Capecitabine/Mitomycin versus 5-Fluorouracil/Mitomycin in Combination with Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy for Anal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Study Treatment
2.3.1. Radiotherapy
2.3.2. Chemotherapy
2.4. Statistical Analysis
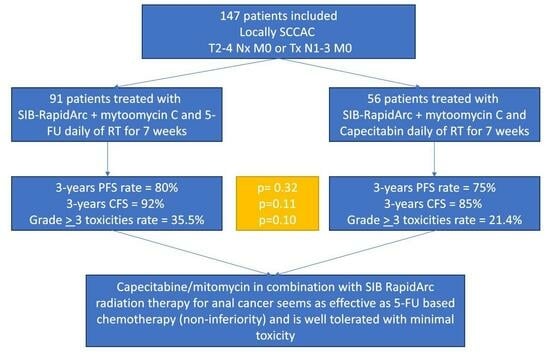
3. Results
3.1. Patient and Treatment Characteristics
3.2. Toxicities
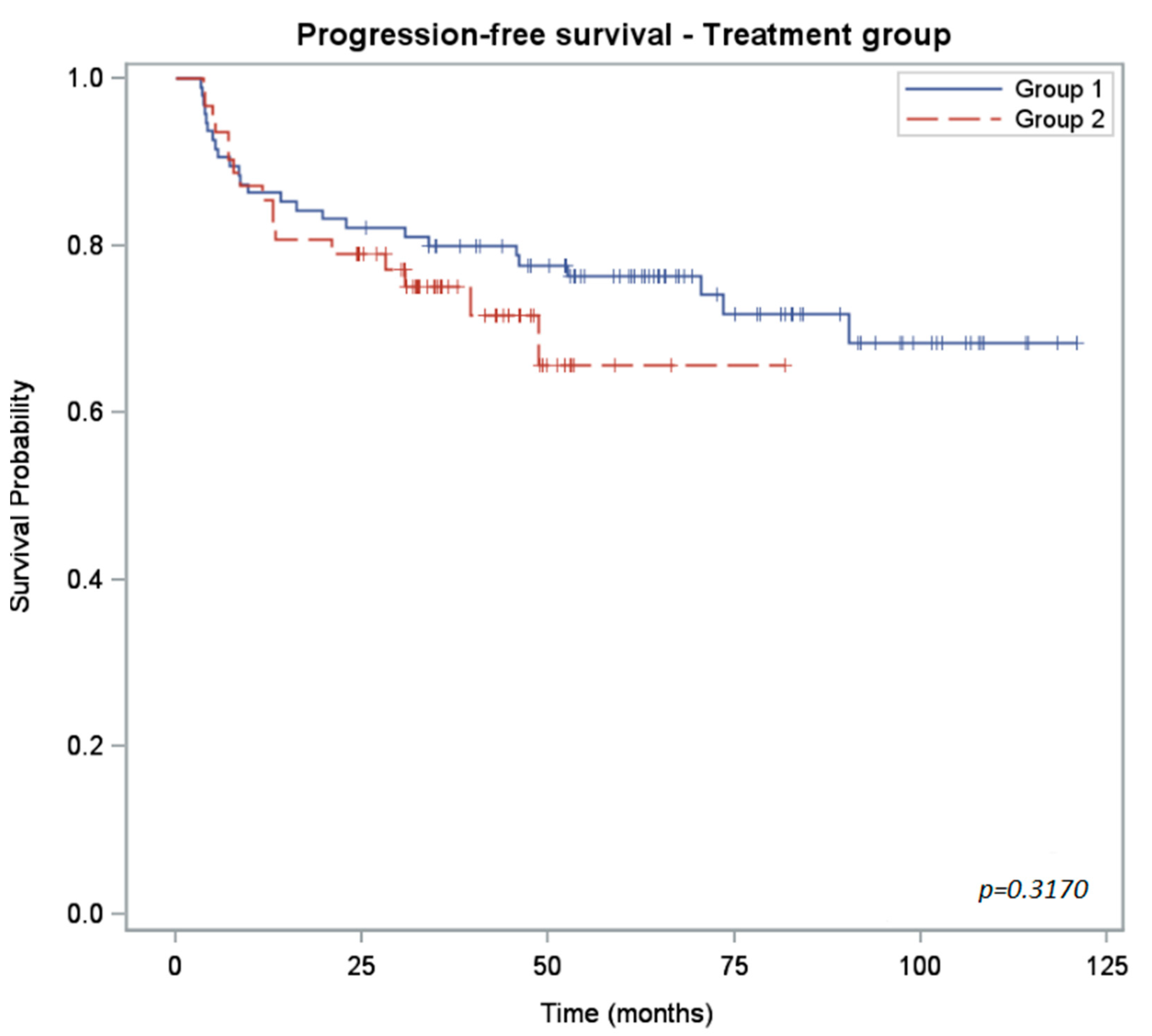
3.3. Outcomes and Survival
3.4. Comparison of Efficacy and Toxicities with Previous Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Valvo, F.; Ciurlia, E.; Avuzzi, B.; Doci, R.; Ducreux, M.; Roelofsen, F.; Roth, A.; Trama, A.; Wittekind, C.; Bosset, J.-F. Cancer of the anal region. Crit. Rev. Oncol. Hematol. 2019, 135, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, A.M.; Belot, A.; Manfredi, S.; Jooste, V.; Uhry, Z.; Faivre, J.; Duport, N.; Grabar, S.; French Network of Cancer Registries FRANCIM. Trends of incidence and survival in squamous-cell carcinoma of the anal canal in France: A population-based study. Eur. J. Cancer. Prev. 2016, 25, 182–187. [Google Scholar] [CrossRef]
- Nigro, N.D.; Seydel, H.G.; Considine, B.; Vaitkevicius, V.K.; Leichman, L.; Kinzie, J.J. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer 1983, 51, 1826–1829. [Google Scholar] [CrossRef]
- UKCCCR Anal Cancer Trial Working Party. Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet 1996, 348, 1049–1054. [Google Scholar] [CrossRef]
- Flam, M.; John, M.; Pajak, T.F.; Petrelli, N.; Myerson, R.; Doggett, S.; Quivey, J.; Rotman, M.; Kerman, H.; Coia, L.; et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J. Clin. Oncol. 1996, 14, 2527–2539. [Google Scholar] [CrossRef]
- Bartelink, H.; Roelofsen, F.; Eschwege, F.; Rougier, P.; Bosset, J.F.; Gonzalez, D.G.; Peiffert, D.; van Glabbeke, M.; Pierart, M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastro. J. Clin. Oncol. 1997, 117, 3342–3351. [Google Scholar] [CrossRef]
- James, R.D.; Glynne-Jones, R.; Meadows, H.M.; Cunningham, D.; Myint, A.S.; Saunders, M.P.; Maughan, T.; McDonald, A.; Essapen, S.; Leslie, M.; et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013, 14, 516–524. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Winter, K.A.; Ajani, J.A.; Pedersen, J.E.; Moughan, J.; Benson, A.B.; Thomas, C.R., Jr.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Long-term update of US GI intergroup RTOG 98-11 Phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J. Clin. Oncol. 2012, 30, 4344–4351. [Google Scholar] [CrossRef]
- Weber, D.C.; Kurtz, J.M.; Allal, A.S. The impact of gap duration on local control of anal cancer treated by split-course radiotherapy and concomitant chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Wust, P.; Hildebrandt, B.; Gögler, H.; Ullrich, R.; Herrmann, R.; Riess, H.; Felix, R. Impact of overall treatment time on local control of anal cancer treated with radiochemotherapy. Oncology 2003, 65, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.G.; Hara, W.; Hsu, A.; Kunz, P.A.; Ford, J.; Fisher, G.A.; Welton, M.L.; Shelton, A.; Kapp, D.S.; Koong, A.C.; et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011, 117, 3342–3351. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.D.; Freilich, J.M.; Hoffe, S.E.; Fulp, W.; Weber, J.M.; Almhanna, K.; Dinwoodie, W.; Rao, N.; Meredith, K.L.; Shridhar, R. Intensity-modulated radiation therapy vs. 3D conformal radiation therapy for squamous cell carcinoma of the anal canal. Gastrointest. Cancer Res. 2013, 6, 39–45. [Google Scholar] [PubMed]
- Zagar, T.M.; Willett, C.G.; Czito, B.G. Intensity-modulated radiation therapy for anal cancer: Toxicity versus outcomes. Oncology 2010, 24, 815–823. [Google Scholar] [PubMed]
- Mitchell, M.P.; Abboud, M.; Eng, C.; Beddar, A.S.; Krishnan, S.; Delclos, M.E.; Crane, C.H.; Das, P. Intensity-modulated radiation therapy with concurrent chemotherapy for anal cancer: Outcomes and toxicity. Am. J. Clin. Oncol. 2014, 37, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Amendola, B.E.; Amendola, M.; Perez, N.; Iglesias, A.; Wu, X. Volumetric arc therapy with Rapid-Arc: An evaluation of treatment delivery efficiency. Rep. Pract. Oncol. Radiother. 2013, 18, 383–386. [Google Scholar] [CrossRef]
- Rich, T.A. Irradiation plus 5-fluorouracil: Cellular mechanisms of action and treatment schedules. Semin. Radiat. Oncol. 1997, 7, 267–273. [Google Scholar] [CrossRef]
- Sawada, N.; Ishikawa, T.; Sekiguchi, F.; Tanaka, Y.; Ishitsuka, H. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin. Cancer Res. 1999, 5, 2948–2953. [Google Scholar]
- Cunningham, D.; Starling, N.; Rao, S.; Chong, G. Capecitabine and oxaliplatin for advanced eosophagogastric cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, W.; Ge, L.; Yang, X.; Wang, Q.; Wang, H. Capecitabine versus 5-5-fluorouracil in neoadjuvant chemoradiotherapy of locally advanced rectal cancer. Medicine 2019, 98, e15241. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Meadows, H.; Wan, S.; Gollins, S.; Leslie, M.; Levine, E.; McDonald, A.C.; Myint, S.; Samuel, L.; Sebag-Montefiore, D. EXTRA—A Multicenter Phase II Study of Chemoradiation Using a 5 Day per Week Oral Regimen of Capecitabine and Intravenous Mitomycin C in Anal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Deenen, M.J.; Dewit, L.; Boot, H.; Beijnen, J.H.; Schellens, J.H.; Cats, A. Simultaneous integrated boost–intensity modulated radiation therapy with concomitant capecitabine and mitomycin C for locally advanced anal carcinoma: A phase 1 study. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, e201–e207. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.D.A.; Wan, D.D.; Schellenberg, D.; Lim, H.J. A comparison between 5-fluorouracil/mitomycin and capecitabine/mitomycin in combination with radiation for anal cancer. J. Gastrointest. Oncol. 2016, 7, 665–672. [Google Scholar] [CrossRef]
- Thind, G.; Johal, B.; Follwell, M.; Kennecke, H.F. Chemoradiation with capecitabine and mitomycin-C for stage I-III anal squamous cell carcinoma. Radiat. Oncol. 2014, 9, 124. [Google Scholar] [CrossRef]
- Oliveira, S.C.R.; Moniz, C.M.V.; Riechelmann, R.; Alex, A.K.; Braghirolli, M.I.; Bariani, G.; Nahas, C.; Hoff, P.M.G. Phase II Study of Capecitabine in Substitution of 5-FU in the Chemoradiotherapy Regimen for Patients with Localized Squamous Cell Carcinoma of the Anal Canal. J. Gastrointest. Cancer 2015, 47, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Meulendijks, D.; Dewit, L.; Tomasoa, N.B.; van Tinteren, H.; Beijnen, J.H.; Schellens, J.H.M.; Cats, A. Chemoradiotherapy with capecitabine for locally advanced anal carcinoma: An alternative treatment option. Br. J. Cancer 2014, 111, 1726–1733. [Google Scholar] [CrossRef]
- Pumpalova, Y.; Kozak, M.M.; von Eyben, R.; Kunz, P.; Fisher, G.; Chang, D.T.; Haraldsdottir, S. Comparison of definitive chemoradiation with 5-fluorouracil versus capecitabine in anal cancer. J. Gastrointest. Oncol. 2019, 10, 605–615. [Google Scholar] [CrossRef]
- Smalley, S.R.; Benedetti, J.K.; Williamson, S.K.; Robertson, J.M.; Estes, N.C.; Maher, T.; Fisher, B.; Rich, T.A.; Martenson, J.A.; Kugler, J.W.; et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J. Clin. Oncol. 2006, 24, 3542–3547. [Google Scholar] [CrossRef]
- Liu, G.; Franssen, E.; Fitch, M.I.; Warner, E. Patient preferences for oral versus intravenous palliative chemotherapy. J. Clin. Oncol. 1997, 15, 110–115. [Google Scholar] [CrossRef]
- Goodman, K.A.; Julie, D.; Cercek, A.; Cambridge, L.; Woo, K.M.; Zhang, Z.; Wu, A.J.; Reidy, D.L.; Segal, N.H.; Stadler, Z.K.; et al. Capecitabine with mitomycin reduces acute hematological toxicity and treatment delays in patients undergoing definitive chemoradiation using Intensity Modulated Radiation Therapy fot Anal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1087–1095. [Google Scholar] [CrossRef] [PubMed]


| 5-FU-Based CT Treatment N = 95 | Capecitabine-Based CT Treatment N = 62 | p-Value | |||
|---|---|---|---|---|---|
| Characteristics | n | % | n | % | |
| Age (years), median (range) | 61 (38–90) | 67 (39–93) | 0.0048 | ||
| Sex Male Female | 17 78 | 17.89 82.11 | 10 52 | 16.13 83.87 | 0.8316 |
| ECOG performance status 0 1–2 | 91 4 | 95.79 4.21 | 57 5 | 91.94 8.06 | 0.3192 |
| History of pelvis/obstetric surgery Yes No/unknown | 49 46 | 51.58 48.42 | 25 37 | 40.32 59.68 | 0.1925 |
| T-classification 1–2 3 4 | 60 22 13 | 63.16 23.16 13.68 | 36 19 7 | 58.06 30.65 11.29 | 0.5583 |
| N-classification 0 1 2–3 | 41 21 33 | 43.16 22.10 34.74 | 31 12 19 | 50.00 19.35 30.65 | 0.7389 |
| HPV status Negative Positive Missing | 3 25 67 | 3.16 26.31 70.53 | 3 36 23 | 4.84 58.06 37.10 | <0.001 |
| HIV status Negative Positive Missing | 21 2 72 | 22.10 2.11 75.79 | 29 2 31 | 46.77 3.23 50.00 | 0.0020 |
| SCC tumor marker Normal Elevated Unknown | 35 23 37 | 36.84 24.21 38.95 | 25 14 23 | 40.32 22.58 37.10 | 0.9342 |
| Radiation technique: boost-integrated Yes No | 86 9 | 90.53 9.47 | 57 5 | 91.94 8.06 | 1.0000 |
| Median RT treatment days (range) | 52 (46–78) | 52 (43–79) | 0.7949 | ||
| RT interruptions > 3 days Yes No | 17 78 | 17.89 82.11 | 8 54 | 12.90 87.10 | 0.5053 |
| Median duration of RT interruption (range), days | 6 (4–25) | 9.5 (5–20) | 0.0203 | ||
| Chemotherapy dose reduction Yes No | 38 57 | 40.00 60.00 | 28 34 | 45.16 54.84 | 0.6200 |
| Capecitabine or 5FU dose received <50% 50–75% 75–100% 100% | 1 12 25 57 | 1.05 12.63 26.32 60.00 | 6 10 12 34 | 9.68 16.13 19.35 54.84 | 0.0641 |
| Toxicity | 5-FU-Based CT Regimen | Capecitabine-Based CT Regimen | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||||
| Number of Patients * N = 95 | 1 | 2 | 3 | 4 | Number of Patients * N = 62 | 1 | 2 | 3 | 4 | |
| Hematological toxicities | ||||||||||
| Anemia | 33 | 23 | 8 | 2 | 0 | 11 | 8 | 3 | 0 | 0 |
| Thrombopenia | 50 | 40 | 6 | 4 | 0 | 12 | 7 | 3 | 1 | 1 |
| Neutropenia | 24 | 17 | 4 | 2 | 1 | 6 | 4 | 1 | 1 | 0 |
| Digestive toxicities | ||||||||||
| Diarrhea | 73 | 31 | 35 | 7 | 0 | 47 | 22 | 17 | 8 | 0 |
| Nausea | 27 | 21 | 5 | 1 | 0 | 11 | 8 | 3 | 0 | 0 |
| Anorexia | 28 | 22 | 5 | 1 | 0 | 13 | 7 | 5 | 1 | 0 |
| Vomiting | 6 | 3 | 1 | 2 | 0 | 2 | 1 | 0 | 1 | 0 |
| Rectitis | 43 | 25 | 17 | 1 | 0 | 25 | 15 | 10 | 0 | 0 |
| Anitis | 80 | 28 | 47 | 5 | 0 | 44 | 22 | 19 | 3 | 0 |
| Other toxicities | ||||||||||
| Renal failure | 6 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Asthenia | 51 | 23 | 22 | 6 | 0 | 26 | 15 | 11 | 0 | 0 |
| Vaginitis | 26 | 17 | 8 | 1 | 0 | 12 | 10 | 1 | 1 | 0 |
| Cystitis | 32 | 24 | 8 | 0 | 0 | 7 | 7 | 0 | 0 | 0 |
| Dermatitis | 77 | 37 | 34 | 6 | 0 | 50 | 31 | 17 | 2 | 0 |
| Alopecia | 20 | 2 | 14 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5-FU-Based CT Treatment N = 95 | Capecitabine-Based CT Treatment N = 62 | p-Value | |
|---|---|---|---|
| Follow-up median (months) CI95% | 67.44 (63.51; 78.03) | 41.51 (34.59; 46.26) | / |
| PFS rate (%) CI95% | 2 years: 82 (73; 88) 3 years: 80 (70; 87) | 2 years: 79 (67; 87) 3 years: 75 (62; 84) | 0.3170 |
| OS rate (%) CI95% | 2 years: 94 (86; 97) 3 years: 91 (83; 96) | 2 years: 95 (86; 98) 3 years: 86 (72; 93) | 0.2431 |
| CFS rate (%) CI95% | 2 years: 93 (86; 97) 3 years: 92 (85; 96) | 2 years: 85 (74; 92) 3 years: 85 (74; 92) | 0.1113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mineur, L.; Vazquez, L.; Belkacemi, M.; Toullec, C.; Bentaleb, N.; Boustany, R.; Plat, F. Capecitabine/Mitomycin versus 5-Fluorouracil/Mitomycin in Combination with Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy for Anal Cancer. Curr. Oncol. 2023, 30, 8563-8574. https://doi.org/10.3390/curroncol30090621
Mineur L, Vazquez L, Belkacemi M, Toullec C, Bentaleb N, Boustany R, Plat F. Capecitabine/Mitomycin versus 5-Fluorouracil/Mitomycin in Combination with Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy for Anal Cancer. Current Oncology. 2023; 30(9):8563-8574. https://doi.org/10.3390/curroncol30090621
Chicago/Turabian StyleMineur, Laurent, Léa Vazquez, Mohamed Belkacemi, Clémence Toullec, Newfel Bentaleb, Rania Boustany, and Frederi Plat. 2023. "Capecitabine/Mitomycin versus 5-Fluorouracil/Mitomycin in Combination with Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy for Anal Cancer" Current Oncology 30, no. 9: 8563-8574. https://doi.org/10.3390/curroncol30090621
APA StyleMineur, L., Vazquez, L., Belkacemi, M., Toullec, C., Bentaleb, N., Boustany, R., & Plat, F. (2023). Capecitabine/Mitomycin versus 5-Fluorouracil/Mitomycin in Combination with Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy for Anal Cancer. Current Oncology, 30(9), 8563-8574. https://doi.org/10.3390/curroncol30090621