Age-Specific Trends of Invasive Cervical Cancer Incidence in British Columbia, Canada, 1971–2017
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Incidence Trends among Ages 15+ Years, 1971–2017
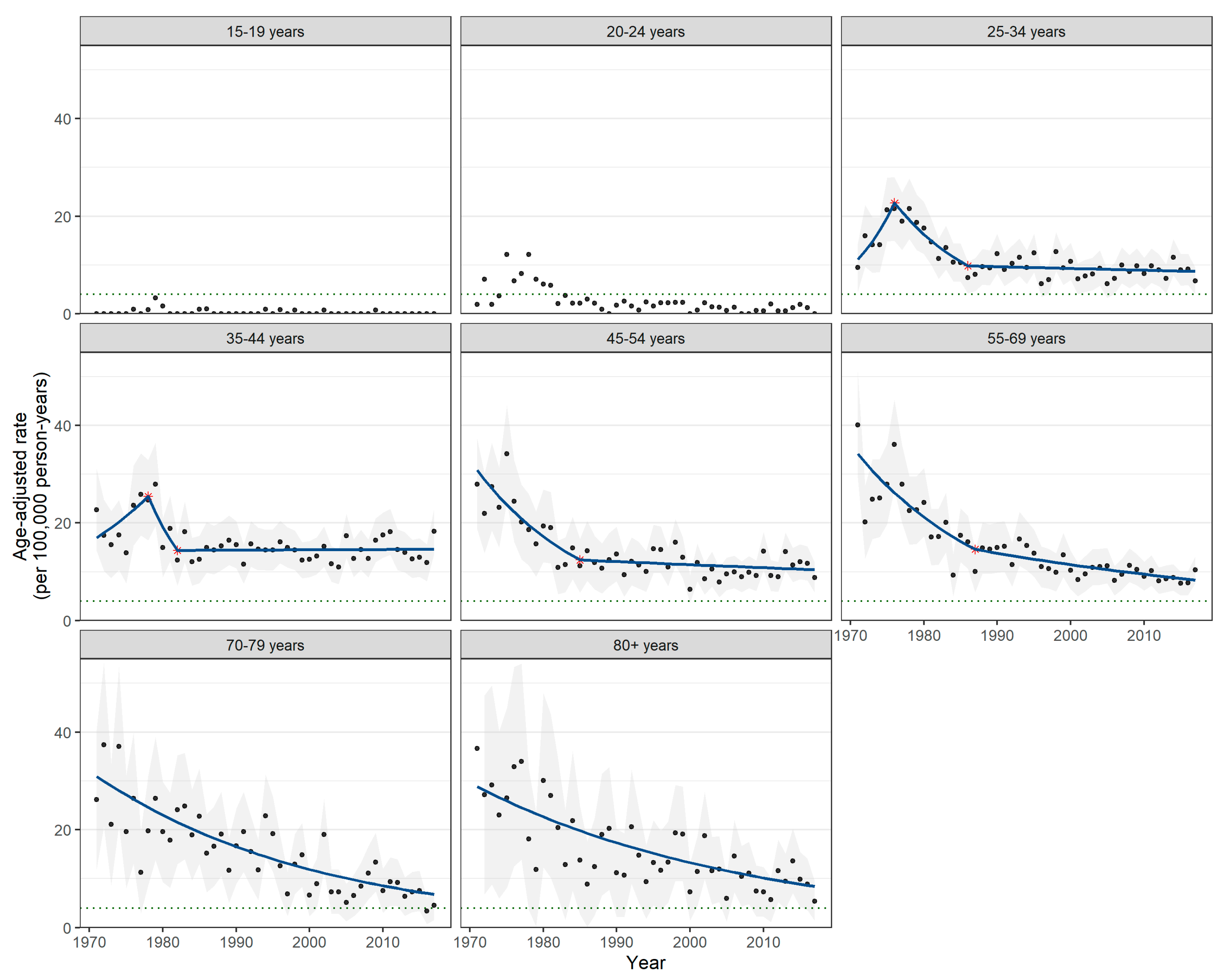
3.2. Incidence Trends by Age
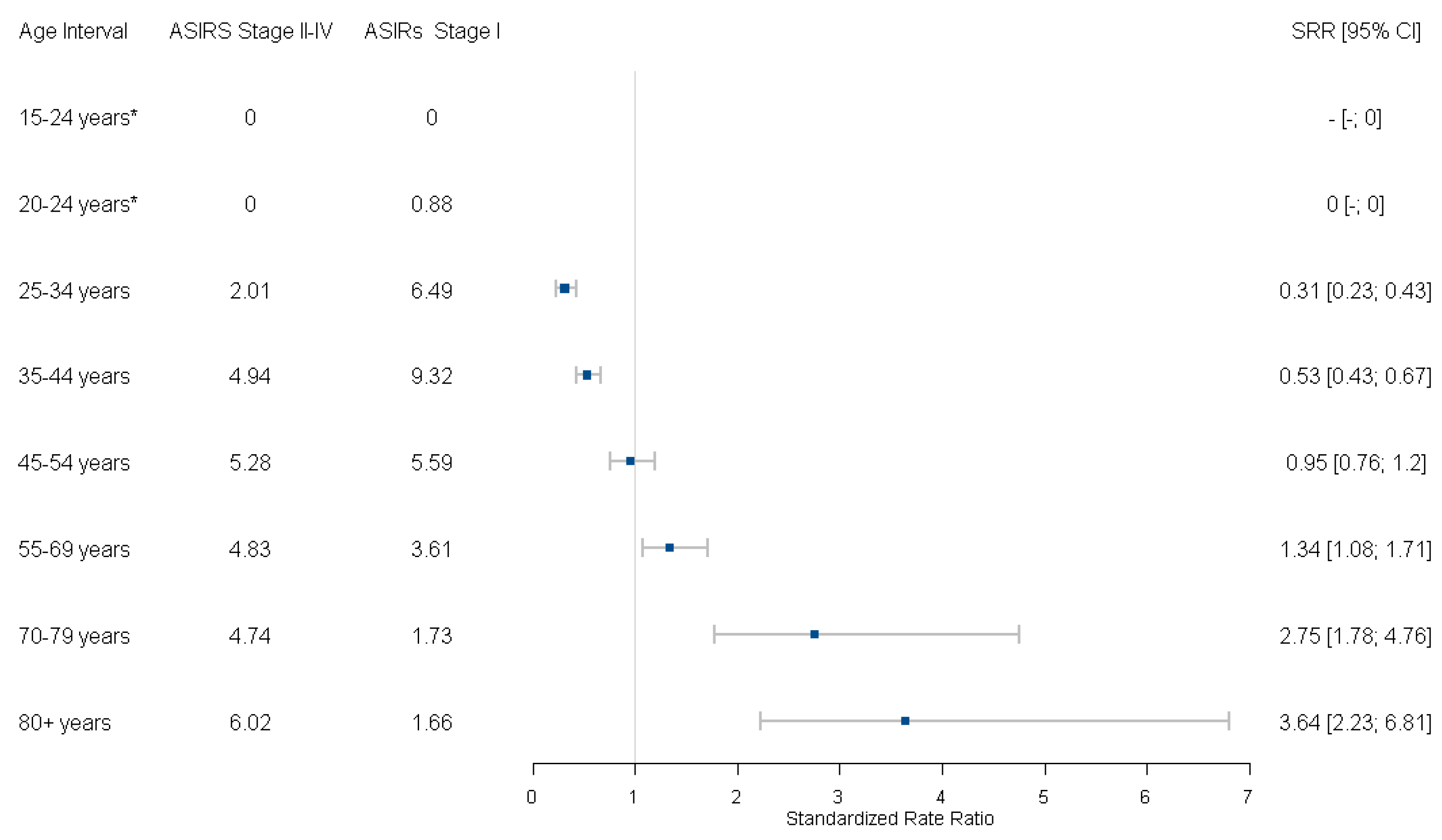
3.3. Incidence Trends by Age and Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Year | ASIR (95% CI)—2011 Canadian Population | ASIR (95% CI)—2015 World Female Population |
|---|---|---|
| 1971 | 18.88 (15.86–21.90) | 14.50 (12.21–16.79) |
| 1972 | 15.31 (12.70–17.92) | 12.40 (10.30–14.50) |
| 1973 | 15.22 (12.63–17.81) | 12.05 (10.01–14.09) |
| 1974 | 15.69 (13.12–18.26) | 12.52 (10.48–14.56) |
| 1975 | 18.07 (15.38–20.76) | 14.91 (12.73–17.09) |
| 1976 | 19.64 (16.86–22.42) | 15.97 (13.74–18.20) |
| 1977 | 16.71 (14.20–19.22) | 14.00 (11.94–16.06) |
| 1978 | 15.92 (13.53–18.31) | 13.67 (11.65–15.69) |
| 1979 | 15.50 (13.17–17.83) | 13.23 (11.27–15.19) |
| 1980 | 14.55 (12.32–16.78) | 11.92 (10.12–13.72) |
| 1981 | 13.07 (10.97–15.17) | 10.76 (9.05–12.47) |
| 1982 | 10.32 (8.50–12.14) | 8.28 (6.83–9.73) |
| 1983 | 11.86 (9.94–13.78) | 9.80 (8.25–11.35) |
| 1984 | 9.20 (7.48–10.92) | 7.50 (6.13–8.87) |
| 1985 | 10.06 (8.34–11.78) | 8.16 (6.79–9.53) |
| 1986 | 9.63 (7.92–11.34) | 7.89 (6.52–9.26) |
| 1987 | 8.34 (6.79–9.89) | 6.92 (5.67–8.17) |
| 1988 | 9.62 (7.97–11.27) | 7.79 (6.48–9.10) |
| 1989 | 9.51 (7.88–11.14) | 7.71 (6.42–9.00) |
| 1990 | 10.10 (8.47–11.73) | 8.38 (7.05–9.71) |
| 1991 | 8.69 (7.20–10.18) | 7.07 (5.87–8.27) |
| 1992 | 9.31 (7.80–10.82) | 7.67 (6.45–8.89) |
| 1993 | 9.60 (8.05–11.15) | 7.87 (6.64–9.10) |
| 1994 | 9.48 (8.01–10.95) | 7.84 (6.64–9.04) |
| 1995 | 10.15 (8.64–11.66) | 8.35 (7.13–9.57) |
| 1996 | 8.66 (7.29–10.03) | 7.12 (6.00–8.24) |
| 1997 | 7.67 (6.42–8.92) | 6.42 (5.38–7.46) |
| 1998 | 9.69 (8.30–11.08) | 8.13 (6.97–9.29) |
| 1999 | 9.14 (7.77–10.51) | 7.40 (6.30–8.50) |
| 2000 | 6.67 (5.53–7.81) | 5.76 (4.78–6.74) |
| 2001 | 7.15 (5.99–8.31) | 5.97 (4.99–6.95) |
| 2002 | 8.22 (6.99–9.45) | 6.78 (5.74–7.82) |
| 2003 | 7.24 (6.08–8.40) | 6.02 (5.04–7.00) |
| 2004 | 6.93 (5.81–8.05) | 5.82 (4.86–6.78) |
| 2005 | 7.24 (6.10–8.38) | 6.13 (5.15–7.11) |
| 2006 | 6.77 (5.67–7.87) | 5.63 (4.71–6.55) |
| 2007 | 7.31 (6.19–8.43) | 6.20 (5.22–7.18) |
| 2008 | 7.55 (6.41–8.69) | 6.22 (5.26–7.18) |
| 2009 | 8.03 (6.87–9.19) | 6.86 (5.84–7.88) |
| 2010 | 8.11 (6.95–9.27) | 6.88 (5.86–7.90) |
| 2011 | 7.97 (6.81–9.13) | 6.89 (5.87–7.91) |
| 2012 | 7.07 (5.99–8.15) | 5.99 (5.05–6.93) |
| 2013 | 7.39 (6.29–8.49) | 6.17 (5.23–7.11) |
| 2014 | 7.70 (6.60–8.80) | 6.55 (5.59–7.51) |
| 2015 | 7.18 (6.12–8.24) | 6.08 (5.16–7.00) |
| 2016 | 6.69 (5.67–7.71) | 5.72 (4.82–6.62) |
| 2017 | 7.08 (6.04–8.12) | 6.04 (5.14–6.94) |
| Age Group | Date Range | APC (95% CI) |
| Overall | 1971–1976 | 2.9 (−2.8 to 8.9) |
| Overall | 1976–1984 | −7.5 (−10.6 to −4.3) |
| Overall | 1984–2017 | −1.1 (−1.4 to −0.8) |
| 25–34 years | 1971–1976 | 15.3 (2.1 to 30.1) |
| 25–34 years | 1976–1986 | −8.0 (−11.8 to −4.1) |
| 25–34 years | 1986–2017 | −0.4 (−1.1 to 0.3) |
| 35–44 years | 1971–1978 | 5.9 (−1.3 to 13.7) |
| 35–44 years | 1978–1982 | −13.3 (−31.8 to 10.3) |
| 35–44 years | 1982–2017 | 0.0 (−0.5 to 0.6) |
| 45–54 years | 1971–1985 | −6.3 (−9.0 to −3.4) |
| 45–54 years | 1985–2017 | −0.5 (−1.3 to 0.3) |
| 55–69 years | 1971–1987 | −5.2 (−6.9 to −3.4) |
| 55–69 years | 1987–2017 | −1.8 (−2.6 to −1.1) |
| 70–79 years | 1971–2017 | −3.2 (−3.9 to −2.6) |
| 80+ years | 1971–2017 | −2.6 (−3.3 to −2.0) |
| Age Group | Date Range | AAPC (95% CI) |
| Overall | 1971–2017 | −1.8 (−2.7 to −1.0) |
| Overall | 2008–2017 | −1.1 (−1.4 to −0.8) |
| 25–34 years | 1971–2017 | −0.5 (−2.1 to 1.1) |
| 25–34 years | 2008–2017 | −0.4 (−1.1 to 0.3) |
| 35–44 years | 1971–2017 | −0.3 (−2.6 to 2.0) |
| 35–44 years | 2008–2017 | 0.0 (−0.5 to 0.6) |
| 45–54 years | 1971–2017 | −2.3 (−3.3 to −1.3) |
| 45–54 years | 2008–2017 | −0.5 (−1.3 to 0.3) |
| 55–69 years | 1971–2017 | −3.0 (−3.8 to −2.3) |
| 55–69 years | 2008–2017 | −1.8 (−2.6 to −1.1) |
| 70–79 years | 1971–2017 | −3.2 (−3.9 to −2.6) |
| 70–79 years | 2008–2017 | −3.2 (−3.9 to −2.6) |
| 80+ years | 1971–2017 | −2.6 (−3.3 to −2.0) |
| 80+ years | 2008–2017 | −2.6 (−3.3 to −2.0) |
| Age Group | Date Range | APC (95% CI) |
| Overall | 1971–1977 | 2.7 (−1.5 to 7.1) |
| Overall | 1977–1984 | −8.5 (−12.4 to −4.5) |
| Overall | 1984–2017 | −0.9 (−1.3 to −0.6) |
| 25–34 years | 1971–1976 | 15.2 (2.3 to 29.7) |
| 25–34 years | 1976–1986 | −8.1 (−11.7 to −4.2) |
| 25–34 years | 1986–2017 | −0.4 (−1.1 to 0.3) |
| 35–44 years | 1971–1978 | 5.9 (−1.2 to 13.6) |
| 35–44 years | 1978–1982 | −13.3 (−31.7 to 10.0) |
| 35–44 years | 1982–2017 | 0.0 (−0.5 to 0.6) |
| 45–54 years | 1971–1985 | −6.3 (−9.0 to −3.4) |
| 45–54 years | 1985–2017 | −0.5 (−1.3 to 0.3) |
| 55–69 years | 1971–1987 | −5.2 (−6.9 to −3.4) |
| 55–69 years | 1987–2017 | −1.8 (−2.6 to −1.1) |
| 70–79 years | 1971–2017 | −3.2 (−3.9 to −2.6) |
| 80+ years | 1971–2017 | −2.6 (−3.2 to −1.9) |
| Age Group | Date Range | AAPC (95% CI) |
| Overall | 1971–2017 | −1.7 (−2.5 to −0.8) |
| Overall | 2008–2017 | −0.9 (−1.3 to −0.6) |
| 25–34 years | 1971–2017 | −0.6 (−2.1 to 1.0) |
| 25–34 years | 2008–2017 | −0.4 (−1.1 to 0.3) |
| 35–44 years | 1971–2017 | −0.3 (−2.6 to 2.0) |
| 35–44 years | 2008–2017 | 0.0 (−0.5 to 0.6) |
| 45–54 years | 1971–2017 | −2.3 (−3.3 to −1.3) |
| 45–54 years | 2008–2017 | −0.5 (−1.3 to 0.3) |
| 55–69 years | 1971–2017 | −3.0 (−3.8 to −2.3) |
| 70–79 years | 1971–2017 | −3.2 (−3.9 to −2.6) |
| 70–79 years | 2008–2017 | −3.2 (−3.9 to −2.6) |
| 80+ years | 1971–2017 | −2.6 (−3.2 to −1.9) |
| 80+ years | 2008–2017 | −2.6 (−3.2 to −1.9) |

References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.-M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected Estimates of Cancer in Canada in 2022. CMAJ 2022, 194, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- The Causal Relation between Human Papillomavirus and Cervical Cancer–PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1769629/ (accessed on 13 June 2023).
- Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://www.who.int/publications-detail-redirect/9789240014107 (accessed on 13 June 2023).
- Demers, A.A.; Brenner, D.R.; Smith, L.; Shaw, A. At-a-Glance—Cancer Trends in Canada, 1984 to 2015. Health Promot. Chronic Dis. Prev. Can. 2019, 39, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.A.; Stankiewicz, A.; Popadiuk, C.; Pogany, L.; Onysko, J.; Miller, A.B. Reduced Cervical Cancer Incidence and Mortality in Canada: National Data from 1932 to 2006. BMC Public Health 2012, 12, 992. [Google Scholar] [CrossRef]
- Kachuri, L.; De, P.; Ellison, L.F.; Semenciw, R.; Advisory Committee on Canadian Cancer Statistics. Cancer Incidence, Mortality and Survival Trends in Canada, 1970–2007. Chronic Dis. Inj. Can. 2013, 33, 69–80. [Google Scholar] [CrossRef]
- Liu, S.; Semenciw, R.; Probert, A.; Mao, Y. Cervical Cancer in Canada: Changing Patterns in Incidence and Mortality. Int. J. Gynecol. Cancer 2001, 11, 24–31. [Google Scholar] [CrossRef]
- Mosavi-Jarrahi, A.; Kliewer, E.V. Cervical Cancer Incidence Trends in Canada: A 30-Year Population-Based Analysis. J. Obstet. Gynaecol. Can. JOGC 2013, 35, 620–626. [Google Scholar] [CrossRef]
- Brenner, D.R.; Ruan, Y.; Shaw, E.; O’Sullivan, D.; Poirier, A.E.; Heer, E.; Villeneuve, P.J.; Walter, S.D.; Friedenreich, C.M.; Smith, L.; et al. Age-Standardized Cancer-Incidence Trends in Canada, 1971–2015. CMAJ 2019, 191, E1262–E1273. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Fedewa, S.A.; Jemal, A. Trends in Cervical Cancer Incidence Rates by Age, Race/Ethnicity, Histological Subtype, and Stage at Diagnosis in the United States. Prev. Med. 2019, 123, 316–323. [Google Scholar] [CrossRef]
- Simard, E.P.; Naishadham, D.; Saslow, D.; Jemal, A. Age-Specific Trends in Black-White Disparities in Cervical Cancer Incidence in the United States: 1975–2009. Gynecol. Oncol. 2012, 127, 611–615. [Google Scholar] [CrossRef]
- Vaccarella, S.; Lortet-Tieulent, J.; Plummer, M.; Franceschi, S.; Bray, F. Worldwide Trends in Cervical Cancer Incidence: Impact of Screening against Changes in Disease Risk Factors. Eur. J. Cancer 2013, 49, 3262–3273. [Google Scholar] [CrossRef]
- Bray, F.; Loos, A.H.; McCarron, P.; Weiderpass, E.; Arbyn, M.; Møller, H.; Hakama, M.; Parkin, D.M. Trends in Cervical Squamous Cell Carcinoma Incidence in 13 European Countries: Changing Risk and the Effects of Screening. Cancer Epidemiol. Biomark. Prev. 2005, 14, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Foley, G.; Alston, R.; Geraci, M.; Brabin, L.; Kitchener, H.; Birch, J. Increasing Rates of Cervical Cancer in Young Women in England: An Analysis of National Data 1982–2006. Br. J. Cancer 2011, 105, 177–184. [Google Scholar] [CrossRef]
- Hansen, B.T.; Campbell, S.; Nygård, M. Long-Term Incidence Trends of HPV-Related Cancers, and Cases Preventable by HPV Vaccination: A Registry-Based Study in Norway. BMJ Open 2018, 8, e019005. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, O.; Kulasingam, S.; Virnig, B. Cervical Cancer Trends in the United States: A 35-Year Population-Based Analysis. J. Women’s Health 2012, 21, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, L.; Pontén, J.; Zack, M.; Adami, H.O. International Incidence Rates of Invasive Cervical Cancer after Introduction of Cytological Screening. Cancer Causes Control CCC 1997, 8, 755–763. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018; Canadian Cancer Society: Toronto, ON, Canada, 2018; Available online: https://cancer.ca/en/research/cancer-statistics/past-editions (accessed on 13 June 2023).
- Benard, V.B.; Watson, M.; Saraiya, M.; Harewood, R.; Townsend, J.S.; Stroup, A.M.; Weir, H.K.; Allemani, C. Cervical Cancer Survival in the United States by Race and Stage (2001–2009): Findings from the CONCORD-2 Study. Cancer 2017, 123 (Suppl. 24), 5119–5137. [Google Scholar] [CrossRef]
- Cervical Cancer: An NCD We Can Overcome. Available online: https://www.who.int/director-general/speeches/detail/cervical-cancer-an-ncd-we-can-overcome (accessed on 13 June 2023).
- Popadiuk, C. Cervical Cancer Screening in Canada. J. Obstet. Gynaecol. Can. JOGC 2019, 41 (Suppl. 2), S177–S180. [Google Scholar] [CrossRef]
- Action Plan for the Elimination of Cervical Cancer in Canada, 2020–2030—Canadian Partnership against Cancer. Available online: https://www.partnershipagainstcancer.ca/topics/elimination-cervical-cancer-action-plan/ (accessed on 14 June 2023).
- Caird, H.; Simkin, J.; Smith, L.; Van Niekerk, D.; Ogilvie, G. The Path to Eliminating Cervical Cancer in Canada: Past, Present and Future Directions. Curr. Oncol. 2022, 29, 1117–1122. [Google Scholar] [CrossRef]
- Popadiuk, C.; Stankiewicz, A.; Dickinson, J.; Pogany, L.; Miller, A.B.; Onysko, J. Invasive Cervical Cancer Incidence and Mortality among Canadian Women Aged 15 to 29 and the Impact of Screening. J. Obstet. Gynaecol. Can. JOGC 2012, 34, 1167–1176. [Google Scholar] [CrossRef]
- BC Population Estimates & Projections. Available online: https://bcstats.shinyapps.io/popApp/ (accessed on 13 June 2023).
- Canadian Partnership against Cancer. Cervical Cancer Screening in Canada: Environmental Scan; Canadian Partnership against Cancer: Toronto, ON, Canada, 2018; Available online: https://www.partnershipagainstcancer.ca/topics/cervical-cancer-screening-environmental-scan-2018/ (accessed on 13 June 2023).
- Canadian Task Force on Preventive Health Care. Recommendations on Screening for Cervical Cancer. CMAJ 2013, 185, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Certified Registries. NAACCR. Available online: https://www.naaccr.org/certified-registries/ (accessed on 14 June 2023).
- Government of Canada, S.C. Population Estimates on July 1st, by Age and Sex. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501 (accessed on 14 June 2023).
- Population Division—United Nations. World Population Prospects 2017. Available online: https://population.un.org/wpp/Download/Archive/Standard/ (accessed on 14 June 2023).
- World Health Organization. International Classification of Diseases for Oncology (ICD-O); World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Canfell, K.; Kim, J.J.; Brisson, M.; Keane, A.; Simms, K.T.; Caruana, M.; Burger, E.A.; Martin, D.; Nguyen, D.T.N.; Bénard, É.; et al. Mortality Impact of Achieving WHO Cervical Cancer Elimination Targets: A Comparative Modelling Analysis in 78 Low-Income and Lower-Middle-Income Countries. Lancet 2020, 395, 591–603. [Google Scholar] [CrossRef]
- Burger, E.A.; Smith, M.A.; Killen, J.; Sy, S.; Simms, K.; Canfell, K.; Kim, J.J. Projected Time to Elimination of Cervical Cancer in the USA: A Comparative Modelling Study. Lancet Public Health 2020, 5, e213–e222. [Google Scholar] [CrossRef]
- Joinpoint Regression Program; Version 4.8.0.1; Statistical Methodology and Applications Branch, Surveillance Research Program; National Cancer Institute: Bethesda, MD, USA.
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation Tests for Joinpoint Regression with Applications to Cancer Rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019; Canadian Cancer Society: Toronto, ON, Canada, 2019; Available online: https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2019-statistics/canadian-cancer-statistics-2019-en.pdf (accessed on 14 June 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; Posit: Boston, MA, USA, 2020. [Google Scholar]
- Aragon, T.J.; Fay, M.; Wollschlaeger, D.; Omidpanah, A. Epitools: Epidemiological Tools; Ausvet: Fremantle, Australia, 2017. [Google Scholar]
- Boyle, P.; Parkin, D.M. Cancer Registration: Principles and Methods. Statistical Methods for Registries. IARC Sci. Publ. 1991, 95, 126–158. [Google Scholar]
- Simms, K.T.; Steinberg, J.; Caruana, M.; Smith, M.A.; Lew, J.-B.; Soerjomataram, I.; Castle, P.E.; Bray, F.; Canfell, K. Impact of Scaled up Human Papillomavirus Vaccination and Cervical Screening and the Potential for Global Elimination of Cervical Cancer in 181 Countries, 2020–2099: A Modelling Study. Lancet Oncol. 2019, 20, 394–407. [Google Scholar] [CrossRef]
- Racey, C.S.; Albert, A.; Donken, R.; Smith, L.; Spinelli, J.J.; Pedersen, H.; de Bruin, P.; Masaro, C.; Mitchell-Foster, S.; Sadarangani, M.; et al. Cervical Intraepithelial Neoplasia Rates in British Columbia Women: A Population-Level Data Linkage Evaluation of the School-Based HPV Immunization Program. J. Infect. Dis. 2020, 221, 81–90. [Google Scholar] [CrossRef]
- Hall, M.T.; Simms, K.T.; Lew, J.-B.; Smith, M.A.; Brotherton, J.M.; Saville, M.; Frazer, I.H.; Canfell, K. The Projected Timeframe until Cervical Cancer Elimination in Australia: A Modelling Study. Lancet Public Health 2019, 4, e19–e27. [Google Scholar] [CrossRef]
- BC Centre for Disease Control. Immunization Coverage in Grade 6 Students: 2011–2020. Available online: http://www.bccdc.ca/resource-gallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/Immunization/Coverage/Grade%206%20Coverage%20Results.pdf (accessed on 14 June 2023).
- Bray, F.; Carstensen, B.; Møller, H.; Zappa, M.; Zakelj, M.P.; Lawrence, G.; Hakama, M.; Weiderpass, E. Incidence Trends of Adenocarcinoma of the Cervix in 13 European Countries. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2191–2199. [Google Scholar] [CrossRef]
- Baldur-Felskov, B.; Munk, C.; Nielsen, T.S.S.; Dehlendorff, C.; Kirschner, B.; Junge, J.; Kjaer, S.K. Trends in the Incidence of Cervical Cancer and Severe Precancerous Lesions in Denmark, 1997–2012. Cancer Causes Control CCC 2015, 26, 1105–1116. [Google Scholar] [CrossRef]
- Simkin, J.; Smith, L.; van Niekerk, D.; Caird, H.; Dearden, T.; van der Hoek, K.; Caron, N.R.; Woods, R.R.; Peacock, S.; Ogilvie, G. Sociodemographic Characteristics of Women with Invasive Cervical Cancer in British Columbia, 2004–2013: A Descriptive Study. Can. Med. Assoc. Open Access J. 2021, 9, E424–E432. [Google Scholar] [CrossRef]
- Drolet, M.; Boily, M.-C.; Greenaway, C.; Deeks, S.L.; Blanchette, C.; Laprise, J.-F.; Brisson, M. Sociodemographic Inequalities in Sexual Activity and Cervical Cancer Screening: Implications for the Success of Human Papillomavirus Vaccination. Cancer Epidemiol. Biomark. Prev. 2013, 22, 641–652. [Google Scholar] [CrossRef]
- Wang, S.S.; Sherman, M.E.; Hildesheim, A.; Lacey, J.V.; Devesa, S. Cervical Adenocarcinoma and Squamous Cell Carcinoma Incidence Trends among White Women and Black Women in the United States for 1976–2000. Cancer 2004, 100, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- BC Cancer Cervix Screening Program. BC Cancer Cervix Screening Program Overview; BC Cancer: Vancouver, BC, Canada, 2022; Available online: http://www.bccancer.bc.ca/screening/Documents/Cervix-Program-Overview.pdf (accessed on 14 June 2023).
- Hammer, A.; Soegaard, V.; Maimburg, R.D.; Blaakaer, J. Cervical Cancer Screening History Prior to a Diagnosis of Cervical Cancer in Danish Women Aged 60 Years and Older-A National Cohort Study. Cancer Med. 2019, 8, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.M.; Castanon, A.; Moss, E.; Redman, C.W.E. Cervical Cancer Is Not Just a Young Woman’s Disease. BMJ 2015, 350, h2729. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Shoemaker, M.L.; Benard, V.B. Cervical Cancer Screening and Incidence by Age: Unmet Needs Near and After the Stopping Age for Screening. Am. J. Prev. Med. 2017, 53, 392–395. [Google Scholar] [CrossRef]
- Gustafson, L.W.; Petersen, L.K.; Bor, P.; Andersen, B.; Hammer, A. Cervical Cancer Prevention among Older Women—Challenges in Screening, Diagnostic Workup and Treatment. Acta Obstet. Gynecol. Scand. 2021, 100, 1364–1368. [Google Scholar] [CrossRef]
- Gravdal, B.H.; Lönnberg, S.; Skare, G.B.; Sulo, G.; Bjørge, T. Cervical Cancer in Women under 30 Years of Age in Norway: A Population-Based Cohort Study. BMC Womens Health 2021, 21, 110. [Google Scholar] [CrossRef]
- Castanon, A.; Leung, V.M.W.; Landy, R.; Lim, A.W.W.; Sasieni, P. Characteristics and Screening History of Women Diagnosed with Cervical Cancer Aged 20–29 Years. Br. J. Cancer 2013, 109, 35–41. [Google Scholar] [CrossRef]
- BC Cancer Agency. BC Cancer Registry 2016 Annual Report; BC Cancer Agency: Vancouver, BC, Canada, 2016; Available online: http://www.bccancer.bc.ca/statistics-and-reports-site/Documents/BCCR_2016_Annual_Report.pdf (accessed on 14 June 2023).
- McGahan, C.E.; Linn, K.; Guno, P.; Johnson, H.; Coldman, A.J.; Spinelli, J.J.; Caron, N.R. Cancer in First Nations People Living in British Columbia, Canada: An Analysis of Incidence and Survival from 1993 to 2010. Cancer Causes Control CCC 2017, 28, 1105–1116. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raveinthiranathan, N.; Simkin, J.; Donken, R.; Ogilvie, G.; Smith, L.; Van Niekerk, D.; Lee, M.; Woods, R.R. Age-Specific Trends of Invasive Cervical Cancer Incidence in British Columbia, Canada, 1971–2017. Curr. Oncol. 2023, 30, 7692-7705. https://doi.org/10.3390/curroncol30080557
Raveinthiranathan N, Simkin J, Donken R, Ogilvie G, Smith L, Van Niekerk D, Lee M, Woods RR. Age-Specific Trends of Invasive Cervical Cancer Incidence in British Columbia, Canada, 1971–2017. Current Oncology. 2023; 30(8):7692-7705. https://doi.org/10.3390/curroncol30080557
Chicago/Turabian StyleRaveinthiranathan, Nivedha, Jonathan Simkin, Robine Donken, Gina Ogilvie, Laurie Smith, Dirk Van Niekerk, Marette Lee, and Ryan R. Woods. 2023. "Age-Specific Trends of Invasive Cervical Cancer Incidence in British Columbia, Canada, 1971–2017" Current Oncology 30, no. 8: 7692-7705. https://doi.org/10.3390/curroncol30080557
APA StyleRaveinthiranathan, N., Simkin, J., Donken, R., Ogilvie, G., Smith, L., Van Niekerk, D., Lee, M., & Woods, R. R. (2023). Age-Specific Trends of Invasive Cervical Cancer Incidence in British Columbia, Canada, 1971–2017. Current Oncology, 30(8), 7692-7705. https://doi.org/10.3390/curroncol30080557





