrolizumab or nivolumab plus chemotherapy in treatment-naive and driver mutation negative patients with potentially resectable NSCLC. | Pembrolizumab MPR: 13/42 = 31%, pCR: 17/42 = 40%, PR: 8/42, NR: 4/42. Nivolumab MPR: 8/34 = 23%, pCR:11/34 = 32%, PR: 13/34, NR: 2/34. | PD-L1 < 1% with Pembrolizumab: 19/42. PD-L1 > 1% with Pembrolizumab: 18/42 = 43%. PD-L1 < 1% with Nivolumab: 11/34. PD-L1 > 1% with Nivolumab: 17/34 = 50%. |
| Zhi-Yong Chen, et al., 2022 [30] | RCS. MPR is defined as ≤ 10% of the viable tumor. | 44 Patients with NSCLC II-III Stage | Immunotherapy +/− chemotherapy: 29 Pat. with Nivolumab, 8 Pat. with Pembrolizumab. | The association of the dynamic changes in PET/CT with MPR in patients receiving different preoperative immunotherapies. | 21/29 = 72% MPR with Nivolumab. 4/8 = 50% MPR with Pembrolizumab. | All patients had > 1% PD-L1 status. |
| Pembrolizumab Compared to Nivolumab for NSCLC | |||
|---|---|---|---|
| Patient or population: Patients with NSCLC Setting: Hospitals and University Hospitals (China) Intervention: Pembrolizumab Comparison: Nivolumab | |||
| Outcome № of participants (studies) | Impact | Certainty | What Happens with immunotherapy |
| Efficacy of Immunotherapy with pembrolizumb vs. nivolumab assessed with: MPR (No more than 10% viable tumor cells in the primary tumor bed), CPR (Absence of viable tumor cells in the primary tumor bed) № of participants: 155 (4 observational studies) | Out of 71 patients that received pembrolizumab, 29 presented MPR and 25 presented CPR. Out of 84 patients that received nivolumab, 38 presented MPR and 23 presented CPR | ⨁◯◯◯ VERY LOW a,b,c | Too heterogenous response to synthesize across studies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaporfyriou, A.; Bartziokas, K.; Apessos, I.; Mueller, J.; Leivaditis, V.; Koletsis, E.; Grapatsas, K. Comparative Efficacy and Safety of Neoadjuvant Immunotherapy with Nivolumab vs. Pembrolizumab in Resectable Non-Small Cell Lung Cancer: A Systematic Review. Curr. Oncol. 2024, 31, 6289-6299. https://doi.org/10.3390/curroncol31100469
Papaporfyriou A, Bartziokas K, Apessos I, Mueller J, Leivaditis V, Koletsis E, Grapatsas K. Comparative Efficacy and Safety of Neoadjuvant Immunotherapy with Nivolumab vs. Pembrolizumab in Resectable Non-Small Cell Lung Cancer: A Systematic Review. Current Oncology. 2024; 31(10):6289-6299. https://doi.org/10.3390/curroncol31100469
Chicago/Turabian StylePapaporfyriou, Anastasia, Konstantinos Bartziokas, Ioulianos Apessos, Jan Mueller, Vasileios Leivaditis, Efstratios Koletsis, and Konstantinos Grapatsas. 2024. "Comparative Efficacy and Safety of Neoadjuvant Immunotherapy with Nivolumab vs. Pembrolizumab in Resectable Non-Small Cell Lung Cancer: A Systematic Review" Current Oncology 31, no. 10: 6289-6299. https://doi.org/10.3390/curroncol31100469
APA StylePapaporfyriou, A., Bartziokas, K., Apessos, I., Mueller, J., Leivaditis, V., Koletsis, E., & Grapatsas, K. (2024). Comparative Efficacy and Safety of Neoadjuvant Immunotherapy with Nivolumab vs. Pembrolizumab in Resectable Non-Small Cell Lung Cancer: A Systematic Review. Current Oncology, 31(10), 6289-6299. https://doi.org/10.3390/curroncol31100469








