Palliative Gastrectomy Improves the Survival of Patients with Metastatic Early-Onset Gastric Cancer: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database and Patients
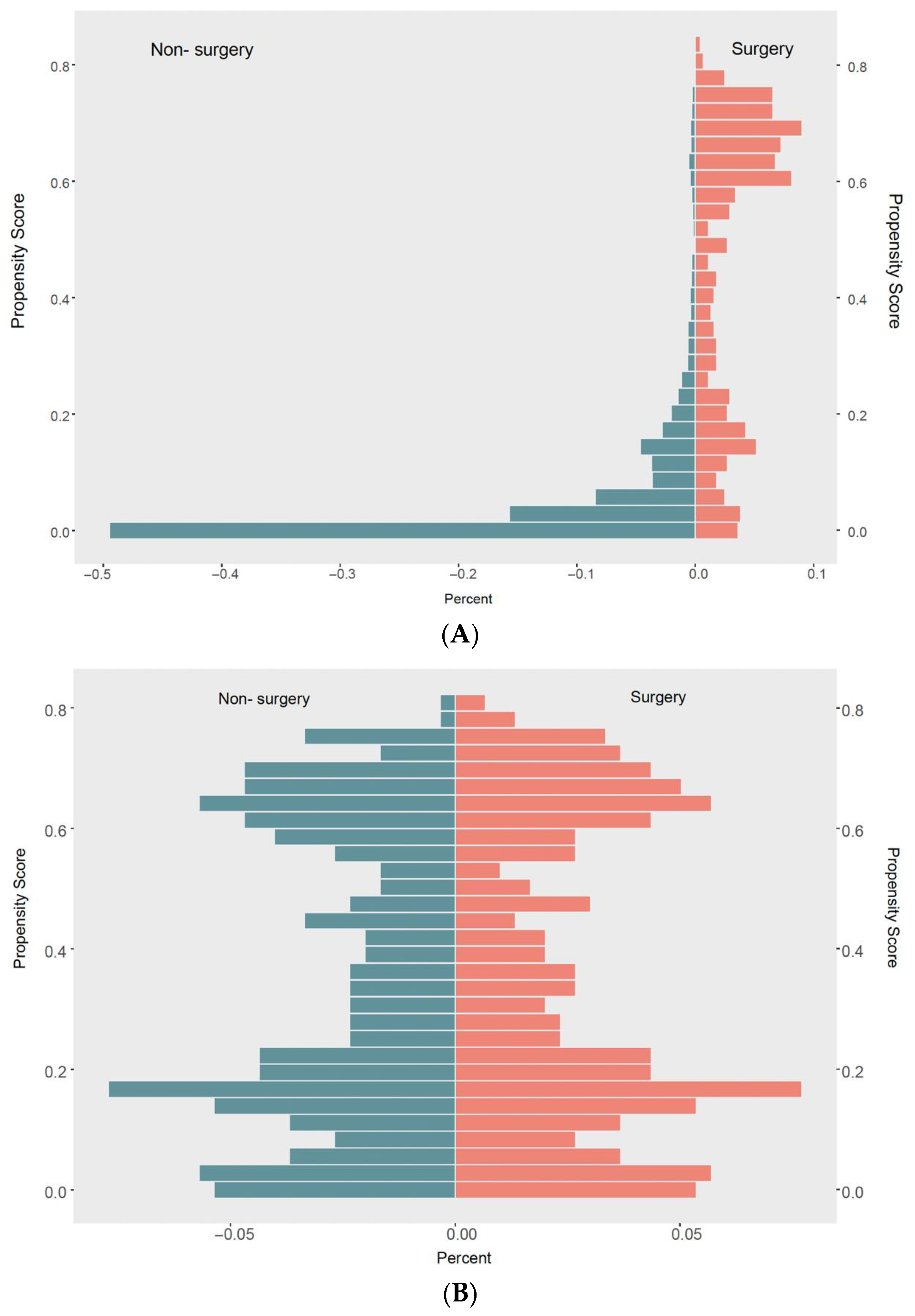
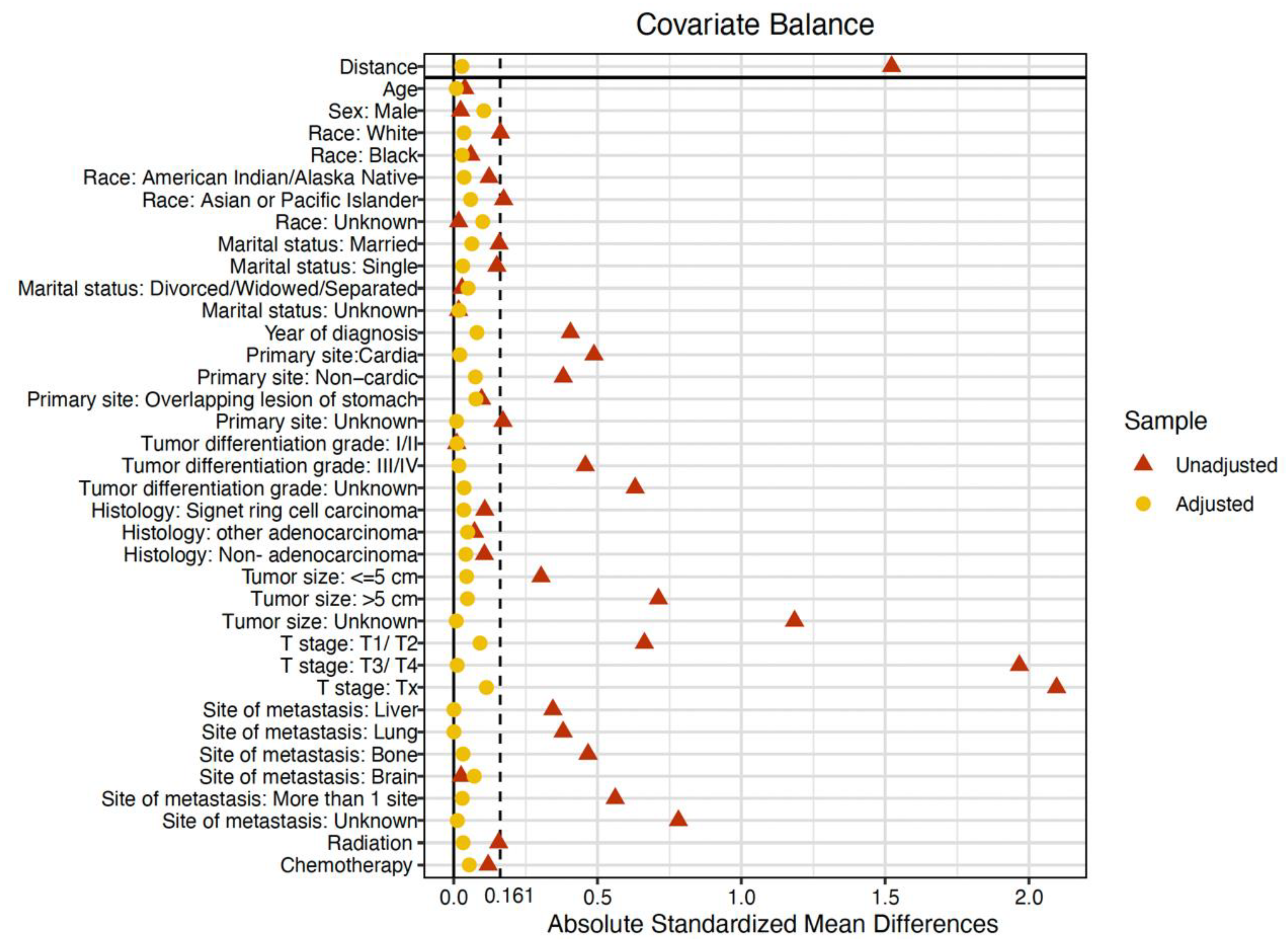
2.2. Propensity Score Matching
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
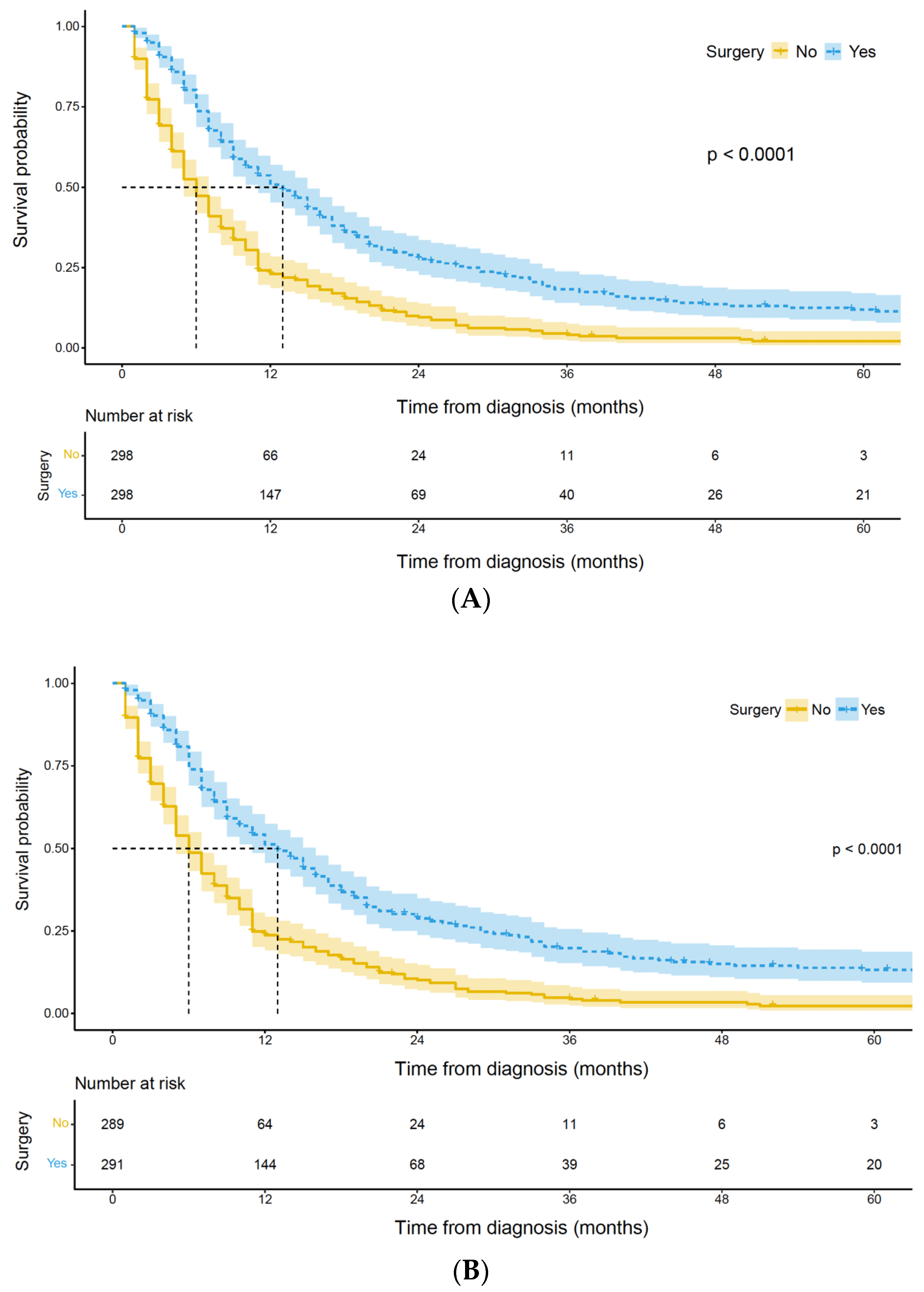
3.2. Survival Analysis of Palliative Gastrectomy
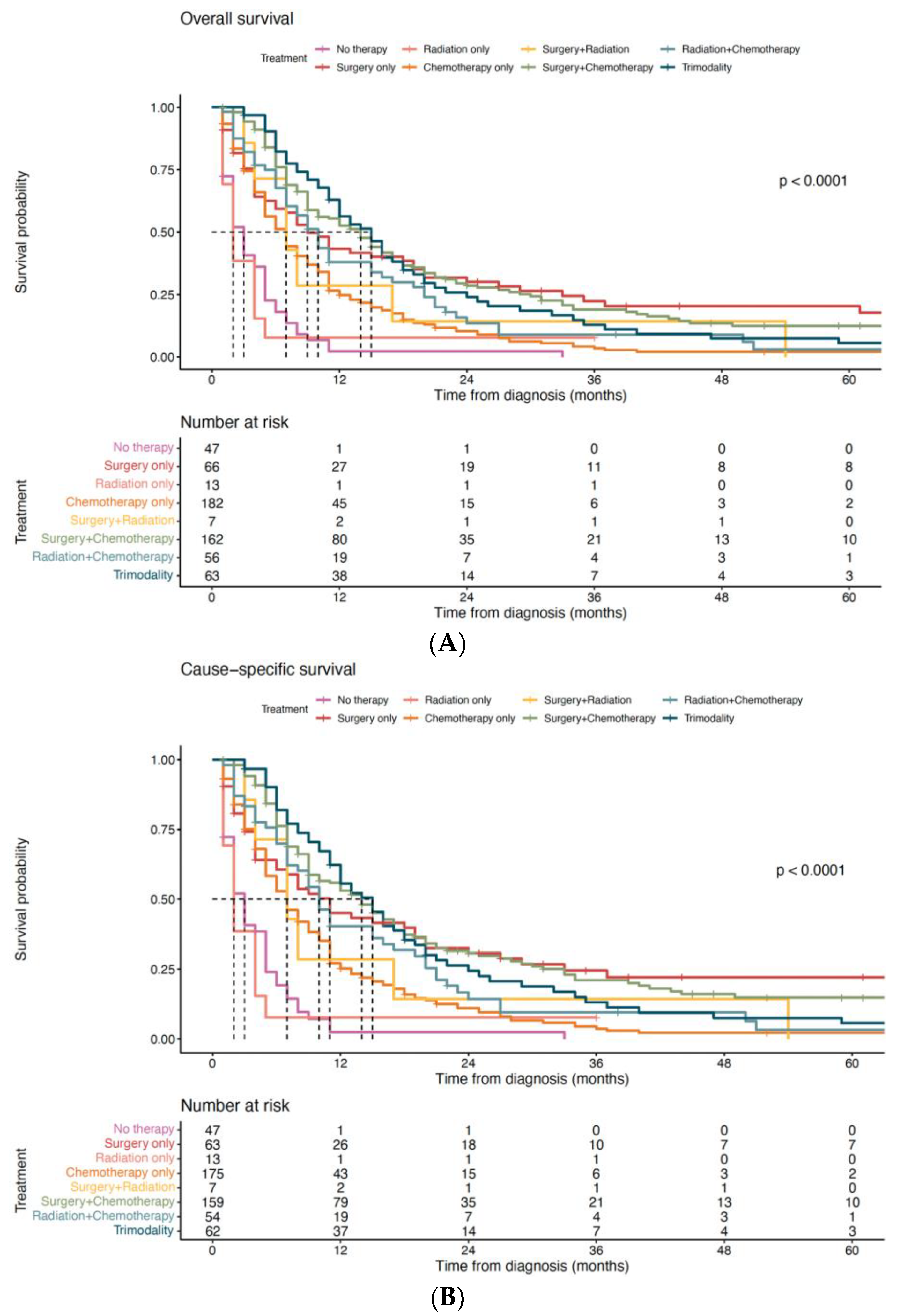
3.3. Impact of Treatment Modality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zheng, C.; Gao, Z.M.; Huang, H.B.; Li, K.; Liu, X.F. Prognostic Significance of Palliative Gastrectomy in Incurable Advanced Gastric Cancer: A Retrospective Cohort Study and Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2299–2312. [Google Scholar]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Thrumurthy, S.G.; Chaudry, M.A.; Chau, I.; Allum, W. Does Surgery Have a Role in Managing Incurable Gastric Cancer? Nat. Rev. Clin. Oncol. 2015, 12, 676–682. [Google Scholar] [CrossRef]
- Li, Q.; Zou, J.; Jia, M.; Li, P.; Zhang, R.; Han, J.; Huang, K.; Qiao, Y.; Xu, T.; Peng, R.; et al. Palliative Gastrectomy and Survival in Patients with Metastatic Gastric Cancer: A Propensity Score-Matched Analysis of a Large Population-Based Study. Clin. Transl. Gastroenterol. 2019, 10, e00048. [Google Scholar] [CrossRef]
- Dittmar, Y.; Rauchfuss, F.; Goetz, M.; Jandt, K.; Scheuerlein, H.; Heise, M.; Settmacher, U. Non-Curative Gastric Resection for Patients with Stage 4 Gastric Cancer—A Single Center Experience and Current Review of Literature. Langenbecks Arch. Surg. 2012, 397, 745–753. [Google Scholar] [CrossRef]
- Nakajima, T.; Ota, K.; Ishihara, S.; Oyama, S.; Nishi, M.; Ohashi, Y.; Yanagisawa, A. Combined Intensive Chemotherapy and Radical Surgery for Incurable Gastric Cancer. Ann. Surg. Oncol. 1997, 4, 203–208. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Markar, S.R.; Phillips, A.W.; Salti, G.I.; Dahdaleh, F.; Griffiths, E.A. Palliative Gastrectomy for Metastatic Gastric Adenocarcinoma: A National Population-Based Cohort Study. Surgery 2021, 170, 1702–1710. [Google Scholar] [CrossRef]
- Cowling, J.; Gorman, B.; Riaz, A.; Bundred, J.R.; Kamarajah, S.K.; Evans, R.P.T.; Singh, P.; Griffiths, E.A. Peri-Operative Outcomes and Survival Following Palliative Gastrectomy for Gastric Cancer: A Systematic Review and Meta-Analysis. J. Gastrointest. Cancer 2021, 52, 41–56. [Google Scholar] [CrossRef]
- Warschkow, R.; Baechtold, M.; Leung, K.; Schmied, B.M.; Nussbaum, D.P.; Gloor, B.; Blazer Iii, D.G.; Worni, M. Selective Survival Advantage Associated with Primary Tumor Resection for Metastatic Gastric Cancer in a Western Population. Gastric Cancer 2018, 21, 324–337. [Google Scholar] [CrossRef]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef]
- Ławniczak, M.; Gawin, A.; Jaroszewicz-Heigelmann, H.; Rogoza-Mateja, W.; Białek, A.; Kulig, J.; Kaczmarczyk, M.; Starzyńska, T. Analysis of Clinicopathologic Characteristics of Gastric Cancer in Patients ≤40 and ≥40 Years of Age. Scand. J. Gastroenterol. 2020, 55, 62–66. [Google Scholar] [CrossRef]
- Koea, J.B.; Karpeh, M.S.; Brennan, M.F. Gastric Cancer in Young Patients: Demographic, Clinicopathological, and Prognostic Factors in 92 Patients. Ann. Surg. Oncol. 2000, 7, 346–351. [Google Scholar] [CrossRef]
- Bergquist, J.R.; Leiting, J.L.; Habermann, E.B.; Cleary, S.P.; Kendrick, M.L.; Smoot, R.L.; Nagorney, D.M.; Truty, M.J.; Grotz, T.E. Early-Onset Gastric Cancer Is a Distinct Disease with Worrisome Trends and Oncogenic Features. Surgery 2019, 166, 547–555. [Google Scholar] [CrossRef]
- Huang, Q.; Zheng, X.; Jiao, Y.; Lei, Y.; Li, X.; Bi, F.; Guo, F.; Wang, G.; Liu, M. A Distinct Clinicopathological Feature and Prognosis of Young Gastric Cancer Patients Aged ≤ 45 Years Old. Front. Oncol. 2021, 11, 674224. [Google Scholar] [CrossRef]
- Zhou, Q.; Tao, F.; Qiu, L.; Chen, H.; Bao, H.; Wu, X.; Shao, Y.; Chi, L.; Song, H. Somatic Alteration Characteristics of Early-Onset Gastric Cancer. J. Oncol. 2022, 2022, 1498053. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, J.W.; Liu, Y.; Park, Y.S.; Kim, J.H.; Yang, H.; Um, H.; Ko, W.R.; Lee, B.I.; Kwon, S.Y.; et al. Sporadic Early-Onset Diffuse Gastric Cancers Have High Frequency of Somatic CDH1 Alterations, but Low Frequency of Somatic RHOA Mutations Compared with Late-Onset Cancers. Gastroenterology 2017, 153, 536–549.e26. [Google Scholar] [CrossRef]
- About the SEER Program. Available online: https://seer.cancer.gov/about/overview.html (accessed on 2 December 2022).
- Park, H.J.; Ahn, J.Y.; Jung, H.-Y.; Lim, H.; Lee, J.H.; Choi, K.-S.; Kim, D.H.; Choi, K.D.; Song, H.J.; Lee, G.H.; et al. Clinical Characteristics and Outcomes for Gastric Cancer Patients Aged 18–30 Years. Gastric Cancer 2014, 17, 649–660. [Google Scholar] [CrossRef]
- Mb, A.; Fl, G.; Sb, E.; Cc, C.; Je, G.; Rk, B.; Meyer, L.; Dm, G.; Dr, B.; Dp, W. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Austin, P.C. Balance Diagnostics for Comparing the Distribution of Baseline Covariates between Treatment Groups in Propensity-Score Matched Samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Amoah, J.; Stuart, E.A.; Cosgrove, S.E.; Harris, A.D.; Han, J.H.; Lautenbach, E.; Tamma, P.D. Comparing Propensity Score Methods Versus Traditional Regression Analysis for the Evaluation of Observational Data: A Case Study Evaluating the Treatment of Gram-Negative Bloodstream Infections. Clin. Infect. Dis. 2020, 71, e497–e505. [Google Scholar] [CrossRef] [PubMed]
- Elze, M.C.; Gregson, J.; Baber, U.; Williamson, E.; Sartori, S.; Mehran, R.; Nichols, M.; Stone, G.W.; Pocock, S.J. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J. Am. Coll. Cardiol. 2017, 69, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, N.; Toubiana, J.; Antona, D.; Javouhey, E.; Madhi, F.; Lorrot, M.; Léger, P.-L.; Galeotti, C.; Claude, C.; Wiedemann, A.; et al. Association of Intravenous Immunoglobulins Plus Methylprednisolone vs. Immunoglobulins Alone with Course of Fever in Multisystem Inflammatory Syndrome in Children. JAMA 2021, 325, 855–864. [Google Scholar] [CrossRef]
- Takeshima, N.; Sozu, T.; Tajika, A.; Ogawa, Y.; Hayasaka, Y.; Furukawa, T.A. Which Is More Generalizable, Powerful and Interpretable in Meta-Analyses, Mean Difference or Standardized Mean Difference? BMC Med. Res. Methodol. 2014, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Samarasam, I.; Chandran, B.S.; Sitaram, V.; Perakath, B.; Nair, A.; Mathew, G. Palliative Gastrectomy in Advanced Gastric Cancer: Is It Worthwhile? ANZ J. Surg. 2006, 76, 60–63. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, D.; Wang, F.; Wang, Z.; Luo, H.; Jin, Y.; Wei, X.; Xu, R. The Role of Non-Curative Surgery in Incurable, Asymptomatic Advanced Gastric Cancer. PLoS ONE 2013, 8, e83921. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ma, T.; Xu, H.; Wu, Z.; Wu, C.; Sun, G. Survival Benefits of Palliative Gastrectomy in Stage IV Gastric Cancer: A Propensity Score Matched Analysis. J. Gastrointest. Oncol. 2020, 11, 376–385. [Google Scholar] [CrossRef]
- He, X.; Lai, S.; Su, T.; Liu, Y.; Ding, Y.; Quan, S.; Si, J.; Sun, L. Survival Benefits of Gastrectomy in Gastric Cancer Patients with Stage IV: A Population-Based Study. Oncotarget 2017, 8, 106577–106586. [Google Scholar] [CrossRef]
- Omori, H.; Tanizawa, Y.; Makuuchi, R.; Irino, T.; Bando, E.; Kawamura, T.; Terashima, M. Role of Palliative Resection in Patients with Incurable Advanced Gastric Cancer Who Are Unfit for Chemotherapy. World J. Surg. 2019, 43, 571–579. [Google Scholar] [CrossRef]
- Fujitani, K.; Yang, H.-K.; Mizusawa, J.; Kim, Y.-W.; Terashima, M.; Han, S.-U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric Cancer with a Single Non-Curable Factor (REGATTA): A Phase 3, Randomised Controlled Trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef]
- Zhou, Q.-P.; Ge, Y.-H.; Liu, C.-Y. Comparison of Metastasis between Early-Onset and Late-Onset Gastric Signet Ring Cell Carcinoma. BMC Gastroenterol. 2020, 20, 380. [Google Scholar] [CrossRef]
- Theuer, C.P.; Kurosaki, T.; Taylor, T.H.; Anton-Culver, H. Unique Features of Gastric Carcinoma in the Young: A Population-Based Analysis. Cancer 1998, 83, 25–33. [Google Scholar] [CrossRef]
- Qu, X.; Zhao, X.; Liu, Y.; Wang, N.; Zhang, L.; Zhu, X.; Dong, Q.; Liu, J.; Shi, Y. The Clinicopathological Characteristics of Early-Onset Gastric Cancer and Its Evolutionary Trends: A Retrospective Study. Am. J. Cancer Res. 2022, 12, 2757–2769. [Google Scholar]
- Seib, C.D.; Rochefort, H.; Chomsky-Higgins, K.; Gosnell, J.E.; Suh, I.; Shen, W.T.; Duh, Q.-Y.; Finlayson, E. Association of Patient Frailty with Increased Morbidity After Common Ambulatory General Surgery Operations. JAMA Surg. 2018, 153, 160–168. [Google Scholar] [CrossRef]
- Froehner, M.; Brausi, M.A.; Herr, H.W.; Muto, G.; Studer, U.E. Complications Following Radical Cystectomy for Bladder Cancer in the Elderly. Eur. Urol. 2009, 56, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.E.; Roth, J.A. Cancer-Induced Immunosuppression: Implications for Therapy? Semin. Surg. Oncol. 1989, 5, 414–419. [Google Scholar] [CrossRef]
- Hsu, J.-T.; Liao, J.-A.; Chuang, H.-C.; Chen, T.-D.; Chen, T.-H.; Kuo, C.-J.; Lin, C.-J.; Chou, W.-C.; Yeh, T.-S.; Jan, Y.-Y. Palliative Gastrectomy Is Beneficial in Selected Cases of Metastatic Gastric Cancer. BMC Palliat. Care 2017, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Cotte, E.; Glehen, O.; Lotti, M.; Poiasina, E.; Catena, F.; Yonemura, Y.; Ansaloni, L. Intraperitoneal Chemotherapy in Advanced Gastric Cancer. Meta-Analysis of Randomized Trials. Eur. J. Surg. Oncol. 2014, 40, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.R.; Han, D.S.; Kong, S.-H.; Lee, H.-J.; Kim, S.H.; Kim, W.H.; Yang, H.-K. The Value of Palliative Gastrectomy in Gastric Cancer with Distant Metastasis. Ann. Surg. Oncol. 2012, 19, 1231–1239. [Google Scholar] [CrossRef]

| Overall (n = 3641) | Non-Surgery (n = 3199) | Surgery (n = 442) | p-Value | Standardized Mean Difference | |
|---|---|---|---|---|---|
| Age, year | 41.7 ± 7.1 | 41.6 ± 7.1 | 41.9 ± 6.7 | 0.483 | 0.036 |
| Sex | 0.688 | 0.023 | |||
| Female | 1395 (38.3%) | 1230 (38.4%) | 165 (37.3%) | ||
| Male | 2246 (61.7%) | 1969 (61.6%) | 277 (62.7%) | ||
| Race | <0.001 | 0.225 | |||
| White | 2564 (70.4%) | 2283 (71.4%) | 281 (63.6%) | ||
| Black | 461 (12.7%) | 397 (12.4%) | 64 (14.5%) | ||
| American Indian/Alaska Native | 70 (1.9%) | 66 (2.1%) | 4 (0.9%) | ||
| Asian or Pacific Islander | 526 (14.4%) | 435 (13.6%) | 91 (20.6%) | ||
| Unknown | 20 (0.5%) | 18 (0.6%) | 2 (0.5%) | ||
| Marital status | 0.021 | 0.161 | |||
| Married | 2013 (55.3%) | 1739 (54.4%) | 274 (62.0%) | ||
| Single | 1157 (31.8%) | 1042 (32.6%) | 115 (26.0%) | ||
| Divorced/Widowed/Separated | 321 (8.8%) | 285 (8.9%) | 36 (8.1%) | ||
| Unknown | 150 (4.1%) | 133 (4.2%) | 17 (3.8%) | ||
| Year of diagnosis | 2011 (2007, 2015) | 2011 (2007, 2015) | 2009 (2005, 2013) | <0.001 | 0.401 |
| Primary site | <0.001 | 0.493 | |||
| Cardia | 955 (26.2%) | 901 (28.2%) | 54 (12.2%) | ||
| Non-cardia | 1491 (41.0%) | 1237 (38.7%) | 254 (57.5%) | ||
| Overlapping lesion of stomach | 419 (11.5%) | 355 (11.1%) | 64 (14.5%) | ||
| Unknown | 776 (21.3%) | 706 (22.1%) | 70 (15.8%) | ||
| Tumor size | <0.001 | 1.172 | |||
| ≤5 cm | 644 (17.7%) | 512 (16.0%) | 132 (29.9%) | ||
| >5 cm | 602 (16.5%) | 391 (12.2%) | 211 (47.7%) | ||
| Unknown | 2395 (65.8%) | 2296 (71.8%) | 99 (22.4%) | ||
| T stage | <0.001 | 1.507 | |||
| T1/ T2 | 660 (18.1%) | 637 (19.9%) | 23 (5.2%) | ||
| T3/ T4 | 1423 (39.1%) | 1024 (32.0%) | 399 (90.3%) | ||
| Tx | 1558 (42.8%) | 1538 (48.1%) | 20 (4.5%) | ||
| N stage | <0.001 | 1.472 | |||
| N0 | 1120 (30.8%) | 1068 (33.4%) | 52 (11.8%) | ||
| N1 | 1208 (33.2%) | 1062 (33.2%) | 146 (33.0%) | ||
| N2 | 221 (6.1%) | 94 (2.9%) | 127 (28.7%) | ||
| N3 | 168 (4.6%) | 64 (2.0%) | 104 (23.5%) | ||
| Nx | 924 (25.4%) | 911 (28.5%) | 13 (2.9%) | ||
| Histology | 0.038 | 0.134 | |||
| Signet ring cell carcinoma | 1306 (35.9%) | 1127 (35.2%) | 179 (40.5%) | ||
| Other adenocarcinoma | 2181 (59.9%) | 1930 (60.3%) | 251 (56.8%) | ||
| Non-adenocarcinoma | 154 (4.2%) | 142 (4.4%) | 12 (2.7%) | ||
| Tumor differentiation grade | |||||
| I/II | 405 (11.1%) | 357 (11.2) | 48 (10.9) | <0.001 | 0.490 |
| III/IV | 2343 (64.4%) | 1988 (62.1) | 355 (80.3) | ||
| Unknown | 893 (24.5%) | 854 (26.7) | 39 (8.8) | ||
| Metastasis to the liver a | <0.001 | 0.426 | |||
| Yes | 655 (18.0%) | 625 (19.5%) | 30 (6.8%) | ||
| No | 1483 (40.7%) | 1312 (41.0%) | 171 (38.7%) | ||
| Unknown | 1503 (41.3%) | 1262 (39.4%) | 241 (54.5%) | ||
| Metastasis to the lung a | <0.001 | 0.404 | |||
| Yes | 265 (7.3%) | 259 (8.1%) | 6 (1.4%) | ||
| No | 1859 (51.1%) | 1665 (52.0%) | 194 (43.9%) | ||
| Unknown | 1517 (41.7%) | 1275 (39.9%) | 242 (54.8%) | ||
| Metastasis to the bone a | <0.001 | 0.454 | |||
| Yes | 361 (9.9%) | 354 (11.1%) | 7 (1.6%) | ||
| No | 1774 (48.7%) | 1580 (49.4%) | 194 (43.9%) | ||
| Unknown | 1506 (41.4%) | 1265 (39.5%) | 241 (54.5%) | ||
| Metastasis to the brain a | <0.001 | 0.313 | |||
| Yes | 39 (1.1%) | 38 (1.2%) | 1 (0.2%) | ||
| No | 2088 (57.3%) | 1888 (59.0%) | 200 (45.2%) | ||
| Unknown | 1514 (41.6%) | 1273 (39.8%) | 241 (54.5%) | ||
| Radiation | <0.001 | 0.165 | |||
| Yes | 638 (17.5%) | 535 (16.7%) | 103 (23.3%) | ||
| No | 3003 (82.5%) | 2664 (83.3%) | 339 (76.7%) | ||
| Chemotherapy | 0.014 | 0.124 | |||
| Yes | 2853 (78.4%) | 2527 (79.0%) | 326 (73.8%) | ||
| No | 788 (21.6%) | 672 (21.0%) | 116 (26.2%) |
| Overall (n = 596) | Non-Surgery (n = 298) | Surgery (n = 298) | p-Value | Standardized Mean Difference | |
|---|---|---|---|---|---|
| Age, year | 41.8 ± 6.7 | 41.7 ± 6.6 | 41.8 ± 6.8 | 0.765 | 0.025 |
| Sex | 0.357 | 0.082 | |||
| Female | 236 (39.6%) | 124 (41.6%) | 112 (37.6%) | ||
| Male | 360 (60.4%) | 174 (58.4%) | 186 (62.4%) | ||
| Race | 0.982 | 0.052 | |||
| White | 392 (65.8%) | 194 (65.1%) | 198 (66.4%) | ||
| Black | 79 (13.3%) | 40 (13.4%) | 39 (13.1%) | ||
| American Indian/Alaska Native | 7 (1.2%) | 4 (1.3%) | 3 (1.0%) | ||
| Asian or Pacific Islander | 113 (19.0%) | 57 (19.1%) | 56 (18.8%) | ||
| Unknown | 5 (0.8%) | 3 (1.0%) | 2 (0.7%) | ||
| Marital status | 0.994 | 0.024 | |||
| Married | 357 (59.9%) | 178 (59.7%) | 179 (60.1%) | ||
| Single | 165 (27.7%) | 83 (27.9%) | 82 (27.5%) | ||
| Divorced/Widowed/Separated | 57 (9.6%) | 29 (9.7%) | 28 (9.4%) | ||
| Unknown | 17 (2.9%) | 8 (2.7%) | 9 (3.0%) | ||
| Year of diagnosis | 2009 (2006, 2014) | 2010 (2006, 2014) | 2009 (2006, 2013) | 0.548 | 0.053 |
| Primary site | 0.779 | 0.086 | |||
| Cardia | 100 (16.8%) | 46 (15.4%) | 54 (18.1%) | ||
| Non-cardia | 315 (52.9%) | 158 (53.0%) | 157 (52.7%) | ||
| Overlapping lesion of stomach | 82 (13.8%) | 44 (14.8%) | 38 (12.8%) | ||
| Unknown | 99 (16.6%) | 50 (16.8%) | 49 (16.4%) | ||
| Tumor size | 0.981 | 0.016 | |||
| ≤5 cm | 174 (29.2%) | 87 (29.2%) | 87 (29.2%) | ||
| >5 cm | 226 (37.9%) | 114 (38.3%) | 112 (37.6%) | ||
| Unknown | 196 (32.9%) | 97 (32.6%) | 99 (33.2%) | ||
| T stage | 0.680 | 0.072 | |||
| T1/ T2 | 43 (7.2%) | 20 (6.7%) | 23 (7.7%) | ||
| T3/ T4 | 508 (85.2%) | 253 (84.9%) | 255 (85.6%) | ||
| Tx | 45 (7.6%) | 25 (8.4%) | 20 (6.7%) | ||
| N stage | <0.001 | 1.119 | |||
| N0 | 139 (23.3%) | 98 (32.9%) | 41 (13.8%) | ||
| N1 | 220 (36.9%) | 116 (38.9%) | 104 (34.9%) | ||
| N2 | 94 (15.8%) | 15 (5.0%) | 79 (26.5%) | ||
| N3 | 70 (11.7%) | 8 (2.7%) | 62 (20.8%) | ||
| Nx | 73 (12.2%) | 61 (20.5%) | 12 (4.0%) | ||
| Histology | 0.410 | 0.110 | |||
| Signet ring cell carcinoma | 242 (40.6%) | 120 (40.3%) | 122 (40.9%) | ||
| Other adenocarcinoma | 325 (54.5%) | 160 (53.7%) | 165 (55.4%) | ||
| Non-adenocarcinoma | 29 (4.9%) | 18 (6.0%) | 11 (3.7%) | ||
| Tumor differentiation grade | 0.659 | 0.075 | |||
| I/II | 72 (12.1%) | 33 (11.1%) | 39 (13.1%) | ||
| III/IV | 449 (75.3%) | 225 (75.5%) | 224 (75.2%) | ||
| Unknown | 75 (12.6%) | 40 (13.4%) | 35 (11.7%) | ||
| Metastasis to the liver a | 0.989 | 0.012 | |||
| Yes | 55 (9.2%) | 28 (9.4%) | 27 (9.1%) | ||
| No | 230 (38.6%) | 115 (38.6%) | 115 (38.6%) | ||
| Unknown | 311 (52.2%) | 155 (52.0%) | 156 (52.3%) | ||
| Metastasis to the lung a | 0.986 | 0.014 | |||
| Yes | 12 (2.0%) | 6 (2.0%) | 6 (2.0%) | ||
| No | 272 (45.6%) | 137 (46.0%) | 135 (45.3%) | ||
| Unknown | 312 (52.3%) | 155 (52.0%) | 157 (52.7%) | ||
| Metastasis to the bone a | 0.986 | 0.014 | |||
| Yes | 14 (2.3%) | 7 (2.3%) | 7 (2.3%) | ||
| No | 272 (45.6%) | 137 (46.0%) | 135 (45.3%) | ||
| Unknown | 310 (52.0%) | 154 (51.7%) | 156 (52.3%) | ||
| Metastasis to the brain a | 0.845 | 0.048 | |||
| Yes | 3 (0.5%) | 2 (0.7%) | 1 (0.3%) | ||
| No | 282 (47.3%) | 141 (47.3%) | 141 (47.3%) | ||
| Unknown | 311 (52.2%) | 155 (52.0%) | 156 (52.3%) | ||
| Radiotherapy | >0.999 | 0.008 | |||
| Yes | 139 (23.3%) | 69 (23.2%) | 70 (23.5%) | ||
| No | 457 (76.7%) | 229 (76.8%) | 228 (76.5%) | ||
| Chemotherapy | 0.238 | 0.105 | |||
| Yes | 463 (77.7%) | 238 (79.9%) | 225 (75.5%) | ||
| No | 133 (22.3%) | 60 (20.1%) | 73 (24.5%) |
| Variable | Univariable Cox Regression Analysis | Multivariable Cox Regression Analysis a | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Age, year | 1.000 | 0.987–1.013 | 0.981 | |||
| Male sex | 0.915 | 0.767–1.092 | 0.326 | |||
| Race | ||||||
| White | reference | |||||
| Black | 0.956 | 0.745–1.229 | 0.727 | |||
| American Indian/Alaska Native | 1.445 | 0.683–3.056 | 0.335 | |||
| Asian or Pacific Islander | 0.852 | 0.675–1.074 | 0.174 | |||
| Unknown | 0.560 | 0.179–1.752 | 0.319 | |||
| Marital status | ||||||
| Married | reference | |||||
| Single | 0.840 | 0.686–1.027 | 0.090 | |||
| Divorced/Widowed/Separated | 0.839 | 0.621–1.135 | 0.254 | |||
| Unknown | 1.018 | 0.615–1.683 | 0.946 | |||
| Year of diagnosis | 0.972 | 0.951–0.993 | 0.008 | 0.980 | 0.940–1.023 | 0.355 |
| Primary site | ||||||
| Cardia | reference | reference | ||||
| Non-cardia | 1.424 | 1.112–1.823 | 0.005 | 1.349 | 1.031–1.766 | 0.029 |
| Overlapping lesion of stomach | 1.517 | 1.099–2.093 | 0.011 | 1.381 | 0.982–1.942 | 0.064 |
| Unknown | 1.599 | 1.182–2.164 | 0.002 | 1.473 | 1.066–2.037 | 0.019 |
| Tumor differentiation grade | ||||||
| I/II | reference | reference | ||||
| III/IV | 1.291 | 0.986–1.690 | 0.063 | |||
| Unknown | 0.996 | 0.685–1.448 | 0.983 | |||
| Histology | ||||||
| Signet ring cell carcinoma | reference | reference | ||||
| Other adenocarcinoma | 0.810 | 0.678–0.969 | 0.021 | 0.854 | 0.706–1.031 | 0.101 |
| Non-adenocarcinoma | 0.586 | 0.370–0.927 | 0.022 | 0.579 | 0.355–0.942 | 0.028 |
| Tumor size | ||||||
| ≤5 cm | reference | |||||
| >5 cm | 1.063 | 0.862–1.312 | 0.568 | |||
| Unknown | 1.115 | 0.893–1.391 | 0.336 | |||
| T stage | ||||||
| T1/ T2 | reference | reference | ||||
| T3/ T4 | 1.421 | 1.005–2.008 | 0.047 | 1.346 | 0.947–1.915 | 0.098 |
| Tx | 1.446 | 0.909–2.300 | 0.120 | 1.547 | 0.965–2.480 | 0.070 |
| Metastasis to the liver b | ||||||
| No | reference | reference | ||||
| Yes | 1.146 | 0.822–1.598 | 0.420 | 1.133 | 0.791–1.623 | 0.496 |
| Unknown | 1.298 | 1.079–1.562 | 0.006 | 1.056 | 0.108–10.332 | 0.963 |
| Metastasis to the lung b | ||||||
| No | reference | reference | ||||
| Yes | 1.933 | 1.024–3.647 | 0.042 | 3.228 | 1.634–6.378 | 0.001 |
| Unknown | 1.302 | 1.091–1.553 | 0.003 | 2.133 | 0.301–15.103 | 0.448 |
| Metastasis to the bone b | ||||||
| No | reference | reference | ||||
| Yes | 1.769 | 1.030–3.038 | 0.039 | 1.918 | 1.099–3.348 | 0.022 |
| Unknown | 1.299 | 1.088–1.551 | 0.004 | 0.703 | 0.068–7.316 | 0.768 |
| Metastasis to the brain b | ||||||
| No | reference | reference | ||||
| Yes | 4.628 | 1.473–14.542 | 0.009 | 4.517 | 1.404–14.538 | 0.011 |
| Unknown | 1.277 | 1.072–1.521 | 0.006 | 0.675 | 0.093–4.903 | 0.698 |
| Receipt of radiation | ||||||
| No | reference | |||||
| Yes | 0.911 | 0.745–1.114 | 0.366 | |||
| Receipt of chemotherapy | ||||||
| No | reference | reference | ||||
| Yes | 0.727 | 0.591–0.894 | 0.003 | 0.640 | 0.512–0.801 | <0.001 |
| Receipt of surgery | ||||||
| No | reference | reference | ||||
| Yes | 0.494 | 0.414–0.590 | <0.001 | 0.459 | 0.382–0.552 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.; Wang, P.-Y.; Liu, Y.-C. Palliative Gastrectomy Improves the Survival of Patients with Metastatic Early-Onset Gastric Cancer: A Retrospective Cohort Study. Curr. Oncol. 2023, 30, 7874-7890. https://doi.org/10.3390/curroncol30090572
An H, Wang P-Y, Liu Y-C. Palliative Gastrectomy Improves the Survival of Patients with Metastatic Early-Onset Gastric Cancer: A Retrospective Cohort Study. Current Oncology. 2023; 30(9):7874-7890. https://doi.org/10.3390/curroncol30090572
Chicago/Turabian StyleAn, Hang, Peng-Yuan Wang, and Yu-Cun Liu. 2023. "Palliative Gastrectomy Improves the Survival of Patients with Metastatic Early-Onset Gastric Cancer: A Retrospective Cohort Study" Current Oncology 30, no. 9: 7874-7890. https://doi.org/10.3390/curroncol30090572
APA StyleAn, H., Wang, P.-Y., & Liu, Y.-C. (2023). Palliative Gastrectomy Improves the Survival of Patients with Metastatic Early-Onset Gastric Cancer: A Retrospective Cohort Study. Current Oncology, 30(9), 7874-7890. https://doi.org/10.3390/curroncol30090572




