Abstract
Background: Risk-reducing surgeries are an option for cancer risk management in BRCA1/2 individuals. However, while adnexectomy is commonly recommended in breast cancer (BC) survivors, risk-reducing bilateral breast surgery (RRBBS) is controversial in ovarian cancer (OC) survivors due to relapse rates and mortality. Methods: We conducted a retrospective analysis of BRCA1/2-OC survivors, with OC as first cancer diagnosis. Results: Median age at OC diagnosis for the 69 BRCA1/2-OC survivors was 54 years. Median overall survival was 8 years, being significantly higher for BRCA2 patients than for BRCA1 patients (p = 0.011). Nine patients (13.2%) developed BC at a median age of 61 years. The mean overall BC-free survival was 15.5 years (median not reached). Eight patients (11.8%) underwent bilateral mastectomy (5 simultaneous with BC treatment; 3 RRBBS) at a median age of 56.5 years. The median time from OC to bilateral mastectomy/RRBBS was 5.5 years. Conclusions: This study adds evidence regarding a lower BC risk after BRCA1/2-OC and higher survival for BRCA2-OC patients. A comprehensive analysis of the competing risks of OC mortality and recurrence against the risk of BC should be individually addressed. Surgical BC risk management may be considered for longer BRCA1/2-OC disease-free survivors. Ultimately, these decisions should always be tailored to patients’ characteristics and preferences.
1. Introduction
Women with hereditary breast and ovarian cancer syndrome have an increased risk of developing cancer, mainly breast cancer (BC)—absolute risk > 60% for BRCA1/2 carriers—and ovarian cancer (OC)—absolute risk of 39–58% for BRCA1 and 13–29% for BRCA2 carriers [1].
Currently, breast imaging, such as ultrasound, mammography and/or magnetic resonance imaging (MRI), is widely recommended to detect malignant lesions at an early stage in BRCA1/2 women [1]. However, the most significant approach to reduce BC risk in BRCA1/2 carriers is risk-reducing bilateral breast surgery (RRBBS) [2]. Some previous reports stated that there was a BC risk reduction of 90 to 95% in BRCA1/2 women that underwent RRBBS, although no significative reduction in mortality was observed [3]. Likewise, risk-reducing adnexectomy is strongly recommended, typically between 35 and 40 years, to manage OC risk in BRCA1/2 women [1]. In addition to a profound decrease in OC incidence, risk-reducing adnexectomy also leads to a substantial reduction in all-cause and OC-related mortality [3]. While risk-reducing adnexectomy is still commonly recommended in BRCA1/2-BC survivors, RRBBS is controversial in BRCA1/2-OC survivors, due to the high relapse rate and mortality associated with OC. In fact, there is a lack of thorough recommendations concerning BC risk and the role of RRBBS in BRCA1/2-OC survivors. In this study, we evaluate the incidence of BC after BRCA1/2-OC and report our experience with RRBBS in these patients.
2. Materials and Methods
All consenting women testing positive for BRCA1 or BRCA2 were invited to participate in long-term prospective follow-up in the Familial Risk Clinic of IPO Lisboa. Patients are kept under surveillance until death, loss to follow-up or consent withdrawal. For this study, patients with OC as first cancer diagnosis and a BRCA1/2-positive test between January 2000 and August 2022 were selected. Women who had had another cancer before OC were excluded. Data before testing were retrospectively collected from available clinical reports. The start of the follow-up period was defined as the date of OC diagnosis. The overall and BC-free survival were calculated using the Kaplan–Meier method. The log-rank test was used to compare survival and BC incidence between different groups. Overall survival was considered as the time from OC diagnosis to the time of death, whereas BC-free survival was defined as the time from OC to BC diagnoses. Statistical analysis was conducted using SigmaPlot software, version 15.0.
3. Results
Over a period of 22 years, a total of 69 women, from 63 different families, were diagnosed with BRCA1/2-OC. The median age at OC diagnosis was 54 years (range: 18–85 years). Most patients for whom data were available were diagnosed with epithelial serous OC (75.9%) and at a III or IV FIGO stage (73.7%). Regarding molecular testing, 33 (47.8%) individuals were identified with a germline BRCA1 variant and 36 (52.2%) with a germline BRCA2 variant. Of the 36 BRCA2 patients, 9 (25%) had the founder variant of Portuguese origin BRCA2:c.156_157insAlu. Among the 69 patients, 55 (79.7%) had at least one relative with BC, while 14 (20.3%) had no known relatives diagnosed with BC. Among those with positive family history of BC, 38 (69.1%) had an affected first-degree relative (Table 1). In the subgroup of nine patients who developed BC, eight (88.9%) reported positive family history, and only one (11.1%) patient had no family history of BC. Among those eight with a positive family history of BC, half had an affected first-degree relative (Table 2).

Table 1.
Characterization of the cohort.

Table 2.
Characterization of BC diagnosed in the cohort.
The median duration of follow-up for all patients since OC diagnosis was 6 years (range: 1–22 years). In this group, there were a total of 35 deaths from all causes (all-cause mortality rate: 50.7%) throughout the follow-up period. Death occurred at a median age of 59 years (range: 40–89 years), at a median of 4 years (range: 1–16 years) after OC diagnosis, and, in 94.3% of the cases (33 patients), within the first 10 years of follow-up. For the entire cohort, the median overall survival was 8.0 years (mean: 11.6 years), being significantly higher for BRCA2-OC patients (median: not reached; mean: 14.3 years) than for BRCA1-OC patients (median: 5 years; mean: 8 years) (p = 0.011).
Further, one of the 69 patients was lost to follow-up more than two years before death, so she is not considered when assessing BC (or other cancer types) risk in this cohort. A total of nine (13.2%) patients developed BC after the OC at a median age of 61 years (range: 44–68 years). The median BC-free survival could not be calculated via Kaplan–Meier survival analysis because of the small number of patients who were diagnosed with BC after OC, with the mean BC-free survival in the total population being 15.5 years (Figure 1). The difference in BC-free survival between BRCA1-OC women (median: not reached; mean: 12.9 years) and BRCA2-OC women (median: not reached; mean: 15.9 years) did not reach statistical significance (p = 0.440). The difference in the overall survival between BRCA1/2-OC women with BC (median: not reached; mean: 16.4 years) and BRCA1/2-OC women without BC (median: 8.0 years; mean: 9.9 years) was also not statistically significant (p = 0.107).
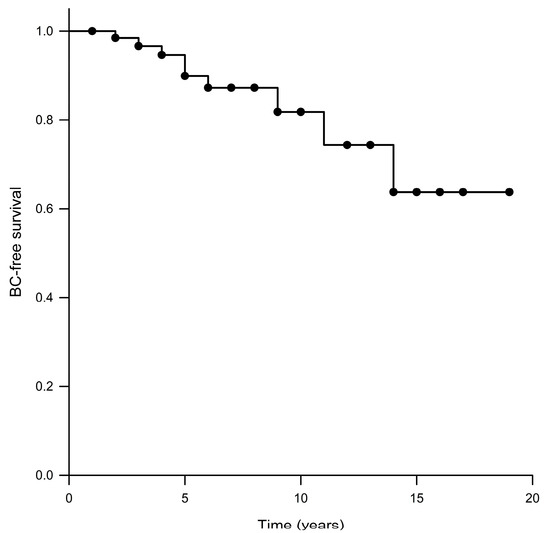
Figure 1.
Kaplan–Meier analysis of BC-free survival.
All diagnosed BCs were unilateral, and two of them were triple-negative (both BRCA1 patients). Of the nine patients with BC, five (55.6%) had a BRCA1 variant, whereas four (44.4%) had a BRCA2 variant (two with the Portuguese founder variant). Three out of the nine BRCA1/2-OC women with BC died at a median age of 51 years (range: 50–61 years) and at a median time of 7 years after the OC diagnosis (range: 5–9 years). The cause of death of these three patients was unrelated to BC: 1—ovarian cancer progression; 2—refractory leukemia; 3—overdose in a patient in remission of both cancers. The characterization of BC diagnosed in our cohort is detailed in Table 2.
Five (7.4%) patients underwent bilateral mastectomy in a unilateral BC context (Table 3). Bilateral mastectomy was performed at a median age of 54 years (range: 44–69 years) and at a median of 6 years (range: 2–15 years) after the OC diagnosis. Three (4.4%) patients, without personal history of BC, were submitted to RRBBS at a median age of 58 years (range: 55–61 years) (Table 3). The median time from OC diagnosis to RRBBS was 5 years (range: 3–15 years).

Table 3.
Characterization of bilateral mastectomy and RRBBS in the cohort.
In this cohort, five other cancers were diagnosed during the follow-up period—a squamous cell carcinoma of the tongue at the age of 51 (one patient), a synchronous high-stage serous carcinoma of the endometrium at the age of 75 (one patient) and basal cell carcinomas of the skin at ages of 59 and 72 (two patients). One patient was diagnosed with acute myeloid leukemia, secondary to chemotherapy for ovarian cancer, at the age of 60 and BC at the age of 61.
4. Discussion
In this study, we observed an incidence of 13.2% of BC in a cohort of 68 BRCA1/2-OC women during a median follow-up period of 6 years. The mean BC-free survival in the total population was 15.5 years (median: not reached), with no significant difference for BRCA1-OC, as compared to BRCA2-OC patients. While we observed a significantly higher overall survival for BRCA2-OC patients as compared with BRCA1-OC patients, the difference in overall survival between BRCA1/2-OC women with BC and BRCA1/2-OC women without BC was not found to be statistically significant.
The incidence of BC in our cohort was slightly higher than that reported in previous studies, such as that described by Vencken et al. [4], who identified 8 primary BCs in 79 BRCA1/2-OC women (10.1%) during a mean period of 6.7 years, and by Domchek et al. [5], who reported 11% of BCs (18 patients) in a group of 164 during a mean follow-up of 5.8 years. Similarly, Gangi et al. [6] described an incidence of 8.9% of BC (12 women) in 135 patients with a mean follow-up period of 6.6 years, and Fong et al. [7] identified 8.3% (16 patients) in 192 BRCA1/2-OC women. More recently, a larger cohort of 502 patients was characterized by Safra et al. [8], who reported a lower incidence (6.2%) of BC in 502 BRCA1/2-OC women, with a median follow-up of 5.0 years. The small number of BRCA1/2-OC women included in most of the studies, including our own, is a limitation regarding conclusions about the incidence of BC in this specific population. However, caution should also be taken when drawing conclusions, as inclusion criteria and mutational BRCA1/2 patterns differ among these studies. For example, Safra et al. [8] included women with BC and other cancers diagnosed prior to OC (representing 17.5% and 1.6% of the cohort, respectively) in their cohort. In our study, any type of cancer diagnosed before OC was an exclusion criterion. Another note that must be emphasized is that women who underwent RRBBS were maintained in our cohort, even after prophylactic surgery. Even after RRBBS, there is a remaining BC risk, and, in our registry, patients are kept in surveillance until death, are lost to follow-up or withdraw their consent.
Regarding mutational BRCA1/2 patterns, our study is the first where the numbers of BRCA2-OC women are higher than BRCA1-OC patients (BRCA2: 52.2% vs BRCA1: 47.8%). In all previous studies, BRCA1-OC patients represented more than 70% of the cohort [4,5,6,7,8]. As we discuss below, data are conflicting regarding BRCA1 and BRCA2 as biomarkers for better survival when compared with sporadic OC. However, a relevant finding in our study is the increased overall survival observed in the BRCA2-OC subgroup when compared to BRCA1-OC patients (14.3 years vs. 8 years, p = 0.011). This observation was previously described in a pooled analysis of 26 studies [9].
Vencken et al. [4] reported a BC risk of 3%, 6% and 11% in BRCA1/2-OC survivors in the following 2, 5 and 10 years after OC diagnosis. The same study reported a significantly higher BC risk in unaffected variant carriers during the same follow-up period (6%, 16% and 28%, respectively) [4]. Domchek et al. [5] also reported a less-than-10% risk of developing BC in the 10-year follow-up period (12% for BRCA1 carriers and 2% for BRCA2 carriers). In a more recent study, McGee et al. [10] reported a risk of 7.8% of developing BC in a 10-year interval, conditional on OC survival and other causes of death. Despite the fact that data are still limited and larger studies are needed, BRCA1/2-OC survivors appear to have a significantly lower risk of developing BC after OC than unaffected individuals. Previous studies suggested several reasons for the apparent lower BC risk in BRCA1/2-OC survivors compared to unaffected women. One of the reasons is the premature termination of ovarian function due to salpingo-oophorectomy, usually performed in an OC treatment context [4,5,6]. In line with that, previous studies reported that risk-reducing adnexectomy reduces the risk of BC in BRCA1/2 patients, mainly if performed at a premenopausal age [4,5,11,12]. Another aspect that is likely to contribute to a lower rate of BC in this subgroup is the effect of the therapy for OC. Some authors argue that platinum-based chemotherapy usually used for OC treatment could contribute to eradicating submicroscopic breast disease, leading to a lower number of BCs in these patients [4,5,6].
Although there are conflicting data in the literature, some studies reported that carrying a BRCA1 or BRCA2 variant leads to better responses to both platinum- and non-platinum-based chemotherapies, as well as better progression-free and overall survival in OC patients [13]. Nonetheless, McLaughlin et al. [14] demonstrated that BRCA1 or BRCA2 variants could be an advantage in OC patients to short-term survival but not to long-term survival. Although there is a trend for increasing OC survival due to recent advances in therapeutic approaches, OC 5-year and 10-year survival rates remain poor (63% and 35%, respectively) [4]. In our study, we report a mortality rate of 50.7% at a median age of 59 years, which is similar to those previously reported (53.2%, 51.1%, 40.7% and 36.3%) [4,6,8,10]. Moreover, most patients in our cohort (74.6%) were diagnosed with a high-stage OC, which is comparable to the 76% reported in Vencken et al. [4] and the most prevalent stage IIIC in Gangi et al. [6]. Currently, patients’ outcome is essentially determined by OC mortality rate and the risk of relapse, mainly during the first years after diagnosis. Noteworthily, 94.3% of deaths in our cohort occurred within the first 10 years of follow-up after OC diagnosis. We registered three deaths among BRCA1/2-OC women with BC but none were linked to the diagnosis of BC. Similarly, Domchek et al. [5] and Safra et al. [8] also stated that none of the deaths in their cohorts of women with OC and BC were related to BC. In the Fong et al. [7] study, only one patient died of BC.
Taking all data into consideration, we propose that BC after BRCA1/2-OC would still require specific surveillance, especially in those women who have better prognosis. Based on simulation studies, McGee et al. [10] concluded that the risk of death from BC after OC is about 1%, and breast MRI screening and RRBBS will have a very small impact on survival. For example, among all BRCA1/2 women diagnosed with stage III/IV OC at the age of 50, breast MRI screening and RRBBS will reduce, by 1% and 2%, respectively, the chance of dying by the age of 80. However, these effects could be greater if OC was diagnosed at an early age or at lower stages, leading several authors to propose that RRBBS or breast MRI screening should be recommended to all patients diagnosed with stage I or II OC and those patients with stage III or IV OC diagnosed at or before the age of 50 and surviving at least 10 years without relapse [10]. It is of note that in our study, eight patients underwent BC risk-reducing surgery, but for five of these patients, surgery was decided in the context of a BC diagnosis and regarding contralateral BC risk reduction.
We are aware that several limitations of this study, particularly the small cohort and the lack of a control group, should be considered when conclusions are discussed. Data regarding individual treatment and OC relapse were also not included in the current discussion. However, this study adds data to the discussion regarding the risk of developing BC in a population of BRCA2-enriched OC survivors. With recent advances in treatment, the number of BRCA1/2-OC survivors may increase in the near future, and this information may help clinicians to provide more accurate counseling regarding BC risk and risk management options to these patients. We are looking forward to in-depth future collaborative studies, including larger cohorts, so as to obtain more robust recommendations for this subgroup.
5. Conclusions
During the early period after OC diagnosis, OC mortality and recurrence rates are significantly high, and BC risk appears to be lower than in unaffected BRCA1/2 individuals. With that in mind, invasive BC risk management could bring an inappropriate burden without significant benefits to these patients. However, with the positive survival impact of new therapeutic advances, we expect a rising number of BRCA1/2-OC survivors with health professionals having to face the dilemma of RRBBS in the context of a potentially life-limiting OC diagnosis. We propose a comprehensive analysis of the competing risks of OC mortality and OC recurrence against the risk of BC that should be individually addressed, particularly in those patients with longer disease-free survival. Ultimately, decisions regarding preventive measures should always be tailored to patients’ characteristics and preferences.
Author Contributions
Writing—original draft preparation, D.O.; writing—review and editing, S.F. (Sofia Fernandes), I.M., S.F. (Sofia Fragoso) and F.V.; supervision, F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this observational study, since all patients included consented previously in data collection during prospective, non-interventional, follow-up. Data collection during follow-up of patients with a positive genetic testing was approved by the Ethics Committee of our Institute (Comissão de Ética do Instituto Português de Oncologia de Lisboa, EPE). Of note, after implementation of the European 679/2016 Regulation, data collection and consent forms were reviewed and approved by the same Ethics Committee.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. All genetic records are protected with restricted access, as per Portuguese law. All forms related to this study were approved by the Ethics Committee of our Institute (Comissão de Ética do Instituto Português de Oncologia de Lisboa, EPE).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We want to thank all patients and their families that contributed to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, (version 1.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (accessed on 30 October 2022).
- Speight, B.; Tischkowitz, M. When to Consider Risk-Reducing Mastectomy in BRCA1/BRCA2 Mutation Carriers with Advanced Stage Ovarian Cancer: A Case Study Illustrating the Genetic Counseling Challenges. J. Genet. Couns. 2017, 26, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.K.; Neuner, J.; Butler, A.; Geurts, J.L.; Kong, A.L. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am. J. Surg. 2016, 212, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Vencken, P.M.L.H.; Kriege, M.; Hooning, M.; Menke-Pluymers, M.B.; Heemskerk-Gerritsen, B.A.M.; Van Doorn, L.C.; Collée, M.M.; Jager, A.; Van Montfort, C.; Burger, C.W.; et al. The risk of primary and contralateral breast cancer after ovarian cancer in BRCA1/BRCA2 mutation carriers: Implications for counseling. Cancer 2013, 119, 955–962. [Google Scholar] [CrossRef]
- Domchek, S.M.; Jhaveri, K.; Patil, S.; Stopfer, J.E.; Hudis, C.; Powers, J.; Stadler, Z.; Goldstein, L.; Kauff, N.; Khasraw, M.; et al. Risk of metachronous breast cancer after BRCA mutation-associated ovarian cancer. Cancer 2013, 119, 1344–1348. [Google Scholar] [CrossRef]
- Gangi, A.; Cass, I.; Paik, D.; Barmparas, G.; Karlan, B.; Dang, C.; Li, A.; Walsh, C.; Rimel, B.J.; Amersi, F.F. Breast Cancer Following Ovarian Cancer in BRCA Mutation Carriers. JAMA Surg. 2014, 149, 1306. [Google Scholar] [CrossRef]
- Fong, A.; Cass, I.; John, C.; Gillen, J.; Moore, K.M.; Gangi, A.; Walsh, C.; Li, A.J.; Rimel, B.J.; Karlan, B.Y.; et al. Breast Cancer Surveillance Following Ovarian Cancer in BRCA Mutation Carriers. Am. Surg. 2020, 86, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Safra, T.; Waissengrin, B.; Gerber, D.; Bernstein-Molho, R.; Klorin, G.; Salman, L.; Josephy, D.; Chen-Shtoyerman, R.; Bruchim, I.; Frey, M.K.; et al. Breast cancer incidence in BRCA mutation carriers with ovarian cancer: A longitudal observational study. Gynecol. Oncol. 2021, 162, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Bolton, K.L. Association Between BRCA1 and BRCA2 Mutations and Survival in Women with Invasive Epithelial Ovarian Cancer. JAMA 2012, 307, 382. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.; Giannakeas, V.; Karlan, B.; Lubinski, J.; Gronwald, J.; Rosen, B.; McLaughlin, J.; Risch, H.; Sun, P.; Foulkes, W.D.; et al. Risk of breast cancer after a diagnosis of ovarian cancer in BRCA mutation carriers: Is preventive mastectomy warranted? Gynecol. Oncol. 2017, 145, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Olopade, O.I.; Artioli, G. Efficacy of Risk-Reducing Salpingo-Oophorectomy in Women with BRCA-1 and BRCA-2 Mutations. Breast J. 2004, 10, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M. Association of Risk-Reducing Surgery in BRCA1 or BRCA2 Mutation Carriers with Cancer Risk and Mortality. JAMA 2010, 304, 967. [Google Scholar] [CrossRef] [PubMed]
- Alsop, K.; Fereday, S.; Meldrum, C.; deFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA Mutation Frequency and Patterns of Treatment Response in BRCA Mutation–Positive Women With Ovarian Cancer: A Report From the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.R.; Rosen, B.; Moody, J.; Pal, T.; Fan, I.; Shaw, P.A.; Risch, H.A.; Sellers, T.A.; Sun, P.; Narod, S.A. Long-Term Ovarian Cancer Survival Associated With Mutation in BRCA1 or BRCA2. JNCI J. Natl. Cancer Inst. 2013, 105, 141–148. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).