The Impact of Neoadjuvant versus Adjuvant Chemotherapy on Survival Outcomes in Locally Advanced Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Studies and Patient Selection for the Combined Statistical Analysis
2.2. Defining Patients with Locally Advanced Breast Cancer (LABC)
- Wolmark et al. [15]: equal to or greater than 4 positive nodes, or clinical tumor size equal to or greater than 5 cm;
- Deo et al. [3]: all patients had LABC (T4a, N0-2, M0);
- Gazet et al. [4]: stage T3a/b, T4b/c;
- Makris et al. [5]: clinical tumor size equal to or greater than 5 cm, or clinical N stage equals 3;
- Mauriac et al. [6]: clinical tumor size equal to or greater than 5 cm;
- Van der Hage et al. [7]: pathological stage T3 or T4a/b/c/d.
2.3. Statistical Analysis Methodology of Combined Individual Patient Data
2.4. Local Data Analysis on the Incidence and Cause of Delay to Surgery
3. Results
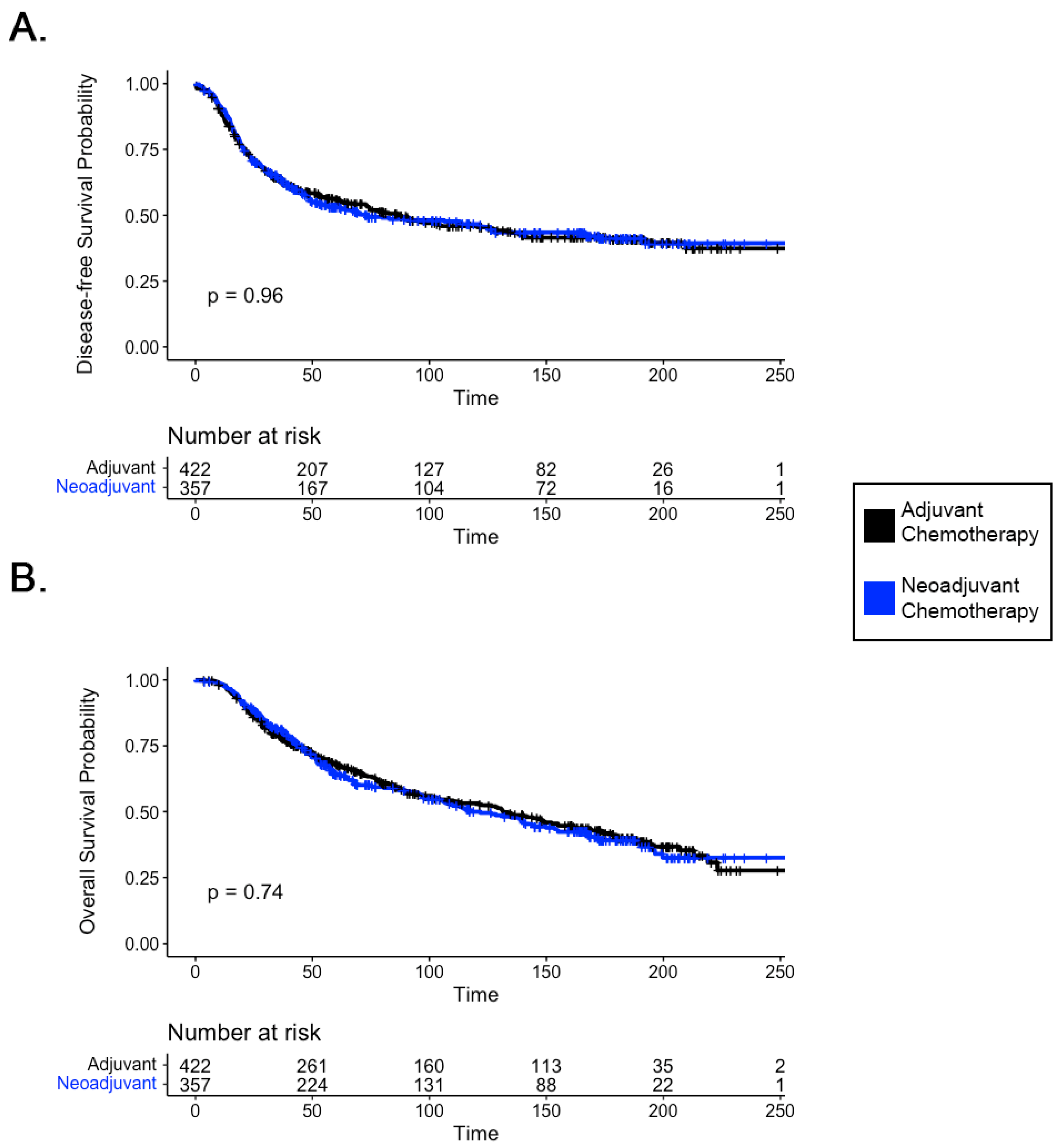
3.1. Survival Outcomes in Patients with LABC Receiving Neoadjuvant Chemotherapy
3.2. Delay to Surgery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikeda, T.; Jinno, H.; Matsui, A.; Masamura, S.; Kitajima, M. The Role of Neoadjuvant Chemotherapy for Breast Cancer Treatment. Breast Cancer 2002, 9, 8–14. [Google Scholar] [CrossRef]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of Preoperative Chemotherapy on Local-Regional Disease in Women with Operable Breast Cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.V.S.; Bhutani, M.; Shukla, N.K.; Raina, V.; Rath, G.K.; Purkayasth, J. Randomized Trial Comparing Neo-adjuvant versus Adjuvant Chemotherapy in Operable Locally Advanced Breast Cancer (T4b N0-2 M0). J. Surg. Oncol. 2003, 84, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Gazet, J.-C.; Ford, H.T.; Gray, R.; McConkey, C.; Sutcliffe, R.; Quilliam, J.; Makinde, V.; Lowndes, S.; Coombes, R.C. Estrogen-Receptor-Directed Neoadjuvant Therapy for Breast Cancer: Results of a Randomised Trial Using Formestane and Methotrexate, Mitozantrone and Mitomycin C (MMM) Chemotherapy. Ann. Oncol. 2001, 12, 685–691. [Google Scholar] [CrossRef]
- Makris, A.; Powles, T.J.; Ashley, S.E.; Chang, J.; Hickish, T.; Tidy, V.A.; Nash, A.G.; Ford, H.T. A Reduction in the Requirements for Mastectomy in a Randomized Trial of Neoadjuvant Chemoendocrine Therapy in Primary Breast Cancer. Ann. Oncol. 1998, 9, 1179–1184. [Google Scholar] [CrossRef]
- Mauriac, L.; MacGrogan, G.; Avril, A.; Durand, M.; Floquet, A.; Debled, M.; Dilhuydy, J.M.; Bonichon, F.; (IBBGS), I.B.B.G.S. Neoadjuvant Chemotherapy for Operable Breast Carcinoma Larger than 3 cm: A Unicentre Randomized Trial with a 124-Month Median Follow-Up. Ann. Oncol. 1999, 10, 47–52. [Google Scholar] [CrossRef]
- van der Hage, J.A.; van de Velde, C.J.; Julien, J.P.; Tubiana-Hulin, M.; Vandervelden, C.; Duchateau, L. Preoperative Chemotherapy in Primary Operable Breast Cancer: Results from the European Organization for Research and Treatment of Cancer Trial 10902. J. Clin. Oncol. 2001, 19, 4224–4237. [Google Scholar] [CrossRef]
- Brackstone, M.; Fletcher, G.G.; Dayes, I.S.; Madarnas, Y.; SenGupta, S.K.; Verma, S.; Members of the Breast Cancer Disease Site Group. Locoregional Therapy of Locally Advanced Breast Cancer: A Clinical Practice Guideline. Curr. Oncol. 2015, 22, 54–66. [Google Scholar] [CrossRef]
- (EBCTCG), E.B.C.T.C.G.; Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; et al. Long-Term Outcomes for Neoadjuvant versus Adjuvant Chemotherapy in Early Breast Cancer: Meta-Analysis of Individual Patient Data from Ten Randomised Trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Bleicher, R.J. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann. Surg. Oncol. 2018, 25, 2829–2838. [Google Scholar] [CrossRef]
- Cullinane, C.; Shrestha, A.; Maksoud, A.A.; Rothwell, J.; Evoy, D.; Geraghty, J.; McCartan, D.; McDermott, E.W.; Prichard, R.S. Optimal Timing of Surgery Following Breast Cancer Neoadjuvant Chemotherapy: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2021, 47, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Norikazu, M.; Soo-Jung, L.; Shoichiro, O.; Young-Hyuck, I.; Eun-Sook, L.; Isao, Y.; Katsumasa, K.; Seock-Ah, I.; Byeong-Woo, P.; Sung-Bae, K.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2018, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative Chemotherapy in Patients With Operable Breast Cancer: Nine-Year Results From National Surgical Adjuvant Breast and Bowel Project B-18. JNCI Monogr. 2001, 2001, 96–102. [Google Scholar] [CrossRef]
- Graham, P.J.; Brar, M.S.; Foster, T.; McCall, M.; Bouchard-Fortier, A.; Temple, W.; Quan, M.L. Neoadjuvant Chemotherapy for Breast Cancer, Is Practice Changing? A Population-Based Review of Current Surgical Trends. Ann. Surg. Oncol. 2015, 22, 3376–3382. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, X.-E.; Tian, J.-H.; Yang, X.-J.; Wang, Y.-F.; Yang, K.-H. Survival Benefit of Neoadjuvant Chemotherapy for Resectable Breast Cancer. Medicine 2018, 97, e10634. [Google Scholar] [CrossRef]
- Buchholz, T.A.; Hunt, K.K.; Whitman, G.J.; Sahin, A.A.; Hortobagyi, G.N. Neoadjuvant Chemotherapy for Breast Carcinoma. Cancer 2003, 98, 1150–1160. [Google Scholar] [CrossRef]
- Aebi, S.; Karlsson, P.; Wapnir, I.L. Locally Advanced Breast Cancer. Breast 2022, 62, S58–S62. [Google Scholar] [CrossRef]
- Nordenskjöld, A.E.; Helena, F.; Arnesson, L.G.; Einbeigi, Z.; Holmberg, E.; Albertsson, P.; Karlsson, P. Breast Cancer Survival Trends in Different Stages and Age Groups—A Population-Based Study 1989–2013. Acta Oncol. 2019, 58, 45–51. [Google Scholar] [CrossRef]
- Cai, L.; Tong, Y.; Zhu, X.; Shen, K.; Zhu, J.; Chen, X. Prolonged Time to Adjuvant Chemotherapy Initiation Was Associated with Worse Disease Outcome in Triple Negative Breast Cancer Patients. Sci. Rep. 2020, 10, 7029. [Google Scholar] [CrossRef] [PubMed]
- Kupstas, A.R.; Hoskin, T.L.; Day, C.N.; Habermann, E.B.; Boughey, J.C. Effect of Surgery Type on Time to Adjuvant Chemotherapy and Impact of Delay on Breast Cancer Survival: A National Cancer Database Analysis. Ann. Surg. Oncol. 2019, 26, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Suleman, K.; Almalik, O.; Haque, E.; Mushtaq, A.; Badran, A.; Alsayed, A.; Ajarim, D.; Al-Tweigeri, T.; Jastaniyah, N.; Elhassan, T.; et al. Does the Timing of Surgery after Neoadjuvant Therapy in Breast Cancer Patients Affect the Outcome? Oncology 2020, 98, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Sanford, R.A.; Lei, X.; Barcenas, C.H.; Mittendorf, E.A.; Caudle, A.S.; Valero, V.; Tripathy, D.; Giordano, S.H.; Chavez-MacGregor, M. Impact of Time from Completion of Neoadjuvant Chemotherapy to Surgery on Survival Outcomes in Breast Cancer Patients. Ann. Surg. Oncol. 2016, 23, 1515–1521. [Google Scholar] [CrossRef]
- Al-Masri, M.; Aljalabneh, B.; Al-Najjar, H.; Al-Shamaileh, T. Effect of Time to Breast Cancer Surgery after Neoadjuvant Chemotherapy on Survival Outcomes. Breast Cancer Res. Treat. 2021, 186, 7–13. [Google Scholar] [CrossRef]
- Caudle, A.S.; Gonzalez-Angulo, A.M.; Hunt, K.K.; Pusztai, L.; Kuerer, H.M.; Mittendorf, E.A.; Hortobagyi, G.N.; Meric-Bernstam, F. Impact of Progression During Neoadjuvant Chemotherapy on Surgical Management of Breast Cancer. Ann. Surg. Oncol. 2011, 18, 932–938. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Newman, L.A.; Smith, T.L.; Ames, F.C.; Hunt, K.K.; Dhingra, K.; Theriault, R.L.; Singh, G.; Binkley, S.M.; Sneige, N.; et al. Clinical Course of Breast Cancer Patients with Complete Pathologic Primary Tumor and Axillary Lymph Node Response to Doxorubicin-Based Neoadjuvant Chemotherapy. J. Clin. Oncol. 1999, 17, 460. [Google Scholar] [CrossRef]
- Chollet, P.; Amat, S.; Cure, H.; de Latour, M.; Bouedec, G.L.; Mouret-Reynier, M.-A.; Ferriere, J.-P.; Achard, J.-L.; Dauplat, J.; Penault-Llorca, F. Prognostic Significance of a Complete Pathological Response after Induction Chemotherapy in Operable Breast Cancer. Br. J. Cancer 2002, 86, 1041–1046. [Google Scholar] [CrossRef]
- Amat, S.; Abrial, S.C.; Abrial, C.; Penault-Llorca, F.; Delva, R.; Bougnoux, P.; Leduc, B.; Mouret-Reynier, M.-A.; Mery-Mignard, D.; Bleuse, J.-P.; et al. High Prognostic Significance of Residual Disease after Neoadjuvant Chemotherapy: A Retrospective Study in 710 Patients with Operable Breast Cancer. Breast Cancer Res. Treat. 2005, 94, 255–263. [Google Scholar] [CrossRef]
- Loibl, S.; Marmé, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.-B.; Bear, H.; McCarthy, N.; Olivé, M.M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer—The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef]
- Foldi, J.; Rozenblit, M.; Park, T.S.; Knowlton, C.A.; Golshan, M.; Moran, M.; Pusztai, L. Optimal Management for Residual Disease Following Neoadjuvant Systemic Therapy. Curr. Treat. Options Oncol. 2021, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kantor, O.; Barrera, E.; Kopkash, K.; Pesce, C.; Barrera, E.; Winchester, D.J.; Yao, K. Are We Overtreating Hormone Receptor Positive Breast Cancer with Neoadjuvant Chemotherapy? Role of OncotypeDx® for Hormone Receptor Positive Patients Undergoing Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2019, 26, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Bear, H.D.; Wan, W.; Robidoux, A.; Rubin, P.; Limentani, S.; White, R.L.; Granfortuna, J.; Hopkins, J.O.; Oldham, D.; Rodriguez, A.; et al. Using the 21-gene Assay from Core Needle Biopsies to Choose Neoadjuvant Therapy for Breast Cancer: A Multicenter Trial. J. Surg. Oncol. 2017, 115, 917–923. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Bandos, H.; White, J.R.; Julian, T.B.; Khan, A.J.; Shaitelman, S.F.; Torres, M.A.; Vicini, F.; Ganz, P.A.; McCloskey, S.A.; et al. NRG Oncology/NSABP B-51/RTOG 1304: Phase III Trial to Determine If Chest Wall and Regional Nodal Radiotherapy (CWRNRT) Post Mastectomy (Mx) or the Addition of RNRT to Whole Breast RT Post Breast-Conserving Surgery (BCS) Reduces Invasive Breast Cancer Recurrence-Free Interval (IBCR-FI) in Patients (Pts) with Pathologically Positive Axillary (PPAx) Nodes Who Are YpN0 after Neoadjuvant Chemotherapy (NC). J. Clin. Oncol. 2019, 37, TPS600. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel Lymph Node Surgery after Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer: The ACOSOG Z1071 (Alliance) Clinical Trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-Lymph-Node Biopsy in Patients with Breast Cancer before and after Neoadjuvant Chemotherapy (SENTINA): A Prospective, Multicentre Cohort Study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boileau, J.-F.; Poirier, B.; Basik, M.; Holloway, C.M.B.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel Node Biopsy After Neoadjuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: The SN FNAC Study. J. Clin. Oncol. 2014, 33, 258–264. [Google Scholar] [CrossRef]
| RCT | Mean Age, Years (SD) | ER Status | PR Status | Surgery Type | ||||
|---|---|---|---|---|---|---|---|---|
| − (freq) | + (freq) | − (freq) | + (freq) | Lumpectomy/ Conservative | Mastectomy | Biopsy/ No Surgery | ||
| Wolmark et al. [15] | 48.9 (11.1) | 166 | 182 | 186 | 161 | 139 | 287 | 2 |
| Deo et al. [3] | 46.6 (10.6) | 77 | 102 | 79 | 100 | 0 | 179 | 0 |
| Gazet et al. [4] | 54.8 (8.8) | 30 | 0 | 0 | 0 | 12 | 12 | 2 |
| Makris et al. [5] | 47.7 (6.1) | 4 | 3 | 0 | 0 | 2 | 5 | 0 |
| Mauriac et al. [6] | 52.8 (5.5) | 36 | 20 | 38 | 18 | 8 | 40 | 8 |
| Van der Hage et al. [7] | 48.6 (9.8) | 0 | 0 | 0 | 0 | 3 | 76 | 12 |
| RCT | Clinical Nodal Status | Pathological N Stage, Number of Positive Nodes | Total | |||
|---|---|---|---|---|---|---|
| Negative (N0) | 0 | 1–3 | 4–9 | >10 | ||
| Wolmark et al. [15] | 232 | 98 | 82 | 158 | 86 | 424 |
| Deo et al. [3] | 0 | 51 | 46 | 38 | 44 | 179 |
| Gazet et al. [4] | 0 | 21 | 8 | 1 | 0 | 30 |
| Makris et al. [5] | 0 | 4 | 1 | 2 | 0 | 7 |
| Mauriac et al. [6] | 27 | 15 | 19 | 9 | 5 | 48 |
| Van der Hage et al. [7] | 0 | 10 | 25 | 24 | 19 | 78 |
| Characteristic | Overall, N = 563 1 | Time to Surgery | p-Value | |
|---|---|---|---|---|
| >8 Weeks, N = 31 (5.5%) 1 | ≤8 Weeks, N = 532 (94.5%) 1 | |||
| Age | 51 (43, 61) | 62 (52, 67) | 50 (42, 60) | <0.001 |
| cT stage | 0.3 | |||
| T1 | 39 (6.9%) | 1 (3.2%) | 38 (7.1%) | |
| T2 | 155 (28%) | 7 (23%) | 148 (28%) | |
| T3 | 204 (36%) | 9 (29%) | 195 (37%) | |
| T4 | 165 (29%) | 14 (45%) | 151 (28%) | |
| cN stage | 0.3 | |||
| N0 | 224 (40%) | 9 (29%) | 215 (40%) | |
| N1 | 274 (49%) | 16 (52%) | 258 (48%) | |
| N2 | 45 (8.0%) | 5 (16%) | 40 (7.5%) | |
| N3 | 20 (3.6%) | 1 (3.2%) | 19 (3.6%) | |
| ER status | >0.9 | |||
| Negative | 203 (36%) | 11 (35%) | 192 (36%) | |
| Positive | 360 (64%) | 20 (65%) | 340 (64%) | |
| PR status | 0.079 | |||
| Negative | 259 (46%) | 19 (61%) | 240 (45%) | |
| Positive | 304 (54%) | 12 (39%) | 292 (55%) | |
| Her2 status | 0.5 | |||
| Negative | 350 (62%) | 21 (68%) | 329 (62%) | |
| Positive | 213 (38%) | 10 (32%) | 203 (38%) | |
| Grade | >0.9 | |||
| 1 | 35 (6.2%) | 1 (3.2%) | 34 (6.4%) | |
| 2 | 240 (43%) | 14 (45%) | 226 (42%) | |
| 3 | 288 (51%) | 16 (52%) | 272 (51%) | |
| Subtype | 0.8 | |||
| Her2 | 75 (13%) | 4 (13%) | 71 (13%) | |
| Lum_A | 151 (27%) | 10 (32%) | 141 (27%) | |
| Lum_B | 217 (39%) | 10 (32%) | 207 (39%) | |
| TN | 120 (21%) | 7 (23%) | 113 (21%) | |
| Time to Surgery (days) | 32 (26, 41) | 79 (63, 115) | 32 (25, 39) | |
| Characteristic | N = 31 1 |
|---|---|
| Reason_for_delay | |
| Administrative/Non-Medical | 9 (29%) |
| Chemotoxicity | 13 (42%) |
| Clinical Trial | 7 (23%) |
| Medical reasons unrelated to chemotherapy | 2 (6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, F.; Brackstone, M. The Impact of Neoadjuvant versus Adjuvant Chemotherapy on Survival Outcomes in Locally Advanced Breast Cancer. Curr. Oncol. 2024, 31, 6007-6016. https://doi.org/10.3390/curroncol31100448
Ghasemi F, Brackstone M. The Impact of Neoadjuvant versus Adjuvant Chemotherapy on Survival Outcomes in Locally Advanced Breast Cancer. Current Oncology. 2024; 31(10):6007-6016. https://doi.org/10.3390/curroncol31100448
Chicago/Turabian StyleGhasemi, Farhad, and Muriel Brackstone. 2024. "The Impact of Neoadjuvant versus Adjuvant Chemotherapy on Survival Outcomes in Locally Advanced Breast Cancer" Current Oncology 31, no. 10: 6007-6016. https://doi.org/10.3390/curroncol31100448
APA StyleGhasemi, F., & Brackstone, M. (2024). The Impact of Neoadjuvant versus Adjuvant Chemotherapy on Survival Outcomes in Locally Advanced Breast Cancer. Current Oncology, 31(10), 6007-6016. https://doi.org/10.3390/curroncol31100448





