Localized Colonic Small-Cell Carcinoma with Pathological Complete Response after Neoadjuvant Cisplatin and Etoposide: A Case Report
Abstract
:1. Introduction
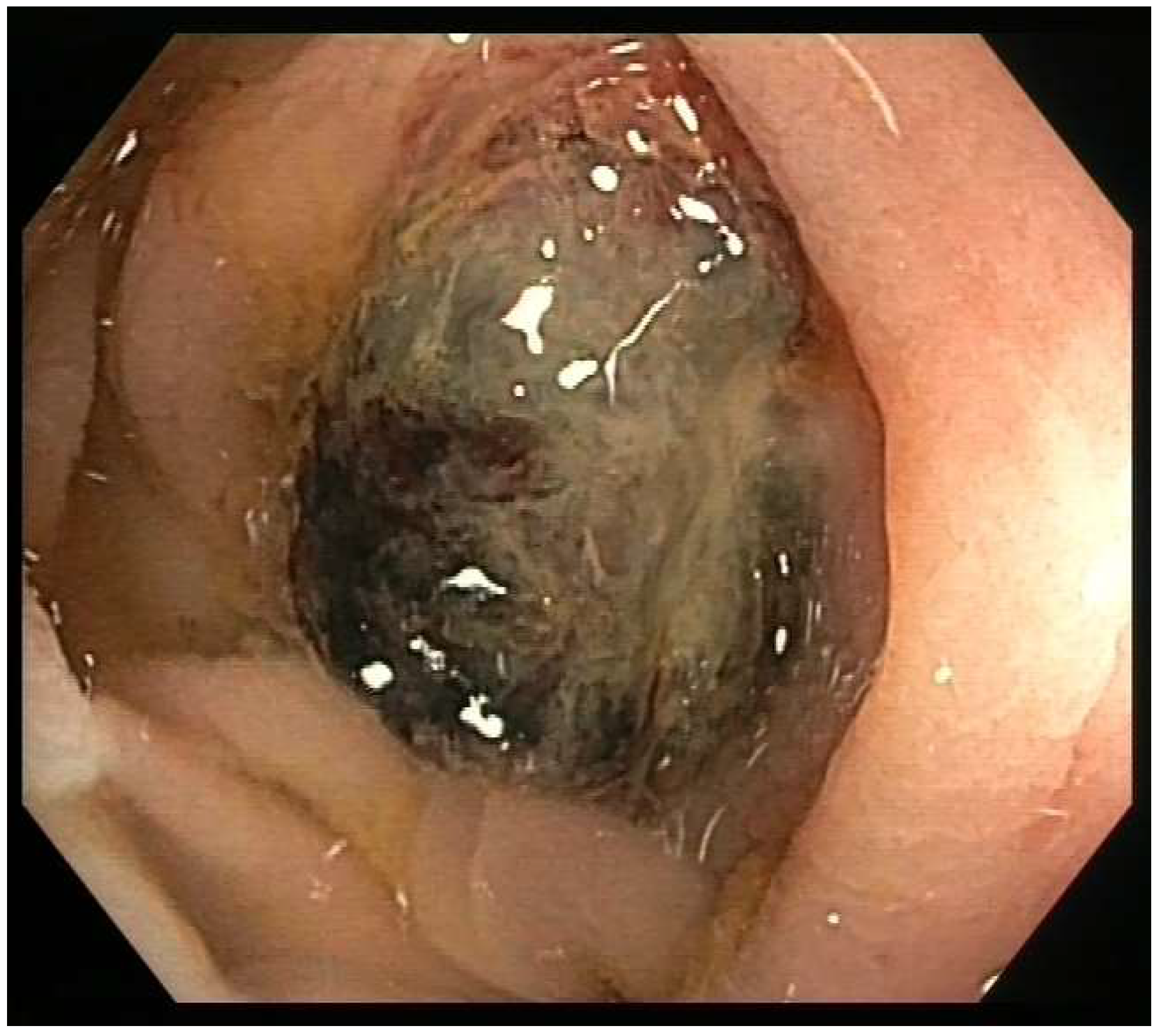
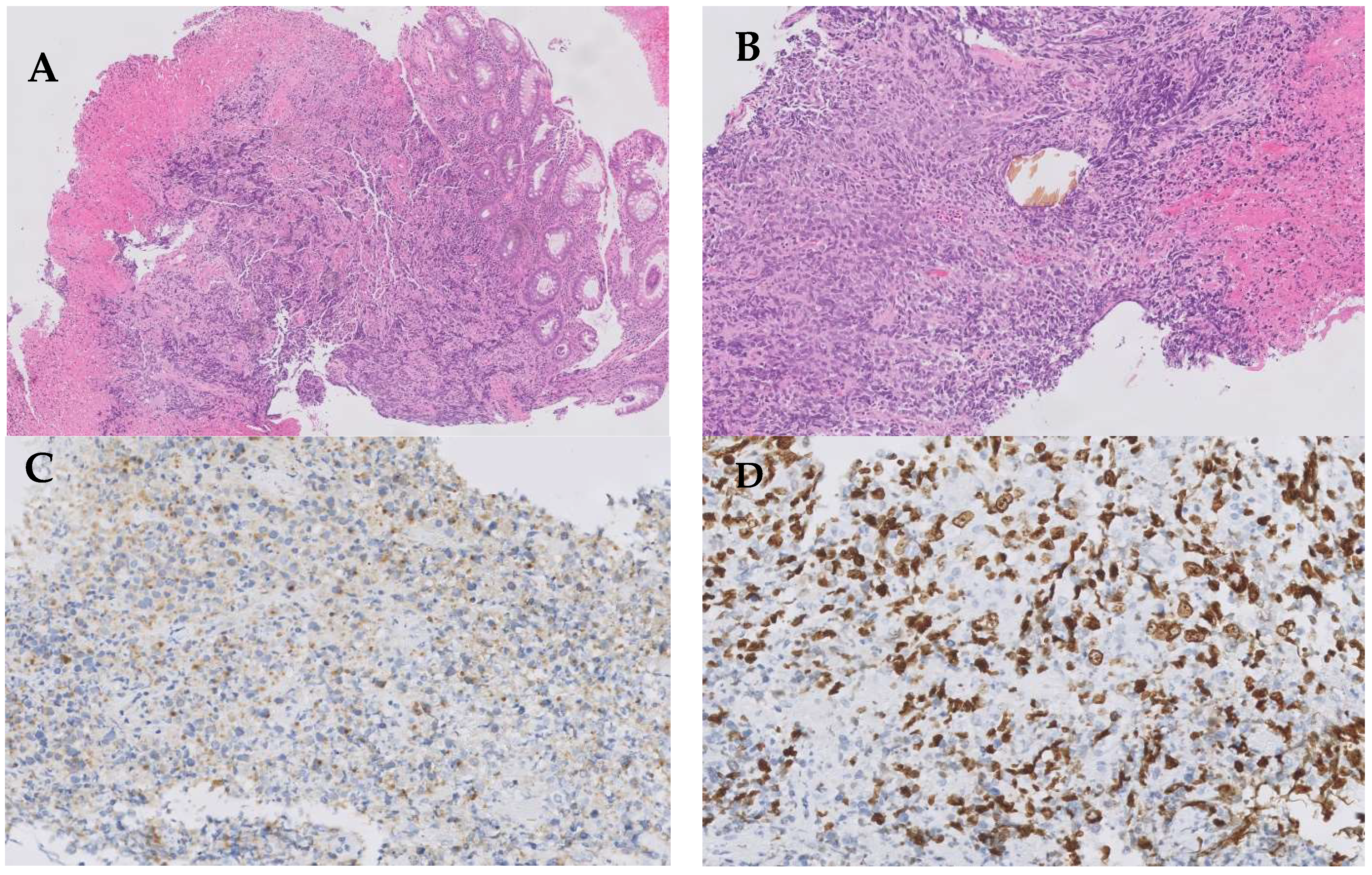
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gazdar, A.F.; Carney, D.N.; Nau, M.M.; Minna, J.D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985, 45, 2924–2930. [Google Scholar] [PubMed]
- Noguchi, M.; Furukawa, K.T.; Morimoto, M. Pulmonary neuroendocrine cells: Physiology, tissue homeostasis and disease. Dis. Model. Mech. 2020, 13, 12. [Google Scholar] [CrossRef]
- Frazier, S.R.; Kaplan, P.A.; Loy, T.S. The pathology of extrapulmonary small cell carcinoma. Semin. Oncol. 2007, 34, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers. 2021, 7, 1. [Google Scholar] [CrossRef]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Howard, S.; O’Regan, K.; Jagannathan, J.; Krajewski, K.; Giardino, A.; Ramaiya, N. Extrapulmonary small cell carcinoma: A picto-rial review. AJR Am. J. Roentgenol. 2011, 197, W392–W398. [Google Scholar] [CrossRef] [PubMed]
- Symeonidis, D.; Papageorgiou, G.; Liatsos, A.; Trihia, H.; Lianos, E.; Kosmas, C. High grade neuroendocrine carcinoma of the breast, first line and maintenance immunotherapy. Anticancer Drugs 2022, 33, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, D.; Meng, Y.; Guo, Y.; Zhao, M. Extrapulmonary small cell carcinoma of the external auditory canal: A case report and review of the literature. J. Int. Med. Res. 2020, 48, 8. [Google Scholar] [CrossRef]
- Meinhardt, A.L.; Uppuluri, A.; Shkolnik, E.; Chang, V.T. Extrapulmonary small cell carcinoma presenting as an orbital mass: A case report. Cureus 2022, 14, 6. [Google Scholar] [CrossRef]
- Han, G.; Wang, Z.; Guo, X.; Wang, M.; Wu, H.; Liu, D. Extrapulmonary small cell neuroendocrine carcinoma of the paranasal sinuses: A case report and review of the literature. J. Oral Maxillofac. Surg. 2022, 70, 2347–2351. [Google Scholar] [CrossRef]
- Silva-Meléndez, P.E.; Escobar, P.F.; None, H.S.; Gutiérrez, S.; Rodríguez, M. Small cell carcinoma of the uterine cervix: A case report and literature review. Bol. Asoc. Med. P.R. 2015, 107, 1. [Google Scholar]
- Reckova, M.; Mego, M.; Rejlekova, K.; Sycova-Mila, Z.; Obertova, Z.; Mardiak, J. Small-cell carcinoma of the ovary with breast metastases: A case report. Klin. Onkol. 2010, 23, 1. [Google Scholar]
- Venkatesh, P.K.; Motwani, B.; Tilahun, E.; Feldman, L.; Ying, S.C.; Garon, J.E.; Feldman, L. Metastatic pure small-cell carcinoma of prostate. Am. J. Med. Sci. 2004, 328, 286–289. [Google Scholar] [CrossRef]
- González-Lois, C.; Madero, S.; Redondo, P.; Alonso, I.; Salas, A.; Montalbán, M.A. Small cell carcinoma of the kidney. Arch. Pathol. Lab. Med. 2001, 125, 796–798. [Google Scholar] [CrossRef]
- Popa, O.; Taban, S.; Pantea, S.; Plopeanu, A.; Barna, R.; Cornianu, M.; Pascu, A.-A.; Dema, A.L.C. The new WHO classification of gastrointestinal neuroendocrine tumors and immunohistochemical expression of somatostatin receptor 2 and 5. Exp. Ther. Med. 2021, 22, 4. [Google Scholar] [CrossRef]
- Wong, Y.N.; Jack, R.H.; Mak, V.; Henrik, M.; Davies, E.A. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970–2004. BMC Cancer 2009, 9, 209. [Google Scholar] [CrossRef]
- Brenner, B.; Tang, L.H.; Klimstra, D.S.; Kelsen, D.P. Small-cell carcinomas of the gastrointestinal tract: A review. J. Clin. Oncol. 2004, 22, 2730–2739. [Google Scholar] [CrossRef]
- Shaun, S.J.; Hung, M.D. Small Cell Carcinoma of the Colon: A Case Report and Literature Review. J. Clin. Gastroenterol. 1989, 11, 335–339. [Google Scholar]
- Rubin, A.; Pandya, P.P. Small cell neuroendocrine carcinoma of the rectum associated with chronic ulcerative colitis. Histopathology. 1990, 16, 95–97. [Google Scholar] [CrossRef]
- Grassia, R.; Bodini, P.; Dizioli, P.; Staiano, T.; Iiritano, E.; Bianchi, G.; Buffoli, F. Neuroendocrine carcinomas arising in ulcerative colitis: Coincidences or possible correlations? World J. Gastroenterol. 2009, 15, 4193–4195. [Google Scholar] [CrossRef]
- Ling, D.; Chirchir, M.; Alzahrani, N.; Morris, D.L. Small cell neuroendocrine carcinoma arising in cystic duplication of colon. ANZ J. Surg. 2022, 92, 608–610. [Google Scholar] [PubMed]
- Peng, C.; Shen, S.; Zhang, X.; Zou, X. Limited stage small cell carcinoma of the gastrointestinal tract: A clinicopathologic and prognostic analysis of 27 cases. Rare Tumors 2013, 5, 21–26. [Google Scholar]
- Yazici, O.; Ozdemir, N.Y.; Sendur, M.A.N.; Aksoy, S.; Zengin, N. Current approaches for prophylactic cranial irradiation in extrapulmonary small cell carcinoma. Curr. Med. Res. Opin. 2014, 30, 1327–1336. [Google Scholar]
- Fenoglio-Preiser, C.M. Gastrointestinal neuroendocrine/neuroectodermal tumors. Am. J. Clin. Pathol. 2001, 115, S79–S93. [Google Scholar] [PubMed]
- Ni, S.-J. Pathologic research update of colorectal neuroendocrine tumors. World J. Gastroenterol. 2010, 16, 14. [Google Scholar]
- Shida, T.; Furuya, M.; Nikaido, T.; Kishimoto, T.; Koda, K.; Oda, K.; Nakatani, Y.; Miyazaki, M.; Ishikura, H. Aberrant expression of human achaete-scute homologue gene 1 in the gastrointestinal neuroendocrine carcinomas. Clin. Cancer Res. 2005, 11, 450–458. [Google Scholar]
- Juhlin, C.C. Second-generation neuroendocrine immunohistochemical markers: Reflections from clinical implementation. Biology 2021, 10, 9. [Google Scholar]
- Balasubramanyam, S.; O’Donnell, B.P.; Musher, B.L.; Jhaveri, P.M.; Ludwig, M.S. Evaluating treatment patterns for small cell carcinoma of the colon using the National Cancer Database (NCDB). J. Gastrointest. Cancer 2019, 50, 244–253. [Google Scholar]
- Grossman, R.A.; Pedroso, F.E.; Byrne, M.M.; Koniaris, L.G.; Misra, S. Does surgery or radiation therapy impact survival for patients with extrapulmonary small cell cancers?: Treating Extrapulmonary Small Cell Cancer. J. Surg. Oncol. 2011, 104, 604–612. [Google Scholar]
- Ulanja, M.B.; Beutler, B.D.; Antwi-Amoabeng, D.; Governor, S.B.; Rahman, G.A.; Djankpa, F.T.; Alese, O.B. ASO author reflections: Prognostic factors in gastrointestinal extrapulmonary small cell carcinoma using real-world data. Ann. Surg. Oncol. 2022, 29, 8261–8262. [Google Scholar]
- Sato, Y.; Fujisawa, J.; Saji, Y.; Misawa, K.; Yabuki, H.; Kotani, H.; Olii, Y.; Minemoto, H.; Kamada, T.; Saito, H. A case of small cell undifferentiated carcinoma (SCUC) of the rectum treated with etoposide, cis-platinum and radiotherapy. Gan To Kagaku Ryoho 1992, 19, 2245–2249. [Google Scholar] [PubMed]
- Jereczek-Fossa, B.; Airoldi, M.; Vasario, E.; Redda, M.G.; Valente, G.; Orecchia, R. Small cell carcinoma of the esophagus: A case report and review of the literature. Tumori 2000, 86, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [PubMed]
- Paz-Ares, L.; Champiat, S.; Lai, W.V.; Izumi, H.; Govindan, R.; Boyer, M.; Hummel, H.-D.; Borghaei, H.; Johnson, M.L.; Steeghs, N.; et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell engager, in recurrent Small-cell lung cancer: An open-label, phase I study. J. Clin. Oncol. 2023, 41, 2893–2903. [Google Scholar]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar]
- Iwase, T.; Masuda, Y.; Suzuki, T.; Takahashi, O.; Miyazaki, M. Advanced small-cell colon carcinoma: A case report. J. Med. Case Rep. 2013, 7, 74. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alía Navarro, V.; Martínez Delfrade, Í.; De Frutos González, B.; Morón García, B.; Barrill Corpa, A.M.; Sotoca Rubio, P.; Peñas García, B.; Ferrer Gómez, A.; Perna Monroy, C.; Ferreiro Monteagudo, R. Localized Colonic Small-Cell Carcinoma with Pathological Complete Response after Neoadjuvant Cisplatin and Etoposide: A Case Report. Curr. Oncol. 2023, 30, 8426-8433. https://doi.org/10.3390/curroncol30090613
Alía Navarro V, Martínez Delfrade Í, De Frutos González B, Morón García B, Barrill Corpa AM, Sotoca Rubio P, Peñas García B, Ferrer Gómez A, Perna Monroy C, Ferreiro Monteagudo R. Localized Colonic Small-Cell Carcinoma with Pathological Complete Response after Neoadjuvant Cisplatin and Etoposide: A Case Report. Current Oncology. 2023; 30(9):8426-8433. https://doi.org/10.3390/curroncol30090613
Chicago/Turabian StyleAlía Navarro, Víctor, Íñigo Martínez Delfrade, Belén De Frutos González, Blanca Morón García, Ana María Barrill Corpa, Pilar Sotoca Rubio, Beatriz Peñas García, Ana Ferrer Gómez, Cristian Perna Monroy, and Reyes Ferreiro Monteagudo. 2023. "Localized Colonic Small-Cell Carcinoma with Pathological Complete Response after Neoadjuvant Cisplatin and Etoposide: A Case Report" Current Oncology 30, no. 9: 8426-8433. https://doi.org/10.3390/curroncol30090613
APA StyleAlía Navarro, V., Martínez Delfrade, Í., De Frutos González, B., Morón García, B., Barrill Corpa, A. M., Sotoca Rubio, P., Peñas García, B., Ferrer Gómez, A., Perna Monroy, C., & Ferreiro Monteagudo, R. (2023). Localized Colonic Small-Cell Carcinoma with Pathological Complete Response after Neoadjuvant Cisplatin and Etoposide: A Case Report. Current Oncology, 30(9), 8426-8433. https://doi.org/10.3390/curroncol30090613




