Primary Diffuse Leptomeningeal Melanomatosis in a Child with Extracranial Metastasis: Case Report
Abstract
1. Introduction
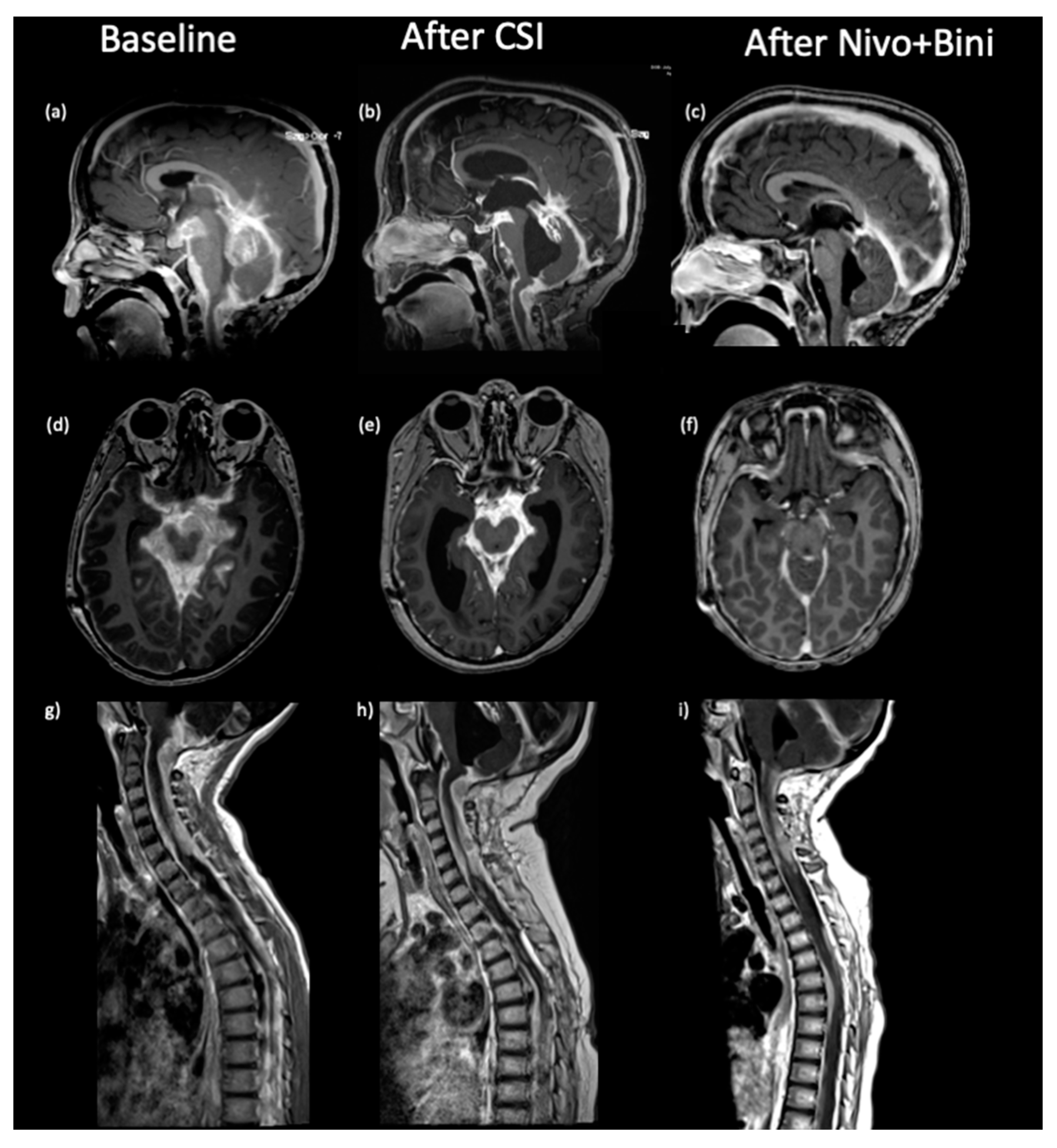
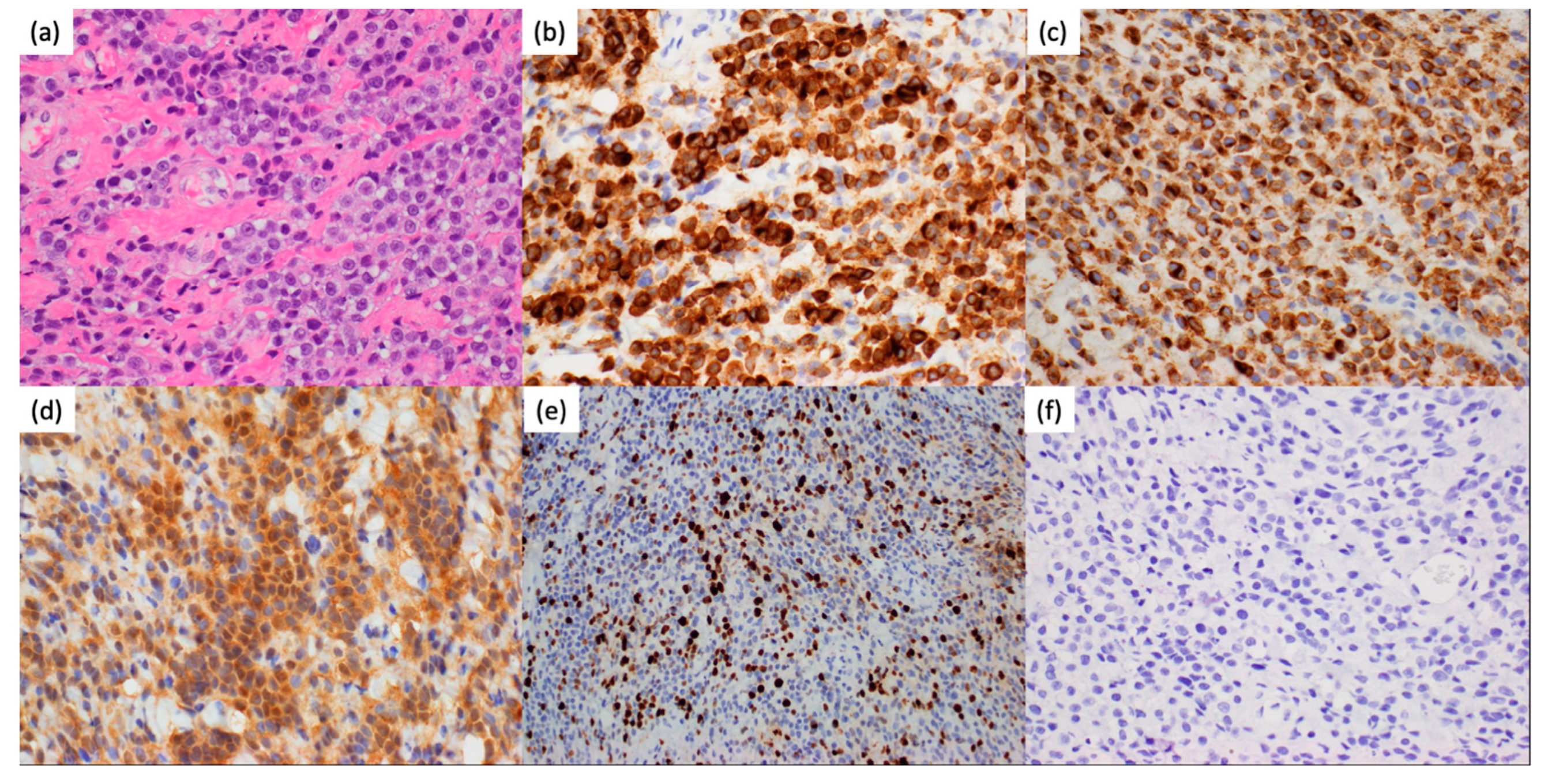
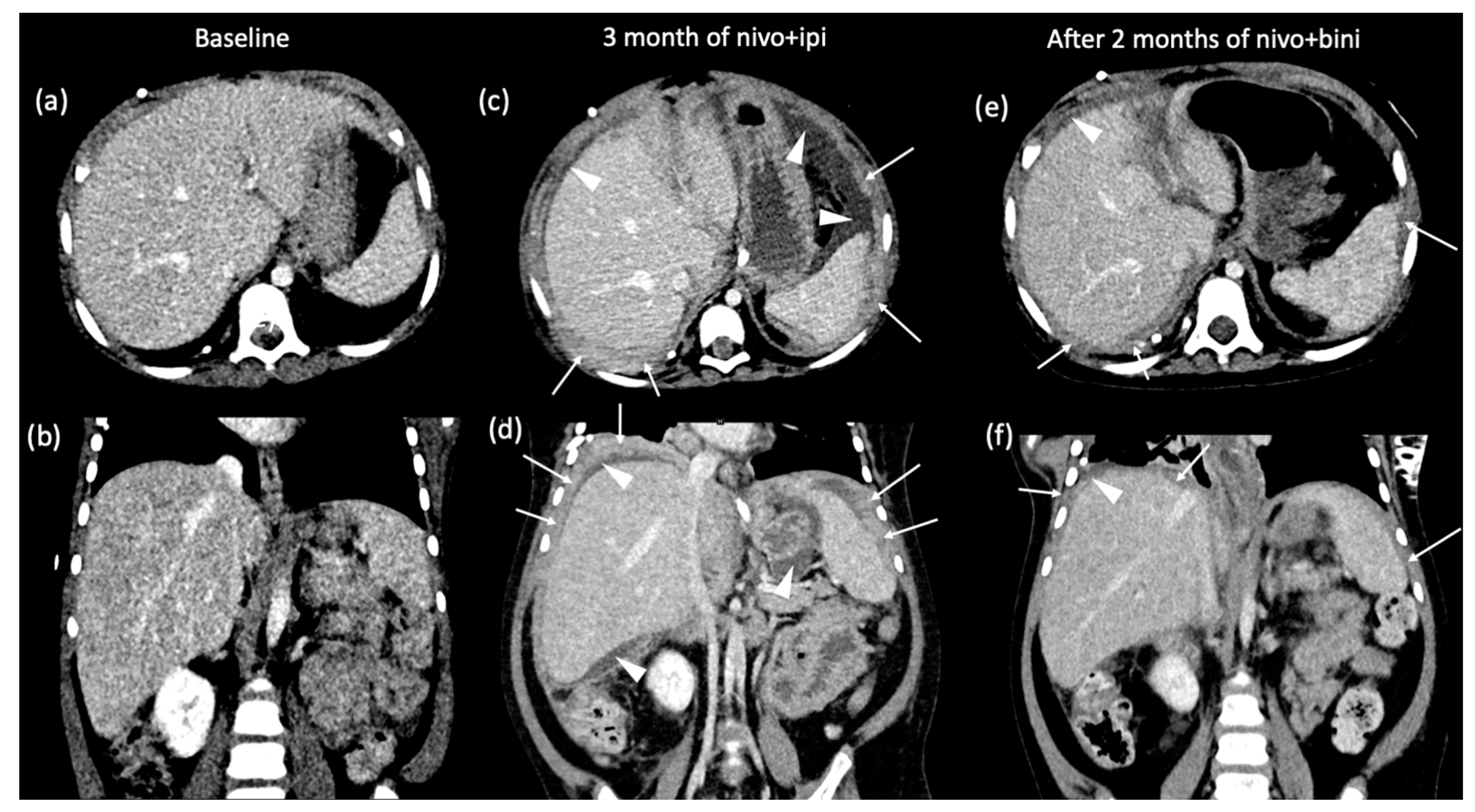
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Puyana, C.; Denyer, S.; Burch, T.; Bhimani, A.D.; McGuire, L.S.; Patel, A.S.; Mehta, A.I. Primary Malignant Melanoma of the Brain: A Population-Based Study. World Neurosurg. 2019, 130, e1091–e1097. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, F.; Desai, S. Intradural Extramedullary Primary Central Nervous System Melanoma of the Craniovertebral Junction during Pregnancy: Observations and Outcomes. Surg. Neurol. Int. 2021, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Andres Sanz, J.A.; Ruiz Gines, J.A.; Iliev, H.; Aguas Valiente, J. Primary intracranial melanoma, amelanotic variant: Case report. Neurocirugia (Astur. Engl. Ed.) 2022, 33, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Porag, R.; Asif, D.S.; Satter, A.M.; Taufiq, M.; Gaddam, S.S. Primary Intracranial Melanoma with Early Leptomeningeal Spread: A Case Report and Treatment Options Available. Case Rep. Oncol. Med. 2015, 2015, 293802. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Stepien, N.; Mayr, L.; Madlener, S.; Dorfer, C.; Schmook, M.T.; Traub-Weidinger, T.; Lotsch-Gojo, D.; Kirchhofer, D.; Reisinger, D.; et al. Novel Insights into Diagnosis, Biology and Treatment of Primary Diffuse Leptomeningeal Melanomatosis. J. Pers. Med. 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Angelino, G.; De Pasquale, M.D.; De Sio, L.; Serra, A.; Massimi, L.; De Vito, R.; Marrazzo, A.; Lancella, L.; Carai, A.; Antonelli, M.; et al. NRAS(Q61K) mutated primary leptomeningeal melanoma in a child: Case presentation and discussion on clinical and diagnostic implications. BMC Cancer 2016, 16, 512. [Google Scholar] [CrossRef]
- Flodmark, O.; Fitz, C.R.; Harwood-Nash, D.C.; Chuang, S.H. Neuroradiological findings in a child with primary leptomeningeal melanoma. Neuroradiology 1979, 18, 153–156. [Google Scholar] [CrossRef]
- Makin, G.W.; Eden, O.B.; Lashford, L.S.; Moppett, J.; Gerrard, M.P.; Davies, H.A.; Powell, C.V.; Campbell, A.N.; Frances, H. Leptomeningeal melanoma in childhood. Cancer 1999, 86, 878–886. [Google Scholar] [CrossRef]
- Szathmari, A.; Perbet, R.; Hermier, M.; Di Rocco, F.; Frappaz, D.; Mottolese, C. Primary Amelanotic Leptomeningeal Melanomatosis in a Child: A Rare but Severe Disease. World Neurosurg. 2016, 92, 581.e15–581.e20. [Google Scholar] [CrossRef]
- Nicolaides, P.; Newton, R.W.; Kelsey, A. Primary malignant melanoma of meninges: Atypical presentation of subacute meningitis. Pediatr. Neurol. 1995, 12, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sakamoto, K.; Kobayashi, N.; Ito, H. Primary melanomas in the central nervous system with peritoneal dissemination through ventriculo-peritoneal shunt. Report of an autopsy case. Neurol. Med. Chir. 1985, 25, 1029–1035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Zheng, Y.; Li, J.; Wang, F.; Li, F. Pediatric primary diffuse leptomeningeal melanomatosis: Case report and review of the literature. Medicine 2020, 99, e19178. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, P.; Carson, H.J.; Attal, H.; Castelli, M.J. Peritoneal implantation of meningeal melanosis via ventriculoperitoneal shunt: A case report and review of the literature. Diagn. Cytopathol. 1995, 13, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ahn, B.C.; Hwang, S.W.; Cho, S.K.; Kim, H.W.; Lee, S.W.; Hwang, J.H.; Lee, J. F-18 fluorodeoxyglucose PET/CT and post hoc PET/MRI in a case of primary meningeal melanomatosis. Korean J. Radiol. 2013, 14, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pirini, M.G.; Mascalchi, M.; Salvi, F.; Tassinari, C.A.; Zanella, L.; Bacchini, P.; Bertoni, F.; D’Errico, A.; Corti, B.; Grigioni, W.F. Primary diffuse meningeal melanomatosis: Radiologic-pathologic correlation. AJNR Am. J. Neuroradiol. 2003, 24, 115–118. [Google Scholar] [PubMed]
- Aebischer, V.; Abu-Ghazaleh, A.; Metzler, G.; Riedl, L.; Garbe, C.; Flatz, L.; Eigentler, T.; Forchhammer, S. Histopathologic abundance of pigmentation correlates with disease-specific survival in malignant melanoma but is not independent of current AJCC pT stage. Pigment. Cell Melanoma Res. 2023, 36, 512–521. [Google Scholar] [CrossRef]
- Narayan, A.; Jallo, G.; Huisman, T.A. Extracranial, peritoneal seeding of primary malignant brain tumors through ventriculo-peritoneal shunts in children: Case report and review of the literature. Neuroradiol. J. 2015, 28, 536–539. [Google Scholar] [CrossRef]
- Hironaka, K.; Tateyama, K.; Tsukiyama, A.; Adachi, K.; Morita, A. Hydrocephalus Secondary to Intradural Extramedullary Malignant Melanoma of Spinal Cord. World Neurosurg. 2019, 130, 222–226. [Google Scholar] [CrossRef]
- Randic, T.; Kozar, I.; Margue, C.; Utikal, J.; Kreis, S. NRAS mutant melanoma: Towards better therapies. Cancer Treat. Rev. 2021, 99, 102238. [Google Scholar] [CrossRef]
- Johnson, D.B.; Lovly, C.M.; Flavin, M.; Panageas, K.S.; Ayers, G.D.; Zhao, Z.; Iams, W.T.; Colgan, M.; DeNoble, S.; Terry, C.R.; et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol. Res. 2015, 3, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef]
- Grossman, J.E.; Vasudevan, D.; Joyce, C.E.; Hildago, M. Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene 2021, 40, 1393–1395. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Magraner, L.; Gumuzio, J.; Miles, J.; Quimi, N.; Martinez Del Prado, P.; Abad-Villar, M.T.; Pikabea, F.; Ortega, L.; Etxezarraga, C.; Martin-Algarra, S.; et al. Functional Engagement of the PD-1/PD-L1 Complex but Not PD-L1 Expression Is Highly Predictive of Patient Response to Immunotherapy in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2023, 41, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Fejza, A.; Polano, M.; Camicia, L.; Poletto, E.; Carobolante, G.; Toffoli, G.; Mongiat, M.; Andreuzzi, E. The Efficacy of Anti-PD-L1 Treatment in Melanoma Is Associated with the Expression of the ECM Molecule EMILIN2. Int. J. Mol. Sci. 2021, 22, 7511. [Google Scholar] [CrossRef]
- Mandala, M.; Merelli, B.; Massi, D. PD-L1 in melanoma: Facts and myths. Melanoma Manag. 2016, 3, 187–194. [Google Scholar] [CrossRef]
- Pedersen, M.; Kusters-Vandevelde, H.V.N.; Viros, A.; Groenen, P.; Sanchez-Laorden, B.; Gilhuis, J.H.; van Engen-van Grunsven, I.A.; Renier, W.; Schieving, J.; Niculescu-Duvaz, I.; et al. Primary melanoma of the CNS in children is driven by congenital expression of oncogenic NRAS in melanocytes. Cancer Discov. 2013, 3, 458–469. [Google Scholar] [CrossRef]
- Knight, A.; Karapetyan, L.; Kirkwood, J.M. Immunotherapy in Melanoma: Recent Advances and Future Directions. Cancers 2023, 15, 1106. [Google Scholar] [CrossRef]
- Wilson, T.G.; Winter, H.; Taylor, H.; Herbert, C. Treating brain metastases in melanoma: What is the optimal CNS-directed and systemic management? J. Radiosurg. SBRT 2021, 7, 279–285. [Google Scholar] [PubMed]
- El Habnouni, C.; Blechet, C.; Bens, G. Pembrolizumab for primary malignant melanoma of the central nervous system. J. Neurooncol. 2018, 139, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Misir Krpan, A.; Rakusic, Z.; Herceg, D. Primary leptomeningeal melanomatosis successfully treated with PD-1 inhibitor pembrolizumab: A case report. Medicine 2020, 99, e22928. [Google Scholar] [CrossRef] [PubMed]
- Saberian, C.; Sperduto, P.; Davies, M.A. Targeted therapy strategies for melanoma brain metastasis. Neurooncol. Adv. 2021, 3, v75–v85. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
| Age (in Years) | Sex (M/F) | Surgery | XRT | CTX | IMMN | Best Response | Time to Death following Diagnosis | Reference |
|---|---|---|---|---|---|---|---|---|
| 2 | F | Biopsy and VP shunt | Y | Temozolomide, cisplatin, vindesine | peg-inter-feron α2b | SD | 11 months | Angelino et al. [7] |
| 3 | M | Biopsy and V-C shunt | N | N | N | PD | Unknown | Flodmark et al. [8] |
| 5 | M | Biopsy | N | vincristine, carboplatin, etoposide | N | PD | 6 months | Makin et al. [9] |
| 5 | F | Biopsy, multiple cyst decompressions and shunts | N | Cisplatin, etoposide, and temozolomide | N | PD | 10 months | Szathmari et al. [10] |
| 5 | M | Biopsy and VP shunt | N | Following regimen for PNET at that time in the UK | N | ? | Unknown (at least 3 months) | Nicolaides et al. [11] |
| 11 | F | Subtotal resection | N/A | N/A | N/A | N/A | 2 months | Tanaka et al. [12] * |
| 13 | M | Resection of temporal and parietal lesions | N | N | N | PD | 5 months | Xu et al. [13] |
| 14 | M | Ommaya | Y focal RT (spine) | Trametinib, everolimus (po), etoposide, cytarabine, topotecan (IVT) | Nivolumab, ipilimumab | CR | 7 months | Baumgartner et al. [6] |
| 16 | F | VP shunt | Y | ? | N | ? | 18 months | Gattuso et al. [14] |
| 17 | M | Biopsy | Y Whole brain | Dacarbazine, carmustine, cisplatin, and tamoxifen | N | PR | Unknown (but tolerated 8 cycles) | Lee et al. [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahab, S.W.; Patil, P.; Fangusaro, J.R.; Patteson, B.; Goldman-Yassen, A.; Eaton, B.R.; Boydston, W.; Schniederjan, M.; Aguilera, D. Primary Diffuse Leptomeningeal Melanomatosis in a Child with Extracranial Metastasis: Case Report. Curr. Oncol. 2024, 31, 579-587. https://doi.org/10.3390/curroncol31010041
Shahab SW, Patil P, Fangusaro JR, Patteson B, Goldman-Yassen A, Eaton BR, Boydston W, Schniederjan M, Aguilera D. Primary Diffuse Leptomeningeal Melanomatosis in a Child with Extracranial Metastasis: Case Report. Current Oncology. 2024; 31(1):579-587. https://doi.org/10.3390/curroncol31010041
Chicago/Turabian StyleShahab, Shubin W., Prabhumallikarjun Patil, Jason R. Fangusaro, Brooke Patteson, Adam Goldman-Yassen, Bree R. Eaton, William Boydston, Matthew Schniederjan, and Dolly Aguilera. 2024. "Primary Diffuse Leptomeningeal Melanomatosis in a Child with Extracranial Metastasis: Case Report" Current Oncology 31, no. 1: 579-587. https://doi.org/10.3390/curroncol31010041
APA StyleShahab, S. W., Patil, P., Fangusaro, J. R., Patteson, B., Goldman-Yassen, A., Eaton, B. R., Boydston, W., Schniederjan, M., & Aguilera, D. (2024). Primary Diffuse Leptomeningeal Melanomatosis in a Child with Extracranial Metastasis: Case Report. Current Oncology, 31(1), 579-587. https://doi.org/10.3390/curroncol31010041





