Intravenous Iron Therapy to Treat Anemia in Oncology: A Mapping Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Population: Adult patients with anemia as defined by the WHO criteria: Hb < 130 g/L for men and Hb < 120 g/L for women and cancer;
- Intervention: IV iron, regardless of dose or frequency;
- Comparators: IV iron, oral iron, placebo, standard of care, or no treatment;
- Outcomes: Studies evaluating the risk of receiving red cell transfusion, hematological measures, and quality of life;
- Study type: randomized controlled trials.
2.2. Search Strategy
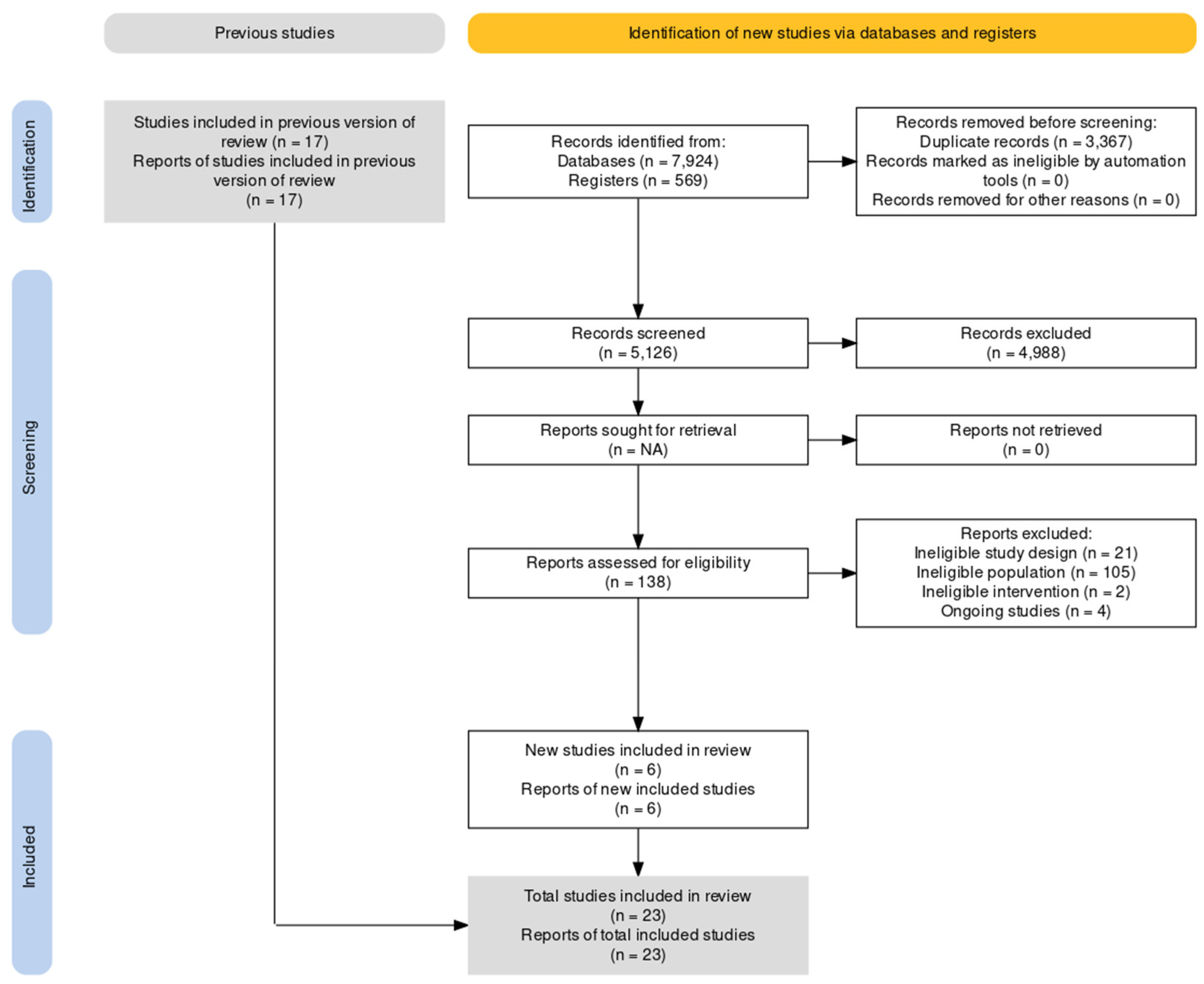
2.3. Study Selection and Data Extraction
2.4. Data Synthesis
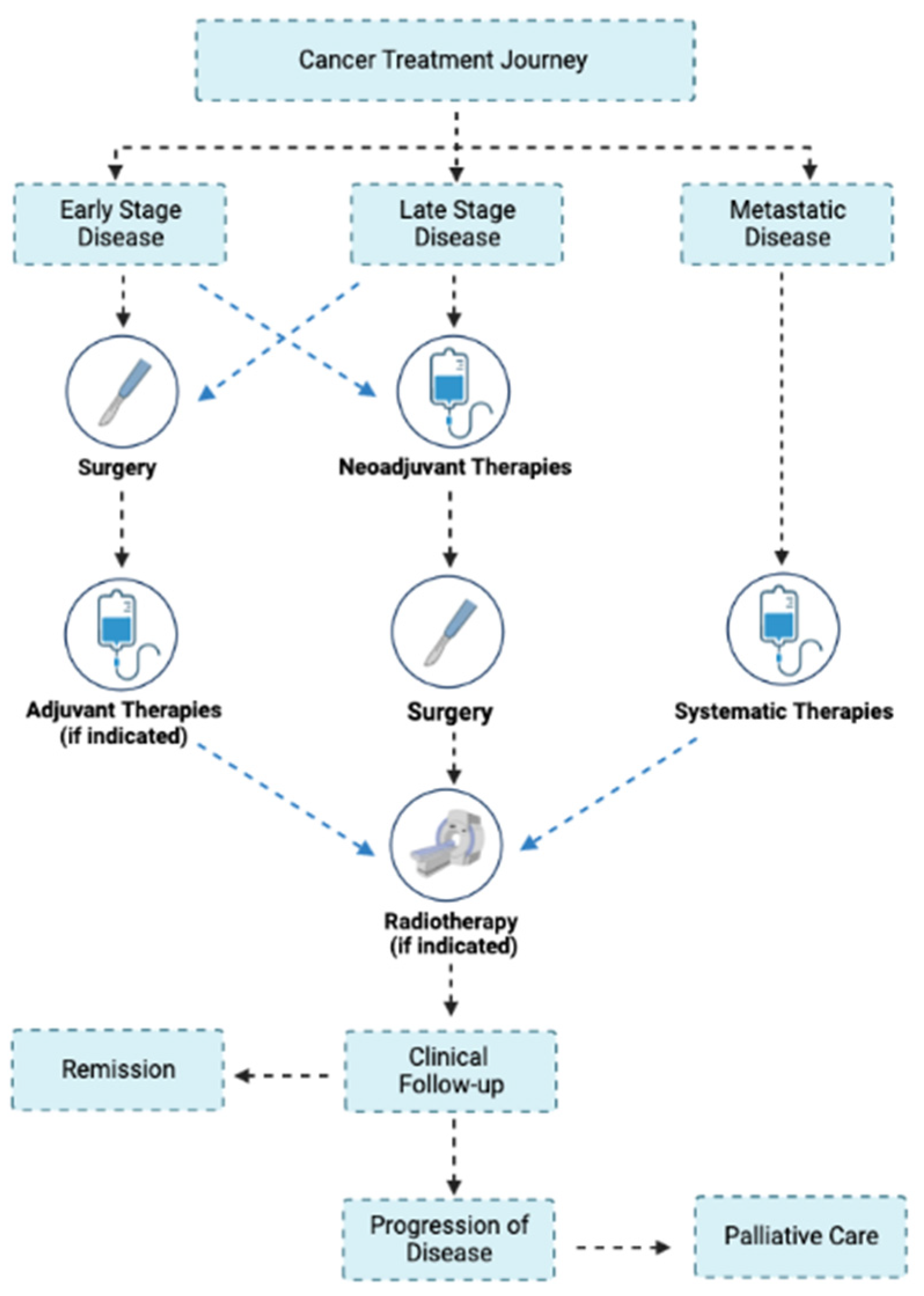
3. Results
3.1. Surgery
Detailed Description of the IVICA and FIT Trials
3.2. Adjuvant Therapy after Surgery
3.3. Adjuvant Therapy with ESAs
Detailed Description of Specific Trials
3.4. Adjuvant Therapy without ESAs
3.5. Radiotherapy
3.6. Palliative
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- exp iron therapy/
- (iron or ferrous or ferric).af.
- 1 or 2
- exp anemia/
- (anemi* OR anaemi*).af.
- 4 or 5
- exp crossover-procedure/or exp double-blind procedure/or exp randomized controlled trial/or single-blind procedure/
- (random* or factorial* or crossover* or placebo*).af.
- 7 or 8
- 3 and 6 and 9
References
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Gilreath, J.A.; Rodgers, G.M. How I treat cancer-associated anemia. Blood 2020, 136, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Kallich, J.; McDermott, A.; Xu, X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: Results from five randomized clinical trials. Ann. Oncol. 2004, 15, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Vernon, C.; Cruickshank, D.; Wilkinson, P.; Littlewood, T.; Stuart, N.; Jenkinson, C.; Grey-Amante, P.; Doll, H.; Wild, D. The level of haemoglobin in anaemic cancer patients correlates positively with quality of life. Br. J. Cancer 2002, 86, 1243–1249. [Google Scholar] [CrossRef]
- West, H.J.; Jin, J.O. JAMA Oncology Patient Page. Performance Status in Patients With Cancer. JAMA Oncol. 2015, 1, 998. [Google Scholar] [CrossRef]
- Nachiappan, S.; Askari, A.; Mamidanna, R.; Munasinghe, A.; Currie, A.; Stebbing, J.; Faiz, O. The impact of adjuvant chemotherapy timing on overall survival following colorectal cancer resection. Eur. J. Surg. Oncol. 2015, 41, 1636–1644. [Google Scholar] [CrossRef]
- Shander, A.; Knight, K.; Thurer, R.; Adamson, J.; Spence, R. Prevalence and outcomes of anemia in surgery: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. S7A), 58S–69S. [Google Scholar] [CrossRef]
- Munoz, M.; Gomez-Ramirez, S.; Campos, A.; Ruiz, J.; Liumbruno, G.M. Pre-operative anaemia: Prevalence, consequences and approaches to management. Blood Transfus. 2015, 13, 370–379. [Google Scholar] [CrossRef]
- Collaborative, P.O.S. The management of peri-operative anaemia in patients undergoing major abdominal surgery in Australia and New Zealand: A prospective cohort study. Med. J. Aust. 2022, 217, 487–493. [Google Scholar] [CrossRef]
- Raphael, M.J.; Biagi, J.J.; Kong, W.; Mates, M.; Booth, C.M.; Mackillop, W.J. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2016, 160, 17–28. [Google Scholar] [CrossRef]
- Biagi, J.J.; Raphael, M.J.; Mackillop, W.J.; Kong, W.; King, W.D.; Booth, C.M. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA 2011, 305, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.M. A perspective on the evolution of management of cancer- and chemotherapy-induced anemia. J. Natl. Compr. Canc. Netw. 2012, 10, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv96–iv110. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.; Laszig, R.; Rube, C.; Schafer, U.; Haase, K.D.; Schilcher, B.; Mose, S.; Beer, K.T.; Burger, U.; Dougherty, C.; et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet 2003, 362, 1255–1260. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Aapro, M.S.; Courdi, A.; Foubert, J.; Link, H.; Osterborg, A.; Repetto, L.; Soubeyran, P. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer. Eur. J. Cancer 2004, 40, 2201–2216. [Google Scholar] [CrossRef]
- Leyland-Jones, B.; Investigators, B.; Study, G. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003, 4, 459–460. [Google Scholar] [CrossRef]
- Bohlius, J.; Langensiepen, S.; Schwarzer, G.; Seidenfeld, J.; Piper, M.; Bennet, C.; Engert, A. Erythropoietin for patients with malignant disease. Cochrane Database Syst. Rev. 2004, CD003407. [Google Scholar] [CrossRef]
- Gascon, P.; Nagarkar, R.; Smakal, M.; Syrigos, K.N.; Barrios, C.H.; Sanchez, J.C.; Zhang, L.; Henry, D.H.; Gordon, D.; Hirsh, V.; et al. A Randomized, Double-Blind, Placebo-Controlled, Phase III Noninferiority Study of the Long-Term Safety and Efficacy of Darbepoetin Alfa for Chemotherapy-Induced Anemia in Patients With Advanced NSCLC. J. Thorac. Oncol. 2020, 15, 190–202. [Google Scholar] [CrossRef]
- Glaspy, J.; Crawford, J.; Vansteenkiste, J.; Henry, D.; Rao, S.; Bowers, P.; Berlin, J.A.; Tomita, D.; Bridges, K.; Ludwig, H. Erythropoiesis-stimulating agents in oncology: A study-level meta-analysis of survival and other safety outcomes. Br. J. Cancer 2010, 102, 301–315. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Glaspy, J.; Henry, D.; Ludwig, H.; Pirker, R.; Tomita, D.; Collins, H.; Crawford, J. Benefits and risks of using erythropoiesis-stimulating agents (ESAs) in lung cancer patients: Study-level and patient-level meta-analyses. Lung Cancer 2012, 76, 478–485. [Google Scholar] [CrossRef]
- Avni, T.; Bieber, A.; Grossman, A.; Green, H.; Leibovici, L.; Gafter-Gvili, A. The safety of intravenous iron preparations: Systematic review and meta-analysis. Mayo Clin. Proc. 2015, 90, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O′Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.A.; Abbasciano, R.G.; Pathak, S.; Oo, S.; Yusoff, S.; Wozniak, M.; Qureshi, S.; Lai, F.Y.; Kumar, T.; Richards, T.; et al. Patient blood management interventions do not lead to important clinical benefits or cost-effectiveness for major surgery: A network meta-analysis. Br. J. Anaesth 2021, 126, 149–156. [Google Scholar] [CrossRef]
- Gurusamy, K.S.; Nagendran, M.; Broadhurst, J.F.; Anker, S.D.; Richards, T. Iron therapy in anaemic adults without chronic kidney disease. Cochrane Database Syst. Rev. 2014, Cd010640. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Ansarinejad, N.; Abbasi, B.; Sadat Rasul, M.S.; Fardad, F.; Ramim, T. The Effectiveness of Ferric Carboxymaltose on the Improvement of Chronic Iron Deficiency Anemia in Patients With Colon Cancer: A Controlled Randomized Clinical Trial. J. Obstet. Gynecol. Cancer Res. 2016, 1. [Google Scholar] [CrossRef]
- Anthony, L.B.; Gabrail, N.Y.; Ghazal, H.; Woytowitz, D.V.; Flam, M.S.; Drelichman, A.; Loesch, D.M.; Niforos, D.; Mangione, A. IV iron sucrose for cancer and/or chemotherapy-induced anemia in patients treated with erythropoiesis-stimulating agents. Community Oncol. 2011, 8, 270–278. [Google Scholar] [CrossRef]
- Auerbach, M.; Ballard, H.; Trout, J.R.; McIlwain, M.; Ackerman, A.; Bahrain, H.; Balan, S.; Barker, L.; Rana, J. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: A multicenter, open-label, randomized trial. J. Clin. Oncol. 2004, 22, 1301–1307. [Google Scholar] [CrossRef]
- Auerbach, M.; Silberstein, P.T.; Webb, R.T.; Averyanova, S.; Ciuleanu, T.E.; Shao, J.; Bridges, K. Darbepoetin alfa 300 or 500 mug once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am. J. Hematol. 2010, 85, 655–663. [Google Scholar] [CrossRef]
- Bastit, L.; Vandebroek, A.; Altintas, S.; Gaede, B.; Pinter, T.; Suto, T.S.; Mossman, T.W.; Smith, K.E.; Vansteenkiste, J.F. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J. Clin. Oncol. 2008, 26, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Dangsuwan, P.; Manchana, T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. Gynecol. Oncol. 2010, 116, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Dickson, E.A.; Ng, O.; Keeler, B.D.; Wilcock, A.; Brookes, M.J.; Acheson, A.G. The ICaRAS randomised controlled trial: Intravenous iron to treat anaemia in people with advanced cancer-feasibility of recruitment, intervention and delivery. Palliat Med. 2023, 37, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.; Mostert, A.E.; Visser, C.; Mouton, A. The severity and optimal management of iron deficiency and anaemia before radiation for cervical cancer at the University of Pretoria Academic Hospitals. South. Afr. J. Gynaecol. Oncol. 2017, 9, 11–15. [Google Scholar] [CrossRef][Green Version]
- Edwards, T.J.; Noble, E.J.; Durran, A.; Mellor, N.; Hosie, K.B. Randomized clinical trial of preoperative intravenous iron sucrose to reduce blood transfusion in anaemic patients after colorectal cancer surgery. Br. J. Surg. 2009, 96, 1122–1128. [Google Scholar] [CrossRef]
- Fung, P.L.P.; Lau, V.N.M.; Ng, F.F.; Leung, W.W.; Mak, T.W.C.; Lee, A. Perioperative changes in haemoglobin and ferritin concentrations from preoperative intravenous iron isomaltoside for iron deficiency anaemia in patients with colorectal cancer: A pilot randomised controlled trial. PLoS ONE 2022, 17, e0270640. [Google Scholar] [CrossRef]
- Hajigholami, A.; Maghsoodi, A.-R.; Ansari, H.; Kafeshani, M. Treatment of chemotherapy induced anemia; a randomized clinical trial to compare quality of life in patients taking intravenous versus oral iron. Immunopathol. Persa 2020, 7, e32. [Google Scholar] [CrossRef]
- Hedenus, M.; Birgegard, G.; Nasman, P.; Ahlberg, L.; Karlsson, T.; Lauri, B.; Lundin, J.; Larfars, G.; Osterborg, A. Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: A randomized multicenter study. Leukemia 2007, 21, 627–632. [Google Scholar] [CrossRef]
- Hedenus, M.; Karlsson, T.; Ludwig, H.; Rzychon, B.; Felder, M.; Roubert, B.; Birgegard, G. Intravenous iron alone resolves anemia in patients with functional iron deficiency and lymphoid malignancies undergoing chemotherapy. Med. Oncol. 2014, 31, 302. [Google Scholar] [CrossRef]
- Henry, D.H.; Dahl, N.V.; Auerbach, M.; Tchekmedyian, S.; Laufman, L.R. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007, 12, 231–242. [Google Scholar] [CrossRef]
- Keeler, B.D.; Simpson, J.A.; Ng, O.; Padmanabhan, H.; Brookes, M.J.; Acheson, A.G.; Group, I.T. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br. J. Surg. 2017, 104, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Kim, S.W.; Yoon, B.S.; Cho, H.J.; Nahm, E.J.; Kim, S.H.; Kim, J.H.; Kim, J.W. Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. Gynecol. Oncol. 2007, 105, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Laso-Morales, M.J.; Vives, R.; Bisbe, E.; Garcia-Erce, J.A.; Munoz, M.; Martinez-Lopez, F.; Carol-Boeris, F.; Pontes-Garcia, C. Single-dose intravenous ferric carboxymaltose infusion versus multiple fractionated doses of intravenous iron sucrose in the treatment of post-operative anaemia in colorectal cancer patients: A randomised controlled trial. Blood Transfus. 2022, 20, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Maccio, A.; Madeddu, C.; Gramignano, G.; Mulas, C.; Sanna, E.; Mantovani, G. Efficacy and safety of oral lactoferrin supplementation in combination with rHuEPO-beta for the treatment of anemia in advanced cancer patients undergoing chemotherapy: Open-label, randomized controlled study. Oncologist 2010, 15, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Makharadze, T.; Boccia, R.; Krupa, A.; Blackman, N.; Henry, D.H.; Gilreath, J.A. Efficacy and safety of ferric carboxymaltose infusion in reducing anemia in patients receiving chemotherapy for nonmyeloid malignancies: A randomized, placebo-controlled study (IRON-CLAD). Am. J. Hematol. 2021, 96, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.; Joshi, A.; Patil, V.M.; Banavali, S.D.; Gupta, S.; Parikh, P.M.; Marfatia, S.; Punatar, S.; More, S.; Goud, S.; et al. Phase III randomized trial comparing intravenous to oral iron in patients with cancer-related iron deficiency anemia not on erythropoiesis stimulating agents. Asia Pac. J. Clin. Oncol. 2018, 14, e129–e137. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, P.; Farris, A.; Del Prete, S.; Del Gaizo, F.; Ferrari, D.; Bianchessi, C.; Colucci, G.; Desogus, A.; Gamucci, T.; Pappalardo, A.; et al. Randomized trial of intravenous iron supplementation in patients with chemotherapy-related anemia without iron deficiency treated with darbepoetin alpha. J. Clin. Oncol. 2008, 26, 1619–1625. [Google Scholar] [CrossRef]
- Steensma, D.P.; Sloan, J.A.; Dakhil, S.R.; Dalton, R.; Kahanic, S.P.; Prager, D.J.; Stella, P.J.; Rowland, K.M., Jr.; Novotny, P.J.; Loprinzi, C.L. Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia. J. Clin. Oncol. 2011, 29, 97–105. [Google Scholar] [CrossRef]
- Talboom, K.; Borstlap, W.A.A.; Roodbeen, S.X.; Bruns, E.R.J.; Buskens, C.J.; Hompes, R.; Tytgat, K.; Tuynman, J.B.; Consten, E.C.J.; Heuff, G.; et al. Ferric carboxymaltose infusion versus oral iron supplementation for preoperative iron deficiency anaemia in patients with colorectal cancer (FIT): A multicentre, open-label, randomised, controlled trial. Lancet Haematol. 2023, 10, e250–e260. [Google Scholar] [CrossRef]
- Keeler, B.D.; Dickson, E.A.; Simpson, J.A.; Ng, O.; Padmanabhan, H.; Brookes, M.J.; Acheson, A.G.; Group, I.T. The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: Outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia 2019, 74, 714–725. [Google Scholar] [CrossRef]
- Steensma, D.P.; Sasu, B.J.; Sloan, J.A.; Tomita, D.; Loprinzi, C.L. The relationship between serum hepcidin levels and clinical outcomes in patients with chemotherapy-associated anemia treated in a controlled trial. J. Clin. Oncol. 2011, 29, 9031. [Google Scholar] [CrossRef]
| Author | Study Type | Indication | Inclusion Criteria | N | Treatment | Results |
|---|---|---|---|---|---|---|
| Ansari Nejad 2016 [27] | Prospective, single-center, open-label RCT | Stage 3/4 colon cancer undergoing chemotherapy | Hb ≤ 120 g/L for women and ≤130 g/L for men, SF < 30 µg/L | 60 | G1: Oral ferrous sulfate 65 mg t.i.d for 8 wk G2: IV FCM (1500 mg for patients weighting < 70 kg or 2000 mg for patients weighting > 70 kg) | Significantly higher Hb in IV FCM group (138.6 ± 7.4 g/L vs. 116.7 ± 12.8 g/L) |
| Anthony 2011 [28] | Prospective, multicentre, open-label RCT | Cancer and/or chemotherapy-induced anemia | Hb ≤ 100 g/L | 375 | G1: IV IS (7 mg/kg up to 500 mg) × 3 times per wk with 1 to 3 week intervals + ESAs G2: No iron + ESAs | Higher Hb in IV IS + ESAs Improved FACIT fatigue scores in IV IS group |
| Auerbach 2004 [29] | Prospective, multicentre, open-label RCT | Cancer and/or chemotherapy-induced anemia | Hb ≤ 105 g/L, SF ≤ 200 µg/L or SF ≤ 300 µg/L with TSAT ≤ 19% | 157 | Epoetin alfa 40,000 U/wk in addition to: G1: No iron G2: Oral ferrous sulfate 325 mg b.i.d G3: IV bolus iron dextran repeated 100 mg G4: IV iron dextran total dose infusion | Greater mean Hb increase in both IV iron groups compared to oral iron and no iron groups Hb response in IV groups compared to oral iron and no iron groups (68% v 25%) QoL improvement in IV iron groups |
| Auerbach 2010 [30] | Prospective, multicentre, double-blind, 2 × 2 factorial RCT | Cancer and/or chemotherapy in active nonmyeloid malignancies | Hb ≤ 100 g/L Exclusion: TSAT < 15% and SF < 10 µg/L | 243 | G1: SC DA 500 μg Q3W + no iron G2: SC DA 500 μg Q3W + IV iron dextran 400 µg Q3W G3: SC DA 300 μg Q3W + no iron G4: SC DA 300 μg Q3W + IV iron dextran 400 µg Q3W | Higher proportion achieved Hb ≥ 110 g/L in IV iron groups (82% v 72%). Clinically significant increase in FACT-F scores were 67%, 100%, 65%, and 63%, respectively |
| Bastit 2008 [31] | Prospective, multicentre, open-label RCT | Nonmyeloid malignancy undergoing chemotherapy | Hb ≤ 110 g/L Exclusion: TSAT < 15% and SF < 10 µg/L), SF > 800 µg/L | 396 | G1: IV FG or IS 200 mg Q3W as single dose or two doses + SC DA 500 μg Q3W G2: Oral iron or no iron + SC DA 500 μg Q3W | Higher proportion achieved Hb target in IV iron group (86% v 73%). Lower transfusion rate in IV iron group from week 4 to end of trial period (9% v 20%) No differences in QoL scores |
| Dangsuwan 2010 [32] | Prospective, single-center, open-label RCT | Gynecological cancer receiving first-line chemotherapy after primary surgery | Hb < 100 g/L | 44 | G1: IV IS 200 mg, single dose G2: Oral ferrous sulfate 200 mg t.i.d | Lower RBC transfusion rate in IV IS (22.7% v 63.6%). Higher mean Hb (100 ± 8 g/L v 95 ± 9 g/L) No difference in change in QoL scores |
| Dickson 2023 [33] | Prospective, multicentre, placebo- controlled feasibility RCT | Advanced solid tumors | Hb < 120 g/L for women and <130 g/L men | 34 | G1: IV FDI 20 mg/kg/week G2: Placebo (250 mL 0.9% sodium chloride) | Feasible trial according to recruitment and attrition rates. Trial was not powered to detect a significant difference in Q5D5L, QLQ-C30, and the FACIT-F scores |
| Dreyer 2017 [34] | Prospective, multicentre, open-label RCT | Locally advanced cervical cancer requiring primary radiation treatment | Hb ≤ 120 g/L | 43 | Limited RBC transfusion to Hb = 60 g/L + IV IS (Ganzoni formula)—most patients 1 g total dose. RBC transfusion to Hb > 120 g/L Both groups received oral ferrous fumarate 400 mg on discharge. | A steady rise of Hb in the IV IS group to week 12. Transfusion group showed a steady decline of about 5 g/L per week post-treatment |
| Edwards 2009 [35] | Prospective, single- center, placebo-controlled RCT | Elective surgery for suspected colorectal cancer | Hb ≤ 125 g/L for women, Hb ≤ 135 g/L for men | 60 | G1: IV IS 600 mg in two divided doses, at least 24 h apart, 14 days preoperatively G2: placebo in two divided doses, at least 24 h apart, 14 days preoperatively | No difference in Hb or transfusion rates |
| Fung, 2022 [36] | Prospective, single-centre, open-label pilot RCT | Elective colorectal cancer surgery | Hb < 130 g/L, SF < 30 µg/L or SF = 30–100 µg/L with TSAT < 20% | 40 | G1: IV IIM 20 mg/kg (up to 1000 mg) preoperatively G2: Usual preoperative care (no iron) | Higher mean Hb change before surgery in IV IIM (7.8 g/L v 1.7 g/L) No differences in QoR-15 and DAH30 at POD 30 |
| Hajigholami 2021 [37] | Prospective Single-centre open-label RCT | Metastatic and non-metastatic carcinoma undergoing chemotherapy | Hb ≤ 120 g/L Exclusion: SF > 500 µg/L | 79 | G1: IV IS 100 mg at each chemotherapy session + SC EPO 150 units/kg SC three times a week) G2: Oral ferrous sulfate 100 mg t.i.d for six wks + EPO 150 units/kg SC three times a week) | No significant between-group differences in Hb increase (114 ± 16 v 112 ± 14 g/L). Physical index score increased in IV group. No significant between-group differences in QLQ-C30 scores |
| Hedenus 2007 [38] | Prospective, multicentre, open-label RCT | Lymphoproliferative malignancy not requiring chemotherapy or blood transfusions | Hb 90–110 g/L Exclusion: SF > 800 µg/L | 60 | G1: IV IS 100 mg/wk for 6 wks followed by 100 mg Q2W for 8 wks + SC EPO 30,000 IU/wk for 16 weeks G2: SC EPO 30,000 IU/wk for 16 weeks | Higher Hb increase in IV iron group (93% v 53%) |
| Hedenus 2014 [39] | Prospective, multicentre, open-label RCT | Indolent lymphoid malignancy with cancer-related anemia | Hb 85–105 g/L and SF > 30 µg/L for women or >40 µg/L for men, TSAT ≤ 20% | 17 | G1: IV FCM 1 g, (>50 kg single dose, 500 mg two weeks apart if <50 kg) G2: Control (no treatment, symptomatic management according to local practice) | Significantly higher mean change in Hb in IV FCM group at 8 weeks (Hb = 21 g/L vs. 11 g/L) |
| Henry 2007 [40] | Prospective, multicentre, open-label RCT | Patients with chemotherapy anemia | Hb ≤ 110 g/L SF ≥ 100 µg/L TSAT ≥ 15% | 187 | G1: IV FG 125 mg/wk for 8 wks + SC EPO 40,000 U/wk for 12 wks G2: Oral ferrous sulfate 325 mg t.i.d SC EPO 40,000 U/wk for 12 wks G3: No iron + SC EPO 40,000 U/wk for 12 wks | Hb response was 73% for FG, 46% for oral iron, and 41% for no iron |
| Keeler 2017 [41] | Prospective, multicentre, open-label RCT | Elective colorectal cancer surgery | Hb ≤ 110 g/L for women and ≤120 g/L for men | 101 | G1: IV FCM 1–2 g (up to two doses with one week apart) preoperatively G2: Oral ferrous sulfate 200 mg b.i.d. at least two weeks before surgery | No difference in transfusion rates Hb increase in IV FCM (median 1.55 (i.q.r. 0.93–2.58) v 0.50 (−0.13 to 1.33) g/dl |
| Kim 2007 [42] | Prospective, single-centre, open-label RCT | Cervical cancer undergoing chemoradiotherapy | Hb ≤ 120 g/L | 75 | G1: IV IS 200 mg single infusion G2: Control (no iron) | Decreased transfusion requirement in IV IS group (40% v 64%) and mean transfusion units (1.87 v 3.58) |
| Laso-Morales 2022 [43] | Prospective single-centre, open-label RCT | Elective colorectal cancer surgery | Hb < 110 g/L after surgery | 104 | G1: IV FCM 1 g, single dose on POD1 G2: IV IS 200 mg (every 48 hrs from POD1 to discharge or up to the total dose equivalent using Ganzoni Formula | No differences in Hb, transfusion rates or length of stay Infection rate lower in IV FC (9.8% v 37.2) |
| Maccio 2010 [44] | Prospective, multicentre, open-label RCT | Advanced solid tumor undergoing chemotherapy | Hb ≤ 100 g/L SF ≥ 100 µg/L and ≤800 mg/dL and/or TSAT > 15% | 148 | G1: IV FG 125 mg weekly + EPO 30,000 UI SC weekly for 12 weeks G2: Oral lactoferrin 100 mg b.i.d + SC EPO 30,000 UI/wk for 12 weeks | No difference in Hb +1.6 g/dL V +1.8 g/dL for lactoferrin |
| Makharadze 2021 [45] | Prospective, multicentre, placebo-controlled RCT | Nonmyeloid malignancy undergoing chemotherapy | Hb: 80–110 g/L, SF:100–800 µg/L TSAT ≤ 35% | 244 | G1: IV FCM 15 mg/kg (single and total doses of 750 mg and ≤1500 mg, respectively) 7 days apart G2: Placebo normal saline 0.9% ≤250 mL of normal saline two infusions 7 days apart | Higher maintained Hb within 0.5 g/dL of baseline in IV FCM group (50.8% v 35.3%) |
| Noronha 2018 [46] | Prospective single-center, open-label RCT | Malignancy requiring chemotherapy | Hb < 120 g/L SF < 100 µg/L TSAT < 20% or hypochromic RBC > 10% | 148 | G1: IV IS (Ganzoni Formula) G2: Oral ferrous sulfate 100 mg t.i.d, started with cycle one of chemotherapy and continued until the end of cycle 2 | No difference between groups in change In Hb at 6 weeks (0.11 g/dL v 0.16 g/dL) No difference in QoL |
| Pedrazzoli 2008 [47] | Prospective multicentre, open-label RCT | Breast, colorectal, lung, or gynaecologic cancer undergoing chemotherapy | Hb ≤ 120 g/L SF ≥ 100 µg/L TSAT ≥ 20% | 149 | G1: IV FG 125 mg/wk for first 6 wks + SC DA 150 µg/wk for 12 wks G2: No iron + SC DA 150 µg/wk for 12 wks | Higher Hb response in IV FG/DA group (76.7% v 61.8%) Faster Hb response from week 5 in IV FG/DA group |
| Steensma 2010 [48] | Prospective multicentre, open-label RCT | Nonmyeloid malignancy undergoing chemotherapy | Hb ≤ 110 g/L | 502 | SC DA 500 μg Q3W in addition to: G1: IV FG 187.5 mg Q3W for 5 doses G2: Oral ferrous sulfate 325 mg for 16 wks G2: Oral placebo for 16 wks | No difference In Hb, transfusion, or QoL on intention-to-treat analysis |
| Talboom, 2023 [49] | Prospective, multicentre, open-label RCT | Elective colorectal cancer surgery | Hb < 120 g/L for women and <130 g/L for men TSAT < 20% | 202 | G1: IV FCM 1–2 g (up to two doses with one wk apart) preoperatively G2: Oral ferrous fumarate 200 mg t.i.d. until day before surgery | No difference In Hb normalization before surgery (17% v 16%), transfusion rates, length of stay, post-op complications. Higher Hb normalized in IV iron at 30 days post-op (60% v 21%). No difference in BFI and EQ5D scores. Improved scores in Role Functioning Scale in EORTC 30 and three symptom scales on the EORTC C29 in oral iron group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Auerbach, M.; MacLean, B.; Al-Sharea, A.; Richards, T. Intravenous Iron Therapy to Treat Anemia in Oncology: A Mapping Review of Randomized Controlled Trials. Curr. Oncol. 2023, 30, 7836-7851. https://doi.org/10.3390/curroncol30090569
Lim J, Auerbach M, MacLean B, Al-Sharea A, Richards T. Intravenous Iron Therapy to Treat Anemia in Oncology: A Mapping Review of Randomized Controlled Trials. Current Oncology. 2023; 30(9):7836-7851. https://doi.org/10.3390/curroncol30090569
Chicago/Turabian StyleLim, Jayne, Michael Auerbach, Beth MacLean, Annas Al-Sharea, and Toby Richards. 2023. "Intravenous Iron Therapy to Treat Anemia in Oncology: A Mapping Review of Randomized Controlled Trials" Current Oncology 30, no. 9: 7836-7851. https://doi.org/10.3390/curroncol30090569
APA StyleLim, J., Auerbach, M., MacLean, B., Al-Sharea, A., & Richards, T. (2023). Intravenous Iron Therapy to Treat Anemia in Oncology: A Mapping Review of Randomized Controlled Trials. Current Oncology, 30(9), 7836-7851. https://doi.org/10.3390/curroncol30090569





