Post-Operative Care of the Cancer Patient: Emphasis on Functional Recovery, Rapid Rescue, and Survivorship
Abstract
1. Introduction
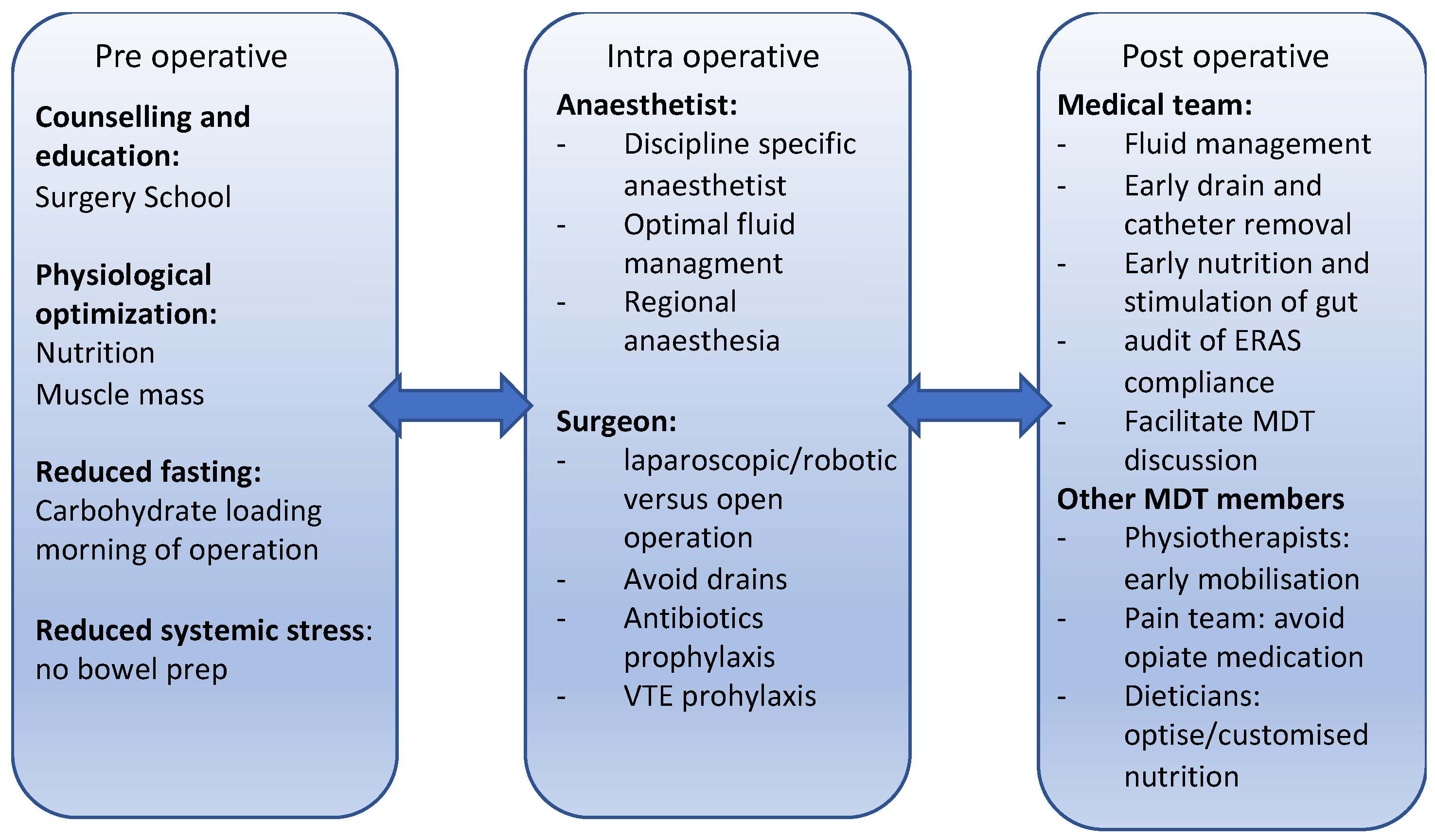
2. Perioperative Medicine
- decrease the metabolic stress and endocrine and inflammatory response to surgery.
- standardise and optimise peri-operative care across different institutes.
- decrease hospital stay, immobility and, therefore, hasten the return to normal function.
- promote pain control and decrease immobility-related complications.
- recommence enteral feeding.
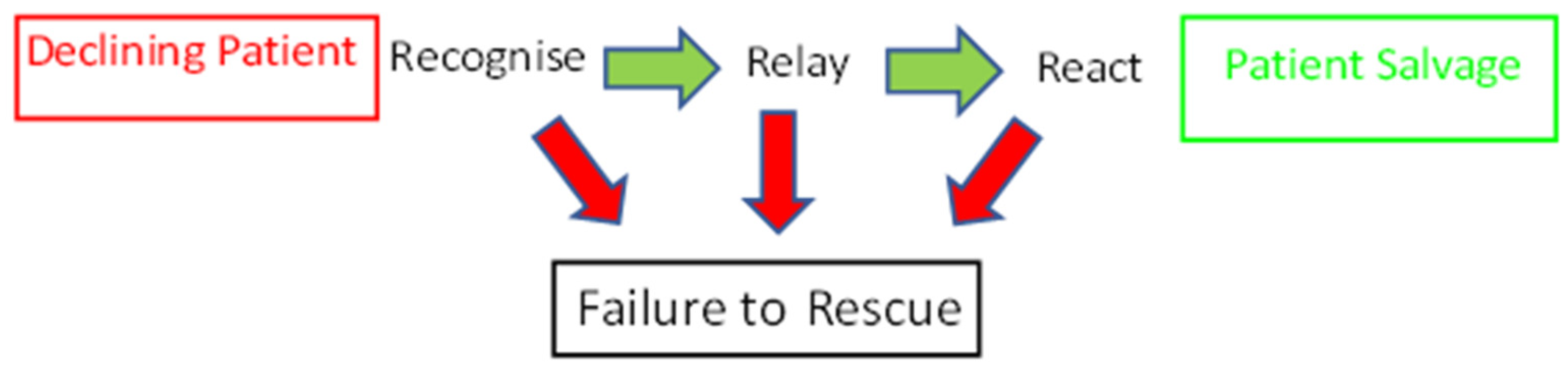
3. Is There a Link between Failure to Rescue and Post-Operative Survival?
4. Functional Recovery after Cancer Surgery
5. Survivorship: Just Because It Is Gone, Does It Mean It Is over?
6. Discussion
Funding
Conflicts of Interest
References
- Zarocostas, J. Global cancer cases and deaths are set to rise by 70% in next 20 years. BMJ 2010, 340, c3041. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034370. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.R.; Shaw, C.A.; Pius, R.; Drake, T.M.; Norman, L.; Ademuyiwa, A.O.; Adisa, A.O.; Aguilera, M.L.; Al-Saqqa, S.W.; Al-Slaibi, I.; et al. Global variation in postoperative mortality and complications after cancer surgery: A multicentre, prospective cohort study in 82 countries. Lancet 2021, 397, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhang, P.; Zhang, R.; Zhang, B.; Xiang, S.; Zhang, Y.; Luo, Z.; Huang, C. Novel hypoxia-induced HIF1α-circTDRD3-positive feedback loop promotes the growth and metastasis of colorectal cancer. Oncogene 2023, 42, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Hu, M.; Yang, R.; Liu, J.; Zeng, P.B.; Zhao, T. Decreasing expression of HIF-1α, VEGF-A, and Ki67 with efficacy of neoadjuvant therapy in locally advanced cervical cancer. Medicine 2023, 102, e33820. [Google Scholar] [CrossRef]
- Koroukian, S.M.; Booker, B.D.; Vu, L.; Schumacher, F.R.; Rose, J.; Cooper, G.S.; Selfridge, J.E.; Markt, S.C. Receipt of Targeted Therapy and Survival Outcomes in Patients with Metastatic Colorectal Cancer. JAMA Netw. Open 2023, 6, e2250030. [Google Scholar] [CrossRef]
- Fukumoto, W.; Yoshino, H.; Horike, S.; Kawakami, I.; Tamai, M.; Arima, J.; Kawahara, I.; Mitsuke, A.; Sakaguchi, T.; Inoguchi, S.; et al. Potential therapeutic target secretogranin II might cooperate with hypoxia-inducible factor 1α in sunitinib-resistant renal cell carcinoma. Cancer Sci. 2023, in press. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Data from Cabozantinib (XL184), a Novel MET and VEGFR2 Inhibitor, Simultaneously Suppresses Metastasis, Angiogenesis, and Tumor Growth. Mol. Cancer Ther. 2023, in press. [Google Scholar] [CrossRef]
- Sun, L.-Y.; Zhang, K.-J.; Xie, Y.-M.; Liu, J.-W.; Xiao, Z.-Q. Immunotherapies for advanced hepatocellular carcinoma. Front. Pharmacol. 2023, 14, 1138493. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Di Petrillo, A.; Gatto, G.; Pilia, L.; Kumar, A. Nanoparticles in Cancer Diagnosis and Treatment. Materials 2023, 16, 5354. [Google Scholar] [CrossRef]
- Cheng, H.; Clymer, J.W.; Chen, B.P.-H.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Seidelman, J.L.; Mantyh, C.R.; Anderson, D.J. Surgical Site Infection Prevention. JAMA 2023, 329, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.W.; Kuhlenschmidt, K.M.; Kubasiak, J.C.; Mossler, L.E.; Taveras, L.R.; Shoultz, T.H.; Phelan, H.A.; Reinke, C.E.; Cripps, M.W. Association of the Risk of a Venous Thromboembolic Event in Emergency vs Elective General Surgery. JAMA Surg. 2020, 155, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Jaconelli, M.; Greenhaff, P.L.; Atherton, P.J.; Lobo, D.N.; Brook, M.S. The effects of elective abdominal surgery on protein turnover: A meta-analysis of stable isotope techniques to investigate postoperative catabolism. Clin. Nutr. 2022, 41, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.R.; Wolfe, R.R.; Ferrando, A.A. Pre- and Post-Surgical Nutrition for Preservation of Muscle Mass, Strength, and Functionality Following Orthopedic Surgery. Nutrients 2021, 13, 1675. [Google Scholar] [CrossRef] [PubMed]
- Raso, K.-L.; Suen, M.; Turner, J.; Khatri, S.; Lin, Y.; Wildbore, C.; Becerril-Martinez, G.; Le Page, P.; Tan, S.Y.; Egger, S.; et al. Prehabilitation Before Gastrointestinal Cancer Surgery: Protocol for an Implementation Study. JMIR Res. Protoc. 2023, 12, e41101. [Google Scholar] [CrossRef] [PubMed]
- Ledet, C.R. Perioperative Care of the Cancer Patient; Elsevier: Amsterdam, The Netherlands, 2023; pp. 419–426. [Google Scholar] [CrossRef]
- Riad, A.; Knight, S.R.; Ghosh, D.; Kingsley, P.A.; Lapitan, M.C.; Parreno-Sacdalan, M.D.; Sundar, S.; Qureshi, A.U.; Valparaiso, A.P.; Pius, R.; et al. Impact of malnutrition on early outcomes after cancer surgery: An international, multicentre, prospective cohort study. Lancet Global Health 2023, 11, e341–e349. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia—Understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef]
- West, M.; Grocott, M.P.; Carli, F. Personalised Multimodal Prehabilitation in Cancer. Front. Res. Top. 2022, in press. [Google Scholar] [CrossRef]
- Knight, S.R.; Shaw, C.A.; Pius, R.; Drake, T.M.; Norman, L.; Ademuyiwa, A.O.; Adisa, A.O.; Aguilera, M.L.; Al-Saqqa, S.W.; Al-Slaibi, I.; et al. Effects of hospital facilities on patient outcomes after cancer surgery: An international, prospective, observational study. Lancet Global Health 2022, 10, e1003–e1011. [Google Scholar] [CrossRef]
- Tamburini, E.; Tassinari, D.; Ramundo, M.; De Stefano, A.; Viola, M.G.; Romano, C.; Elia, M.T.; Zanaletti, N.; Rudnas, B.; Casadei-Gardini, A.; et al. Adjuvant chemotherapy after neoadjuvant chemo-radiotherapy and surgery in locally advanced rectal cancer. A systematic review of literature with a meta-analysis of randomized clinical trials. Crit. Rev. Oncol. 2022, 172, 103627. [Google Scholar] [CrossRef]
- West, M.; Bates, A.; Grimmett, C.; Allen, C.; Green, R.; Hawkins, L.; Moyses, H.; Leggett, S.; Levett, D.Z.; Rickard, S.; et al. The Wessex Fit-4-Cancer Surgery Trial (WesFit): A protocol for a factorial-design, pragmatic randomised-controlled trial investigating the effects of a multi-modal prehabilitation programme in patients undergoing elective major intra-cavity cancer surgery. F1000Research 2022, 10, 952. [Google Scholar] [CrossRef]
- Wong, H.-Y.; Starsmore, L.; Tetlow, N.; Dewar, A.; Chapman, O.; Lipman, J.; Pidding, J.; Montanheiro, K.; Bellin, U.; Whittle, J. Implementation of a surgical prehabilitation pathway at a tertiary surgical centre during a global pandemic. BJA Open 2022, 4, 100069. [Google Scholar] [CrossRef]
- Melgar, P.; Rodríguez-Laiz, G.P.; Lluís, N.; Alcázar-López, C.; Franco-Campello, M.; Villodre, C.; Pascual, S.; Rodríguez-Soler, M.; Bellot, P.; Miralles, C.; et al. Textbook outcome among patients undergoing enhanced recovery after liver transplantation stratified by risk. A single-center retrospective observational cohort study. Int. J. Surg. 2022, 99, 106266. [Google Scholar] [CrossRef]
- Bristow, Z.; Neck, C.; Cullum, D.; Lau, B.; Groves, K.R.; Foxley, A.; Repperday, C.; Graham, P.; Dawson, L.G.; Merchant, Z.; et al. Evaluating the Success of the Pioneering Prehab4Cancer (P4C) Programme Using innovative, Collaborative Methodology. Available online: https://gmcancer.org.uk/wp-content/uploads/2022/10/71.-Prehab4Cancer-A1-v3.pdf (accessed on 31 May 2023).
- Kehlet, H. Multimodal approach to control postoperative pathophysiology and rehabilitation. BJA Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef]
- Moningi, S.; Patki, A.; Padhy, N.; Ramachandran, G. Enhanced recovery after surgery: An anesthesiologist’s perspective. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S5–S13. [Google Scholar] [CrossRef]
- Fearon, K.C.H.; Ljungqvist, O.; Von Meyenfeldt, M.; Revhaug, A.; Dejong, C.H.C.; Lassen, K.; Nygren, J.; Hausel, J.; Soop, M.; Andersen, J.; et al. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoingcolonic resection. Clin. Nutr. 2005, 24, 466–477. [Google Scholar] [CrossRef]
- Oodit, R.; Biccard, B.M.; Panieri, E.; Alvarez, A.O.; Sioson, M.R.; Maswime, S.; Thomas, V.; Kluyts, H.L.; Peden, C.J.; de Boer, H.D.; et al. Guidelines for Perioperative Care in Elective Abdominal and Pelvic Surgery at Primary and Secondary Hospitals in Low–Middle-Income Countries (LMIC’s): Enhanced Recovery After Surgery (ERAS) Society Recommendation. World J. Surg. 2022, 46, 1826–1843. [Google Scholar] [CrossRef]
- Carmichael, J.C.; Keller, D.S.; Baldini, G.; Bordeianou, L.; Weiss, E.; Lee, L.; Boutros, M.; McClane, J.; Feldman, L.S.; Steele, S.R. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis. Colon Rectum 2017, 60, 761–784. [Google Scholar] [CrossRef]
- Yuan, Q.; Dan, X.; Chen, L. Evidence Summary for Prevention of Gastrointestinal Dysfunction After Laparoscopic Surgery. Indian J. Surg. 2023, 85, 262–272. [Google Scholar] [CrossRef]
- Romain, B.; Grass, F.; Addor, V.; Demartines, N.; Hübner, M. Impact of weekday surgery on application of enhanced recovery pathway: A retrospective cohort study. BMJ Open 2016, 6, e011067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kehlet, H.; Wilmore, D.W. Evidence-Based Surgical Care and the Evolution of Fast-Track Surgery. Ann. Surg. 2008, 248, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.; Hvass, K.E.; Hansen, T.B.; Thomsen, P.B.; Søballe, K. Effectiveness of accelerated perioperative care and rehabilitation intervention compared to current intervention after hip and knee arthroplasty. A before-after trial of 247 patients with a 3-month follow-up. BMC Musculoskelet. Disord. 2008, 9, 59. [Google Scholar] [CrossRef]
- Fry, B.T.; Smith, M.E.; Thumma, J.R.; Ghaferi, A.A.; Dimick, J.B. Ten-year Trends in Surgical Mortality, Complications, and Failure to Rescue in Medicare Beneficiaries. Ann. Surg. 2020, 271, 855–861. [Google Scholar] [CrossRef]
- Ghaferi, A.A.; Birkmeyer, J.D.; Dimick, J.B. Variation in Hospital Mortality Associated with Inpatient Surgery. N. Engl. J. Med. 2009, 361, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.H.; Williams, S.V.; Krakauer, H.; Schwartz, S. Hospital and Patient Characteristics Associated with Death After Surgery: A Study of Adverse Occurrence and Failure to Rescue. Med. Care 1992, 30, 615–629. [Google Scholar] [CrossRef]
- Ghaferi, A.A.; Birkmeyer, J.D.; Dimick, J.B. Hospital Volume and Failure to Rescue with High-risk Surgery. Med. Care 2011, 49, 1076–1081. [Google Scholar] [CrossRef]
- Mouch, C.A.; Regenbogen, S.E.; Revels, S.L.; Wong, S.L.; Lemak, C.H.; Morris, A.M. The quality of surgical care in safety net hospitals: A systematic review. Surgery 2014, 155, 826–838. [Google Scholar] [CrossRef]
- Ede, J.; Watkinson, P.; Endacott, R. Protocol for a mixed methods exploratory study of success factors to escalation of care: The SUFFICE study. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Hall, K.K.; Lim, A.; Gale, B. The Use of Rapid Response Teams to Reduce Failure to Rescue Events: A Systematic Review. J. Patient Saf. 2020, 16, S3–S7. [Google Scholar] [CrossRef]
- Burke, J.R.; Downey, C.; Almoudaris, A.M. Failure to Rescue Deteriorating Patients: A Systematic Review of Root Causes and Improvement Strategies. J. Patient Saf. 2022, 18, e140–e155. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.; Griffiths, P. Effectiveness of Critical Care Outreach Services—A Literature Review. Work. Pap. Health Sci. 2014, 1, 1–10. [Google Scholar]
- Bansal, D.; Ghai, B.; Gudala, K.; Asrar, M.M.; Chanana, N.; Kanukula, R. Development, validation and evaluation of a novel self-instructional module in patients with chronic non-specific low back pain. Indian J. Anaesth. 2020, 64, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.C.; Scott, N.W.; Powell, R.; Johnston, M.; Sutton, A.J.; Cooper, N.J. Component network meta-analysis identifies the most effective components of psychological preparation for adults undergoing surgery under general anesthesia. J. Clin. Epidemiol. 2018, 98, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Rasulić, L.; Nikolić, Ž.; Lepić, M.; Savić, A.; Vitošević, F.; Novaković, N.; Radojević, S.; Mićić, A.; Lepić, S.; Mandić-Rajčević, S. Useful functional recovery and quality of life after surgical treatment of peroneal nerve injuries. Front. Surg. 2022, 9, 1005483. [Google Scholar] [CrossRef]
- Meropol, N.J.; Egleston, B.L.; Buzaglo, J.S.; Benson, A.B.; Cegala, D.J.; Diefenbach, M.A.; Fleisher, L.; Miller, S.M.; Sulmasy, D.P.; Weinfurt, K.P.; et al. Cancer patient preferences for quality and length of life. Cancer 2008, 113, 3459–3466. [Google Scholar] [CrossRef]
- NHS. Shared Decision Making: Summary Guide. 2019. Available online: www.england.nhs.uk/shared-decision-making-summary-guide/ (accessed on 29 June 2023).
- Royse, C.F.; Newman, S.; Chung, F.; Stygall, J.; McKay, R.E.; Boldt, J.; Servin, F.S.; Hurtado, I.; Hannallah, R.; Yu, B.; et al. Development and Feasibility of a Scale to Assess Postoperative Recovery. Anesthesiology 2010, 113, 892–905. [Google Scholar] [CrossRef]
- Allvin, R.; Ehnfors, M.; Rawal, N.; Idvall, E. Experiences of the Postoperative Recovery Process: An Interview Study. Open Nurs. J. 2008, 2, 1–7. [Google Scholar] [CrossRef]
- Myles, P.S.; Hunt, J.O.; Nightingale, C.E.; Fletcher, H.; Beh, T.; Tanil, D.; Nagy, A.; Rubinstein, A.; Ponsford, J.L. Development and Psychometric Testing of a Quality of Recovery Score after General Anesthesia and Surgery in Adults. Anesth. Analg. 1999, 88, 83–90. [Google Scholar] [CrossRef]
- Myles, P.S. More than just morbidity and mortality—Quality of recovery and long-term functional recovery after surgery. Anaesthesia 2020, 75, e143–e150. [Google Scholar] [CrossRef]
- Maillard, J.; Elia, N.; Ris, F.; Courvoisier, D.S.; Zekry, D.; Galy, I.L.; Toso, C.; Mönig, S.; Zaccaria, I.; Walder, B. Changes of health-related quality of life 6 months after high-risk oncological upper gastrointestinal and hepatobiliary surgery: A single-centre prospective observational study (ChangeQol Study). BMJ Open 2023, 13, e065902. [Google Scholar] [CrossRef]
- CRUK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/survival (accessed on 29 June 2023).
- Macmillan. Prevelance of Cancer. Available online: www.macmillan.org.uk/about-us/what-we-do/research/cancer-prevalence (accessed on 29 June 2023).
- National Cancer Research Initiative. Available online: https://www.ncri.org.uk (accessed on 29 June 2023).
- Breidenbach, C.; Heidkamp, P.; Hiltrop, K.; Pfaff, H.; Enders, A.; Ernstmann, N.; Kowalski, C. Prevalence and determinants of anxiety and depression in long-term breast cancer survivors. BMC Psychiatry 2022, 22, 101. [Google Scholar] [CrossRef]
- National Cancer Institute. USA. Available online: https://www.cancer.gov/ (accessed on 29 June 2023).
- Pranjic, N.; Bajraktarevic, A.; Ramic, E. Distress and Ptsd in Patients with Cancer: Cohort Study Case. Mater. Socio-Med. 2016, 28, 12–16. [Google Scholar] [CrossRef]
- Grassi, L.; Spiegel, D.; Riba, M. Advancing psychosocial care in cancer patients. F1000Research 2017, 6, 2083. [Google Scholar] [CrossRef]
- Cherry, M.G.; Salmon, P.; Byrne, A.; Ullmer, H.; Abbey, G.; Fisher, P.L. Qualitative Evaluation of Cancer Survivors’ Experiences of Metacognitive Therapy: A New Perspective on Psychotherapy in Cancer Care. Front. Psychol. 2019, 10, 949. [Google Scholar] [CrossRef]
- Törnävä, M.; Harju, E.; Vasarainen, H.; Pakarainen, T.; Perttilä, I.; Kaipia, A. Men’s experiences of the impact of penile cancer surgery on their lives: A qualitative study. Eur. J. Cancer Care 2022, 31, e13548. [Google Scholar] [CrossRef]
- Stuopelytė, R.; Žukienė, G.; Breivienė, R.; Rudaitis, V.; Bartkevičienė, D. Quality of Life in Cervical Cancer Survivors Treated with Concurrent Chemoradiotherapy. Medicina 2023, 59, 777. [Google Scholar] [CrossRef]
- Heyne, S.; Taubenheim, S.; Dietz, A.; Lordick, F.; Götze, H.; Mehnert-Theuerkauf, A. Physical and psychosocial factors associated with sexual satisfaction in long-term cancer survivors 5 and 10 years after diagnosis. Sci. Rep. 2023, 13, 2011. [Google Scholar] [CrossRef]
- Turner, D.; Adams, E.; Boulton, M.; Harrison, S.; Khan, N.; Rose, P.; Ward, A.; Watson, E.K. Partners and close family members of long-term cancer survivors: Health status, psychosocial well-being and unmet supportive care needs. Psycho Oncol. 2013, 22, 12–19. [Google Scholar] [CrossRef]
- Feiler, B. Scanxiety. Fear of a postcancer ritual. Time 2011, 177, 56. [Google Scholar]
- Baník, G.; Dědová, M. Cancer as a trauma? Risk factors of posttraumatic stress disorder (PTSD) in cancer survivors. PsyArXiv 2019. preprint. [Google Scholar] [CrossRef]
- Neuman, H.B.; Schumacher, J.R. Follow-up and Cancer Survivorship. Surg. Clin. N. Am. 2023, 103, 169–185. [Google Scholar] [CrossRef]
- Li, C.; Jing, B.; Ke, L.; Li, B.; Xia, W.; He, C.; Qian, C.; Zhao, C.; Mai, H.; Chen, M.; et al. Long- versus short-interval follow-up after resection of hepatocellular carcinoma: A retrospective cohort study. Cancer Commun. 2018, 38, 1–11. [Google Scholar]
- Shand, L.K.; Cowlishaw, S.; Brooker, J.E.; Burney, S.; Ricciardelli, L.A. Correlates of post-traumatic stress symptoms and growth in cancer patients: A systematic review and meta-analysis. Psycho Oncol. 2015, 24, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Powell-Chandler, A.; Boyce, K.; James, O.; Scourfield, L.; Torkington, J.; Bisson, J.; Cornish, J.A.; PISA Trial Management Group. Psychological sequelae of colonic resections. Color. Dis. 2020, 22, 945–951. [Google Scholar] [CrossRef]
- Faller, H.; Weis, J.; Koch, U.; Brähler, E.; Härter, M.; Keller, M.; Schulz, H.; Wegscheider, K.; Boehncke, A.; Hund, B.; et al. Perceived need for psychosocial support depending on emotional distress and mental comorbidity in men and women with cancer. J. Psychosom. Res. 2016, 81, 24–30. [Google Scholar] [CrossRef]
- Simin, T.; Jin, Y.; Aidi, Z.; Xiaofang, T.; Chunhong, R.; Lezhi, L. Gender Comparison of Psychological Reaction Between Breast Cancer Survivors and Their Spouses. Front. Psychol. 2021, 12, 722877. [Google Scholar] [CrossRef]
- Fitch, M. Perspectives of survivors: Relationship changes following cancer diagnosis and treatment. Can. Oncol. Nurs. J. Rev. Can. Nurs. Oncol. 2023, 33, 279–282. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrison-Jones, V.; West, M. Post-Operative Care of the Cancer Patient: Emphasis on Functional Recovery, Rapid Rescue, and Survivorship. Curr. Oncol. 2023, 30, 8575-8585. https://doi.org/10.3390/curroncol30090622
Morrison-Jones V, West M. Post-Operative Care of the Cancer Patient: Emphasis on Functional Recovery, Rapid Rescue, and Survivorship. Current Oncology. 2023; 30(9):8575-8585. https://doi.org/10.3390/curroncol30090622
Chicago/Turabian StyleMorrison-Jones, Victoria, and Malcolm West. 2023. "Post-Operative Care of the Cancer Patient: Emphasis on Functional Recovery, Rapid Rescue, and Survivorship" Current Oncology 30, no. 9: 8575-8585. https://doi.org/10.3390/curroncol30090622
APA StyleMorrison-Jones, V., & West, M. (2023). Post-Operative Care of the Cancer Patient: Emphasis on Functional Recovery, Rapid Rescue, and Survivorship. Current Oncology, 30(9), 8575-8585. https://doi.org/10.3390/curroncol30090622




