The Impact of Oral Antibiotics Prior to Cancer Diagnosis on Overall Patient Survival: Findings from an English Population-Based Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Study Design
2.2. Study Sample
2.3. Exposure and Outcome Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Viaud, S.; Vétizou, M.; Daillère, R.; Merad, M.; Kroemer, G. Cancer and the gut microbiota: An unexpected link. Sci. Transl. Med. 2015, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Singer, M. Do antibiotics cause mitochondrial and immune cell dysfunction? A literature review. J. Antimicrob. Chemother. 2022, 77, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.-G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; De Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association and Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano GA, D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Elkrief, A.; Derosa, L.; Kroemer, G.; Zitvogel, L.; Routy, B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: A new independent prognostic factor? Ann. Oncol. 2019, 30, 1572–1579. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 2019, 5, 1774–1778. [Google Scholar] [CrossRef]
- Raymond, F.; Ouameur, A.A.; Déraspe, M.; Iqbal, N.; Gingras, H.; Dridi, B.; Leprohon, P.; Plante, P.-L.; Giroux, R.; Bérubé, È.; et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 2016, 10, 707–720. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4554–4561. [Google Scholar] [CrossRef]
- Tinsley, N.; Zhou, C.; Tan, G.; Rack, S.; Lorigan, P.; Blackhall, F.; Krebs, M.; Carter, L.; Thistlethwaite, F.; Graham, D.; et al. Cumulative Antibiotic Use Significantly Decreases Efficacy of Checkpoint Inhibitors in Patients with Advanced Cancer. Oncologist 2020, 25, 55–63. [Google Scholar] [CrossRef]
- Williams, T.; van Staa, T.; Puri, S.; Eaton, S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther. Adv. Drug Saf. 2012, 3, 89–99. [Google Scholar] [CrossRef]
- Herrett, E.; Gallagher, A.M.; Bhaskaran, K.; Forbes, H.; Mathur, R.; van Staa, T.; Smeeth, L. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int. J. Epidemiol. 2015, 44, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Radjabzadeh, D.; Boer, C.G.; Beth, S.A.; van der Wal, P.; Jong, J.C.K.-D.; Jansen, M.A.E.; Konstantinov, S.R.; Peppelenbosch, M.P.; Hays, J.P.; Jaddoe, V.W.V.; et al. Diversity, compositional and functional differences between gut microbiota of children and adults. Sci. Rep. 2020, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Symptoms and risk factors to identify men with suspected cancer in primary care: Derivation and validation of an algorithm. Br. J. Gen. Pract. 2013, 63, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Rogers, M.A.; Aronoff, D.M. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect. 2016, 22, 178.e1–178.e9. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2015, 65, 740–748. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Kurita, A.; Kado, S.; Matsumoto, T.; Asakawa, N.; Kaneda, N.; Kato, I.; Uchida, K.; Onoue, M.; Yokokura, T. Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of β-glucuronidase activity in intestinal lumen. Cancer Chemother. Pharmacol. 2011, 67, 201–213. [Google Scholar] [CrossRef]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Frank, M.; Hennenberg, E.M.; Eyking, A.; Rünzi, M.; Gerken, G.; Scott, P.; Parkhill, J.; Walker, A.W.; Cario, E. TLR Signaling Modulates Side Effects of Anticancer Therapy in the Small Intestine. J. Immunol. 2015, 194, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Hilovska, L.; Jendzelovsky, R.; Fedorocko, P. Potency of non-steroidal anti-inflammatory drugs in chemotherapy. Mol. Clin. Oncol. 2015, 3, 3–12. [Google Scholar] [CrossRef]
- Pollak, M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia 2017, 60, 1662–1667. [Google Scholar] [CrossRef]
- Cancer Research UK. Your Cancer Type|All Cancer Types. 2018. Available online: http://www.cancerresearchuk.org/about-cancer/type (accessed on 11 September 2023).
- American Cancer Society. Cancer. 2018. Available online: https://www.cancer.org/cancer/all-cancer-types.html (accessed on 11 September 2023).
| Leukaemia | Lymphoma | Myeloma | Melanoma | Ovary | Bladder | Colorectal | Uterus | Breast | |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 3303 | 5499 | 2022 | 4701 | 2917 | 4894 | 15,903 | 3151 | 20,635 |
| Age (mean) a | 68 | 66 | 71 | 62 | 65 | 74 | 72 | 67 | 64 |
| Sex = female (%) | 1394 (42.2) | 2636 (47.9) | 956 (47.3) | 2607 (55.5) | 2917 (100.0) | 1477 (30.2) | 7701 (48.4) | 3151 (100.0) | 20,495 (99.3) |
| Diabetes (%) | 461 (14.0) | 786 (14.3) | 305 (15.1) | 488 (10.4) | 311 (10.7) | 881 (18.0) | 2765 (17.4) | 593 (18.8) | 2136 (10.4) |
| COPD (%) | 546 (16.5) | 942 (17.1) | 338 (16.7) | 682 (14.5) | 454 (15.6) | 869 (17.8) | 2826 (17.8) | 422 (13.4) | 3378 (16.4) |
| Asthma (%) | 391 (11.8) | 730 (13.3) | 255 (12.6) | 589 (12.5) | 386 (13.2) | 532 (10.9) | 2064 (13.0) | 365 (11.6) | 2844 (13.8) |
| CVD (%) | 290 (8.8) | 408 (7.4) | 150 (7.4) | 284 (6.0) | 175 (6.0) | 527 (10.8) | 1462 (9.2) | 158 (5.0) | 1121 (5.4) |
| Heart failure (%) | 158 (4.8) | 227 (4.1) | 104 (5.1) | 121 (2.6) | 120 (4.1) | 275 (5.6) | 845 (5.3) | 87 (2.8) | 525 (2.5) |
| Renal disease (%) | 344 (10.4) | 612 (11.1) | 327 (16.2) | 380 (8.1) | 267 (9.2) | 798 (16.3) | 2027 (12.7) | 353 (11.2) | 1679 (8.1) |
| Cancer Type | Time Period | Patients at Risk | Deaths | Censored | Proportion That Died | Proportion That Survived | Survival Probability | % Survival |
|---|---|---|---|---|---|---|---|---|
| Leukaemia | 0–1 years | 3303 | 1036 | 0 | 0.31 | 0.69 | 0.69 | 69% |
| 1–5 years | 2267 | 835 | 271 | 0.39 | 0.61 | 0.42 | 42% | |
| 5–10 years | 1161 | 318 | 474 | 0.34 | 0.66 | 0.28 | 28% | |
| Lymphoma | 0–1 years | 5499 | 1287 | 0 | 0.23 | 0.77 | 0.77 | 77% |
| 1–5 years | 4212 | 992 | 628 | 0.25 | 0.75 | 0.58 | 58% | |
| 5–10 years | 2592 | 512 | 1116 | 0.25 | 0.75 | 0.43 | 43% | |
| Myeloma | 0–1 years | 2022 | 547 | 0 | 0.27 | 0.73 | 0.73 | 73% |
| 1–5 years | 1475 | 620 | 166 | 0.45 | 0.55 | 0.40 | 40% | |
| 5–10 years | 689 | 282 | 234 | 0.49 | 0.51 | 0.20 | 20% | |
| Melanoma | 0–1 years | 4701 | 196 | 0 | 0.04 | 0.96 | 0.96 | 96% |
| 1–5 years | 4505 | 678 | 768 | 0.16 | 0.84 | 0.81 | 81% | |
| 5–10 years | 3059 | 353 | 1440 | 0.15 | 0.85 | 0.69 | 69% | |
| Ovarian | 0–1 years | 2917 | 875 | 0 | 0.30 | 0.70 | 0.70 | 70% |
| 1–5 years | 2042 | 867 | 208 | 0.45 | 0.55 | 0.39 | 39% | |
| 5–10 years | 967 | 190 | 414 | 0.25 | 0.75 | 0.29 | 29% | |
| Bladder | 0–1 years | 4894 | 1434 | 0 | 0.29 | 0.71 | 0.71 | 71% |
| 1–5 years | 3460 | 1345 | 310 | 0.41 | 0.59 | 0.42 | 42% | |
| 5–10 years | 1805 | 534 | 601 | 0.35 | 0.65 | 0.27 | 27% | |
| Colorectal | 0–1 years | 15,903 | 4068 | 0 | 0.26 | 0.74 | 0.74 | 74% |
| 1–5 years | 11,835 | 4065 | 1365 | 0.36 | 0.64 | 0.47 | 47% | |
| 5–10 years | 6405 | 1472 | 2615 | 0.29 | 0.71 | 0.34 | 34% | |
| Uterus | 0–1 years | 3151 | 299 | 0 | 0.09 | 0.91 | 0.91 | 91% |
| 1–5 years | 2852 | 524 | 414 | 0.20 | 0.80 | 0.73 | 73% | |
| 5–10 years | 1914 | 247 | 825 | 0.16 | 0.84 | 0.61 | 61% | |
| Breast | 0–1 years | 20,635 | 1197 | 0 | 0.06 | 0.94 | 0.94 | 94% |
| 1–5 years | 19,438 | 3165 | 2953 | 0.18 | 0.82 | 0.77 | 77% | |
| 5–10 years | 13,320 | 1780 | 5727 | 0.17 | 0.83 | 0.64 | 64% |
| Cancer Registry | Primary Care | ||||
|---|---|---|---|---|---|
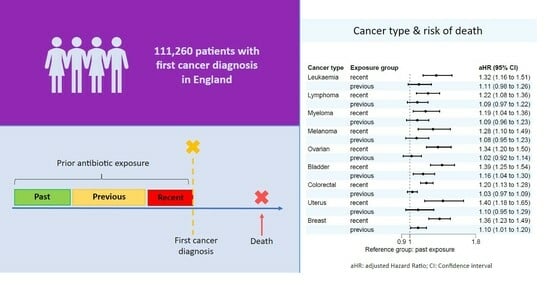
| Cancer Type | Exposure Group | Crude HR [95% CI] | Adjusted HR [95% CI] | Crude HR [95% CI] | Adjusted HR [95% CI] |
| Leukaemia | Recent | 1.34 [1.17–1.52] | 1.32 [1.16–1.51] | 1.55 [1.37–1.75] | 1.53 [1.35–1.73] |
| Previous | 1.12 [0.98–1.26] | 1.11 [0.98–1.26] | 1.10 [0.98–1.24] | 1.17 [1.04–1.32] | |
| Past | reference | reference | reference | reference | |
| Lymphoma | Recent | 1.26 [1.12–1.41] | 1.22 [1.08–1.36] | 1.27 [1.16–1.40] | 1.16 [1.13–1.30] |
| Previous | 1.13 [1.01–1.26] | 1.09 [0.97–1.22] | 1.07 [0.98–1.18] | 1.09 [0.97–1.22] | |
| Past | reference | reference | reference | reference | |
| Myeloma | Recent | 1.22 [1.05–1.43] | 1.19 [1.04–1.36] | 1.32 [1.16–1.51] | 1.14 [0.99–1.32] |
| Previous | 1.07 [0.93–1.24] | 1.09 [0.96–1.23] | 1.06 [0.94–1.19] | 1.00 [0.88–1.15] | |
| Past | reference | reference | reference | reference | |
| Melanoma | Recent | 1.28 [1.11–1.49] | 1.28 [1.10–1.49] | 1.16 [1.03–1.31] | 1.37 [1.21–1.56] |
| Previous | 1.08 [0.95–1.22] | 1.08 [0.95–1.23] | 1.02 [0.92–1.15] | 1.07 [0.96–1.20] | |
| Past | reference | reference | reference | reference | |
| Ovarian | Recent | 1.40 [1.22–1.60] | 1.34 [1.20–1.50] | 1.26 [1.13–1.40] | 1.27 [1.11–1.44] |
| Previous | 1.07 [0.94–1.23] | 1.02 [0.92–1.14] | 1.06 [0.95–1.18] | 1.09 [0.96–1.24] | |
| Past | reference | reference | reference | reference | |
| Bladder | Recent | 1.20 [1.07–1.40] | 1.39 [1.25–1.54] | 1.28 [1.16–1.40] | 1.34 [1.23–1.45] |
| Previous | 1.15 [1.00–1.30] | 1.16 [1.04–1.30] | 1.12 [1.01–1.23] | 1.08 [0.99–1.18] | |
| Past | reference | reference | reference | reference | |
| Colorectal | Recent | 1.22 [1.14–1.30] | 1.20 [1.13–1.28] | 1.19 [1.09–1.28] | 1.22 [1.14–1.30] |
| Previous | 1.04 [0.98–1.10] | 1.03 [0.97–1.09] | 1.07 [1.00–1.14] | 1.05 [0.99–1.11] | |
| Past | reference | reference | reference | reference | |
| Uterus | Recent | 1.43 [1.22–1.69] | 1.40 [1.18–1.65] | 1.29 [1.06–1.57] | 1.23 [1.01–1.50] |
| Previous | 1.14 [0.98–1.32] | 1.10 [0.95–1.29] | 1.07 [0.89–1.28] | 1.03 [0.86–1.24] | |
| Past | reference | reference | reference | reference | |
| Breast | Recent | 1.49 [1.36–1.63] | 1.36 [1.23–1.49] | 1.39 [1.25–1.54] | 1.18 [0.96–1.27] |
| Previous | 1.16 [1.07–1.26] | 1.10 [1.01–1.20] | 1.13 [1.03–1.24] | 1.39 [1.27–1.51] | |
| Past | reference | reference | reference | reference | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domzaridou, E.; Van Staa, T.; Renehan, A.G.; Cook, N.; Welfare, W.; Ashcroft, D.M.; Palin, V. The Impact of Oral Antibiotics Prior to Cancer Diagnosis on Overall Patient Survival: Findings from an English Population-Based Cohort Study. Curr. Oncol. 2023, 30, 8434-8443. https://doi.org/10.3390/curroncol30090614
Domzaridou E, Van Staa T, Renehan AG, Cook N, Welfare W, Ashcroft DM, Palin V. The Impact of Oral Antibiotics Prior to Cancer Diagnosis on Overall Patient Survival: Findings from an English Population-Based Cohort Study. Current Oncology. 2023; 30(9):8434-8443. https://doi.org/10.3390/curroncol30090614
Chicago/Turabian StyleDomzaridou, Eleni, Tjeerd Van Staa, Andrew G. Renehan, Natalie Cook, William Welfare, Darren M. Ashcroft, and Victoria Palin. 2023. "The Impact of Oral Antibiotics Prior to Cancer Diagnosis on Overall Patient Survival: Findings from an English Population-Based Cohort Study" Current Oncology 30, no. 9: 8434-8443. https://doi.org/10.3390/curroncol30090614
APA StyleDomzaridou, E., Van Staa, T., Renehan, A. G., Cook, N., Welfare, W., Ashcroft, D. M., & Palin, V. (2023). The Impact of Oral Antibiotics Prior to Cancer Diagnosis on Overall Patient Survival: Findings from an English Population-Based Cohort Study. Current Oncology, 30(9), 8434-8443. https://doi.org/10.3390/curroncol30090614