Narrative Review of Immunotherapy in Gastroentero-Pancreatic Neuroendocrine Neoplasms
Abstract
1. Introduction
2. Description of the Literature Search
3. Tumor Microenvironment and Scientific Rationale of Immunotherapy of GEP-NEN
4. Clinical Trials of Immune Checkpoint Inhibitors in NENs (Table 1)
5. Combination Therapy (Table 1)
- -
- Dual Immune checkpoint inhibitors (ICI) (-Targeting CTLA-4 + PDL-1/PD-1)
- -
- Combination of ICI with TKI
- -
- Combination of ICI with somatostatin analogs
- -
- Combination of ICI with chemotherapy in G3 NEN
6. Current Treatment Paradigm with NCCN Recommendations (Figure 1)
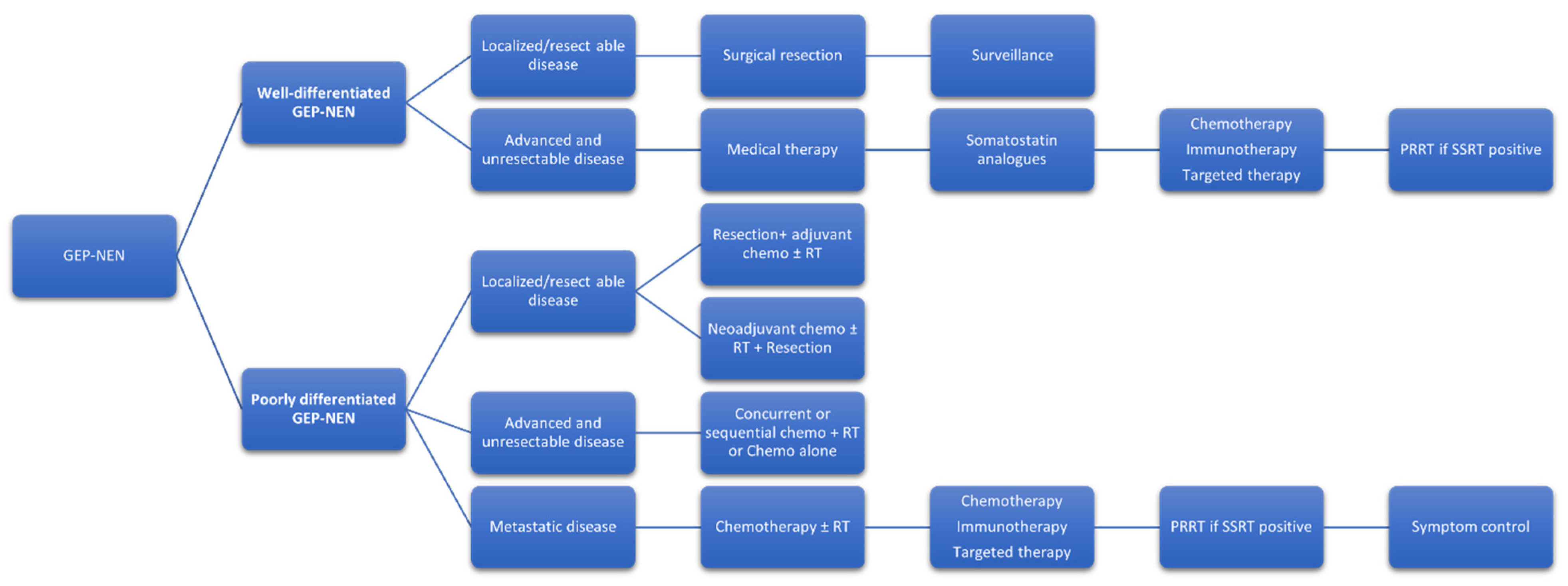
7. Future Direction
- -
- Combination of ICI with lutathera
- -
- Vaccine and CART cell therapy
| Trial | Disease Site | Grade | Phase, Study Design | No. | Drug | ORR (95% CI) | PFS (95% CI) | OS (95% CI) | Toxicities Grade > 3 |
|---|---|---|---|---|---|---|---|---|---|
| Monotherapy -Targeting PDL-1 and PD-1 | |||||||||
| Mehnert et al 2020 [18] Keynote-028 NCT02054806 | Carcinoid tumor | NA | I, open label, single group assignment | 25 | Pembrolizumab 10 mg/Kg every 2 weeks | 12 (2.5–31.2) | 5.6 m (3.5–10.7) | NA | 4% |
| pNET | NA | 16 | 6.3% (0.2–30.2) | 4.5 m (3.6–8.3) | NA | 6.3% | |||
| Strosberg, et al, 2020 [19] Keynote -158 NCT02628067 | NET | 1,2 | II, open label, non-RCT | 24 | Pembrolizumab 200 mg every 3 weeks | 3.7% (1–9.3) | 4.1 m (3.5–5.4) | 24.2 (15.8–32.5) | 21.5% |
| GEP-NEN | 83 | ||||||||
| Yao, et al 2021 [21] NCT02955069 | NET | 1,2 | II, open label, single group assignment | 95 | Spartalizumab 400 mg every 4 weeks | 7.4% (3.0–14.6), | 19.5% (12 m PFS) | 73.5% at 12 months (63–81.4) | 20 (21.1%) |
| GEP-NEC | 3 | 21 | 4.8% (0.1–23.8) | 0% (12 m PFS) | 19.1% at 12 months (4.8–40.6) | 4 (19%); | |||
| Lu et al 2020 [22] NCT03167853 | WD-NEN | 2,3 | Ib, open label, single group assignment | 8 | Toripalimab 3 mg/kg every 2 weeks | 25% | 2.5 (1.9–3.1) | 7.8 (5–10.8) | 11 (27.5%) |
| PD-NEN | 3 | 32 | 18.7% | ||||||
| Fottner et al 2019 [23] AVENEC NCT03352934 | GEP | 3 | II, open label, single group assignment | 27 | Avelumab 10 mg/kg every 2 weeks | -- | 3.3 m (1.2–24.6) | 14.2 m | 10% |
| Dual Immune check point inhibitors (-Targeting CTLA-4 + PDL-1/PD-1) | |||||||||
| Patel et al 2020 [28] DART trial NCT02834013 | NEN | 1,2,3 | II | 32 | Nivolumab 240 mg every 2 weeks plus Ipilimumab 1 mg every 6 weeks | 25% (13–42) | 6% at 6 months (19–52%) | 11 m (6-NE) | 16 (50%) |
| Klein et al 2020 [29] CA209-538 NCT02923934 | NEN | 1,2,3 | II | 29 | Nivolumab 3 mg/kg plus Ipilimumab 1 mg/kg every 3 weeks for four doses followed by Nivolumab 3 mg/kg every 2 weeks upto 96 weeks | 24% | 4.8 m (2.7–10.5) | 14.8 M (4.1–21.3) | 10 (34%) |
| Girard et al., 2021 [46] NCT03591731 | GEPNET and Lung NEC (PD) | NEC | II | 170 | Nivolumab plus Ipilimumab | 14.9% (8.2–24.2) | 1.9 m (1.6–2.1) | 7.2 m (3.7–14.1) | -- |
| NEC | Nivolumab | 7.2% (2.7–15.1) | 1.8 m (1.7–2.0) | 5.8 m (3.3–7.6) | -- | ||||
| Capdevila et al 2020 [30,31] DUNE trial NCT03095274 | Lung NEN | 1,2 | II | 27 | Durvalumab 20 mg/kg every 4 weeks plus Tremelimumab 1 mg/Kg every 4 weeks | 11.1% | 5.6 m (4.9–6.2) | NR (0.3–41.3) | 12.2% |
| GI-NET | 1,2 | 31 | 0% | 5.8 (3.1–8.5) | 29.5 (19.6–39.4) | ||||
| p-NET | 1,2 | 32 | 6.3% | 5.5 (2.4–8.7) | 23.8 (16.4–31.2) | ||||
| GEP-NEN | 3 | 33 | 9.1% | 2.4 (2.1–2.8) | 9 months OS 36.1% (19.6–52.6) | ||||
| ICI combined with TKI | |||||||||
| Halperin et al. 2022 [32] NCT03074513 | p-NET | 1,2 | II, open label, single group assignment | 20 | Atezolizumab 1200 mg plus Bevacizumab 15 mg/kg every 3 weeks | 20% (5.7–43.7) | 14.9 (4.4–32.0) | 30.1 m (17.7 m-NR) | -- |
| Ep-NET | 20 | 15% (3.2–37.9) | 14.2 (10.2–19.6) | NR | -- | ||||
| Al-Toubah et al. 2022 [33] NCT03290079 | NET | -- | II, open label, single group assignment | 20 | Pembrolizumab 200 mg every 3 weeks plus Lenvatinib 20 mg daily | 10% | 10 m (5.9–14.1 m) | -- | 12 (60%) |
| Morse et al 2021 [34] PLANET trial NCT03043664 | GEP-NEN | -- | II, open label, single group assignment | 22 | Pembrolizumb 200 mg every 3 weeks plus Lanreotide 90 mg every 3 weeks | 39% | 5.4 m (1.7–8.3) | 15 m (NR) | -- |
| Raj et al. 2023 [35] NCT03136055 | NEN | -- | II, open label, Non-RCT | 14 | Pembrolizumab 200 mg every 3 weeks for 24 months or 35 administrations | 7% (0.02–33.9) | 1.8 m (1.7–21.4), | 7.8 (3.1-NR) | 2 (14%) |
| -- | 22 | Pembrolizumab 200 mg every 3 weeks for 24 months or 35 administrations plus Irinotecan 125 mg/m2 day 1 and day 8 every 3 weeks plus paclitaxel day 1, 8 and 15 every 3 weeks | 5% (0–22.8) | 2.0 m (1.9–3.4), | 4.8 (4.1–8.2) | 10 (45%) | |||
| Trial | Disease Site | Phase | No. | Drug | Primary Outcome | Secondary Outcome | Result/Status |
|---|---|---|---|---|---|---|---|
| NCT01174121 Parallel arm design | Metastatic cancer (NET) | 2 | 332 | TIL, Pembro, Aldesleuki + Chemo | Response rate | Safety and efficacy | Accruing |
| NCT03980925; Single group | GEPNET | 2 | 38 | Nivo + platinum doublet | OS at 12 months | PFS, ORR | Accruing |
| NCT03290079; Single group | NET | 2 | 28 | Pembro + Lenvatinib | ORR | DOR, PFS, OS | Active not accruing |
| NCT03475953; Sequential assignment | Solid tumor (GEPNET) | 2 | 482 | Regorafenib + Avelumab | RP2D, antitumor activity of regorafenib | MTD, DLT | Accruing |
| NCT04579757 | Solid tumor (NET) | 2 | 135 | Surufatinib and Tislelizumab | ORR, dose limiting toxicity | PFS | Active not accruing |
| NCT05058651 | Extrapulmonary NEC | 2/3 | 189 | Atezolizumab, etoposide, platinum | OS | PFS, ORR, DOR | Accruing |
| NCT04079712 | NET | 2 | 30 | Cabozantinib +Nivolumab +Ipilimumab | ORR | PFS, AE | Active not accruing |
| NCT04525638 | NET (G3 WD) | 2 | 30 | 177Lu-DOTATATE and Nivolumab | ORR | PFS, OS, AE | Accruing |
| NCT02749331 RADNET | NET | 1/2a | 35 | Recombinant Adenovirus AdVince | AE | PFS, changes in tumor size, | Accruing |
| NCT03879694 | NET (metastatic) | 1 | 14 | Survivin Long Peptide Vaccine (SurVaxM) | AE | TTP, DOR, ORR | Accruing |
8. Scientific Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kunz, P.L. Making Sense of a Complex Disease: A Practical Approach to Managing Neuroendocrine Tumors. JCO Oncol. Pract. 2022, 18, 258–264. [Google Scholar] [CrossRef]
- Hijioka, S.; Morizane, C.; Ikeda, M.; Ishii, H.; Okusaka, T.; Furuse, J. Current status of medical treatment for gastroenteropancreatic neuroendocrine neoplasms and future perspectives. Jpn. J. Clin. Oncol. 2021, 51, 1185–1196. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wang, W.Q.; Gao, H.L.; Yu, X.J.; Liu, L. The tumor immune microenvironment in gastroenteropancreatic neuroendocrine neoplasms. Biochim. Biophys. Acta. Rev. Cancer 2019, 1872, 188311. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Heatherton, K.R.; O’Connell, K.P.; Alexander, I.S.; Katz, S.C. Assessing the Future of Solid Tumor Immunotherapy. Biomedicines 2022, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Al-Toubah, T.; Cives, M.; Strosberg, J. Novel immunotherapy strategies for treatment of neuroendocrine neoplasms. Transl. Gastroenterol. Hepatol. 2020, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Albertelli, M.; Dotto, A.; Nista, F.; Veresani, A.; Patti, L.; Gay, S.; Sciallero, S.; Boschetti, M.; Ferone, D. Present and future of immunotherapy in Neuroendocrine Tumors. Rev. Endocr. Metab. Disord. 2021, 22, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Cives, M.; Pelle, E.; Quaresmini, D.; Rizzo, F.M.; Tucci, M.; Silvestris, F. The Tumor Microenvironment in Neuroendocrine Tumors: Biology and Therapeutic Implications. Neuroendocrinology 2019, 109, 83–99. [Google Scholar] [CrossRef]
- Katz, S.C.; Donkor, C.; Glasgow, K.; Pillarisetty, V.G.; Gönen, M.; Espat, N.J.; Klimstra, D.S.; D’Angelica, M.I.; Allen, P.J.; Jarnagin, W.; et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB 2010, 12, 674–683. [Google Scholar] [CrossRef]
- Lamarca, A.; Nonaka, D.; Breitwieser, W.; Ashton, G.; Barriuso, J.; McNamara, M.G.; Moghadam, S.; Rogan, J.; Mansoor, W.; Hubner, R.A.; et al. PD-L1 expression and presence of TILs in small intestinal neuroendocrine tumours. Oncotarget 2018, 9, 14922–14938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cortez, E.; Gladh, H.; Braun, S.; Bocci, M.; Cordero, E.; Björkström, N.K.; Miyazaki, H.; Michael, I.P.; Eriksson, U.; Folestad, E.; et al. Functional malignant cell heterogeneity in pancreatic neuroendocrine tumors revealed by targeting of PDGF-DD. Proc. Natl. Acad. Sci. USA 2016, 113, E864–E873. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Gonzalez, R.S.; Das, S.; Berlin, J.; Shi, C. Expression of PD-1 and PD-L1 in poorly differentiated neuroendocrine carcinomas of the digestive system: A potential target for anti-PD-1/PD-L1 therapy. Hum. Pathol. 2017, 70, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Dong, B.; Sun, W.; Yang, X.; Liu, R.; Zhou, L.; Huang, X.; Jia, L.; Lin, D. Prognostic significance of PD-L1 expression and CD8+ T cell infiltration in pulmonary neuroendocrine tumors. Diagn. Pathol. 2018, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Bösch, F.; Brüwer, K.; Altendorf-Hofmann, A.; Auernhammer, C.J.; Spitzweg, C.; Westphalen, C.B.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Heinemann, V.; et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr. Relat. Cancer 2019, 26, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J.; Diffalha, S.A.; Coppola, D. Analysis of the immune landscape of small bowel neuroendocrine tumors. Endocr. Relat. Cancer 2019, 26, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Vijayvergia, N.; Boland, P.M.; Handorf, E.; Gustafson, K.S.; Gong, Y.; Cooper, H.S.; Sheriff, F.; Astsaturov, I.; Cohen, S.J.; Engstrom, P.F. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: A Fox Chase Cancer Center Pilot Study. Br. J. Cancer 2016, 115, 564–570. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef]
- Strosberg, J.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.P.; Shapira-Frommer, R.; Bergsland, E.; Shah, M.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Vijayvergia, N.; Dasari, A.; Deng, M.; Litwin, S.; Al-Toubah, T.; Alpaugh, R.K.; Dotan, E.; Hall, M.J.; Ross, N.M.; Runyen, M.M.; et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: Joint analysis of two prospective, non-randomised trials. Br. J. Cancer 2020, 122, 1309–1314. [Google Scholar] [CrossRef]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Bergsland, E.; Ruszniewski, P.; Halperin, D.M.; Li, D.; Tafuto, S.; Raj, N.; et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr.-Relat. Cancer 2021, 28, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, P.; Zhang, Y.; Li, Z.; Gong, J.; Li, J.; Li, J.; Li, Y.; Zhang, X.; Lu, Z.; et al. Efficacy, Safety, and Biomarkers of Toripalimab in Patients with Recurrent or Metastatic Neuroendocrine Neoplasms: A Multiple-Center Phase Ib Trial. Clin. Cancer Res. 2020, 26, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Fottner, C.; Apostolidis, L.; Ferrata, M.; Krug, S.; Michl, P.; Schad, A.; Roth, W.; Jaeger, D.; Galle, P.R.; Weber, M.M. A phase II, open label, multicenter trial of avelumab in patients with advanced, metastatic high-grade neuroendocrine carcinomas NEC G3 (WHO 2010) progressive after first-line chemotherapy (AVENEC). J. Clin. Oncol. 2019, 37, 4103. [Google Scholar] [CrossRef]
- Chan, D.L.; Rodriguez-Freixinos, V.; Doherty, M.; Wasson, K.; Iscoe, N.; Raskin, W.; Hallet, J.; Myrehaug, S.; Law, C.; Thawer, A.; et al. Avelumab in unresectable/metastatic, progressive, grade 2–3 neuroendocrine neoplasms (NENs): Combined results from NET-001 and NET-002 trials. Eur. J. Cancer 2022, 169, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wright, K. FDA Approves Nivolumab Plus Ipilimumab for the Treatment of Advanced HCC. Oncology 2020, 34, 693606. [Google Scholar]
- Wright, K. FDA Approves Nivolumab Plus Ipilimumab for Previously Untreated Unresectable Malignant Pleural Mesothelioma. Oncology 2020, 34, 502–503. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, X.; Huang, M.; Dai, X.; Gao, J.; Li, S.; Sheng, L.; Huang, K.; Wang, J.; Liu, L. Comparison of Efficacy and Safety of Single and Double Immune Checkpoint Inhibitor-Based First-Line Treatments for Advanced Driver-Gene Wild-Type Non-Small Cell Lung Cancer: A Systematic Review and Network Meta-Analysis. Front. Immunol. 2021, 12, 731546. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Capdevila, J.; Teule, A.; López, C.; García-Carbonero, R.; Benavent, M.; Custodio, A.; Cubillo, A.; Alonso, V.; Gordoa, T.A.; Carmona-Bayonas, A. 1157O A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601). Ann. Oncol. 2020, 31, S770–S771. [Google Scholar] [CrossRef]
- Capdevila, J.; Hernando, J.; Teule, A.; Lopez, C.; Garcia-Carbonero, R.; Benavent, M.; Custodio, A.; Garcia-Alvarez, A.; Cubillo, A.; Alonso, V.; et al. Durvalumab plus tremelimumab for the treatment of advanced neuroendocrine neoplasms of gastroenteropancreatic and lung origin. Nat. Commun. 2023, 14, 2973. [Google Scholar] [CrossRef] [PubMed]
- Halperin, D.M.; Liu, S.; Dasari, A.; Fogelman, D.; Bhosale, P.; Mahvash, A.; Estrella, J.S.; Rubin, L.; Morani, A.C.; Knafl, M.; et al. Assessment of Clinical Response Following Atezolizumab and Bevacizumab Treatment in Patients With Neuroendocrine Tumors: A Nonrandomized Clinical Trial. JAMA Oncol. 2022, 8, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Al-Toubah, M.T.; Morse, M.B.; Haider, M.M.; Valone, P.T.; Strosberg, M.J. Phase II Study of Pembrolizumab and Lenvatinib in Advanced Well-Differentiated Neuroendocrine Tumors. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2023. [Google Scholar]
- Morse, M.; Halperin, D.M.; Uronis, H.E.; Hsu, D.S.; Hurwitz, H.; Bolch, E.; Warren, D.; Haley, S.; John, L.; Moyer, A.; et al. Phase Ib/II study of pembrolizumab with lanreotide depot for advanced, progressive gastroenteropancreatic neuroendocrine tumors (PLANET). J. Clin. Oncol. 2021, 39, 369. [Google Scholar] [CrossRef]
- Raj, N.; Chan, J.A.; Wang, S.J.; Aggarwal, R.R.; Calabrese, S.; DeMore, A.; Fong, L.; Grabowsky, J.; Hope, T.A.; Kolli, K.P.; et al. Pembrolizumab alone and pembrolizumab plus chemotherapy in previously treated, extrapulmonary poorly differentiated neuroendocrine carcinomas. Br. J. Cancer 2023, 129, 291–300. [Google Scholar] [CrossRef]
- Ridolfi, L.; Petrini, M.; Granato, A.M.; Gentilcore, G.; Simeone, E.; Ascierto, P.A.; Pancisi, E.; Ancarani, V.; Fiammenghi, L.; Guidoboni, M.; et al. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J. Transl. Med. 2013, 11, 135. [Google Scholar] [CrossRef]
- Owen, D.H.; Benner, B.; Wei, L.; Sukrithan, V.; Goyal, A.; Zhou, Y.; Pilcher, C.; Suffren, S.-A.; Christenson, G.; Curtis, N. A Phase II Clinical Trial of Nivolumab and Temozolomide for Neuroendocrine Neoplasms. Clin. Cancer Res. 2023, 29, 731–741. [Google Scholar] [CrossRef]
- NCCN Guidelines Neuroendocrine and Adrenal Gland [Internet]. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (accessed on 30 June 2023).
- Rassy, E.; Flippot, R.; Albiges, L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920907504. [Google Scholar] [CrossRef]
- Stefanini, B.; Ielasi, L.; Chen, R.; Abbati, C.; Tonnini, M.; Tovoli, F.; Granito, A. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev. Anticancer Ther. 2023, 23, 279–291. [Google Scholar] [CrossRef]
- Ahn, R.; Ursini-Siegel, J. Clinical Potential of Kinase Inhibitors in Combination with Immune Checkpoint Inhibitors for the Treatment of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 2608. [Google Scholar] [CrossRef]
- Mittra, E.S. Neuroendocrine Tumor Therapy: (177)Lu-DOTATATE. AJR Am. J. Roentgenol. 2018, 211, 278–285. [Google Scholar] [CrossRef]
- Ohtaki, Y.; Kaira, K.; Atsumi, J.; Nagashima, T.; Kawashima, O.; Ibe, T.; Kamiyoshihara, M.; Onozato, R.; Fujita, A.; Yazawa, T.; et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocytes in large cell neuroendocrine carcinoma of lung. Am. J. Transl. Res. 2018, 10, 3243–3253. [Google Scholar] [PubMed]
- Feng, Z.; He, X.; Zhang, X.; Wu, Y.; Xing, B.; Knowles, A.; Shan, Q.; Miller, S.; Hojnacki, T.; Ma, J.; et al. Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues. Nat. Cancer 2022, 3, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Mandriani, B.; Pellè, E.; Mannavola, F.; Palazzo, A.; Marsano, R.M.; Ingravallo, G.; Cazzato, G.; Ramello, M.C.; Porta, C.; Strosberg, J.; et al. Development of anti-somatostatin receptors CAR T cells for treatment of neuroendocrine tumors. J. Immunother. Cancer 2022, 10, e004854. [Google Scholar] [CrossRef]
- Girard, N.; Mazieres, J.; Otto, J.; Lena, H.; Lepage, C.; Egenod, T.; Smith, D.; Madelaine, J.; Gérinière, L.; El Hajbi, F. LBA41 Nivolumab (nivo) ± ipilimumab (ipi) in pre-treated patients with advanced, refractory pulmonary or gastroenteropancreatic poorly differentiated neuroendocrine tumors (NECs)(GCO-001 NIPINEC). Ann. Oncol. 2021, 32, S1318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, J.; Vijayvergia, N. Narrative Review of Immunotherapy in Gastroentero-Pancreatic Neuroendocrine Neoplasms. Curr. Oncol. 2023, 30, 8653-8664. https://doi.org/10.3390/curroncol30090627
Kaur J, Vijayvergia N. Narrative Review of Immunotherapy in Gastroentero-Pancreatic Neuroendocrine Neoplasms. Current Oncology. 2023; 30(9):8653-8664. https://doi.org/10.3390/curroncol30090627
Chicago/Turabian StyleKaur, Jasmeet, and Namrata Vijayvergia. 2023. "Narrative Review of Immunotherapy in Gastroentero-Pancreatic Neuroendocrine Neoplasms" Current Oncology 30, no. 9: 8653-8664. https://doi.org/10.3390/curroncol30090627
APA StyleKaur, J., & Vijayvergia, N. (2023). Narrative Review of Immunotherapy in Gastroentero-Pancreatic Neuroendocrine Neoplasms. Current Oncology, 30(9), 8653-8664. https://doi.org/10.3390/curroncol30090627





