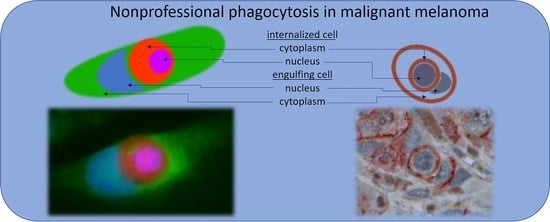
Non-Professional Phagocytosis Increases in Melanoma Cells and Tissues with Increasing E-Cadherin Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. CIC Induction in Adherent Cells
2.3. CIC Induction in Suspended Cells
2.4. Imaging and Image Analysis
2.5. Criteria for CIC Evaluation
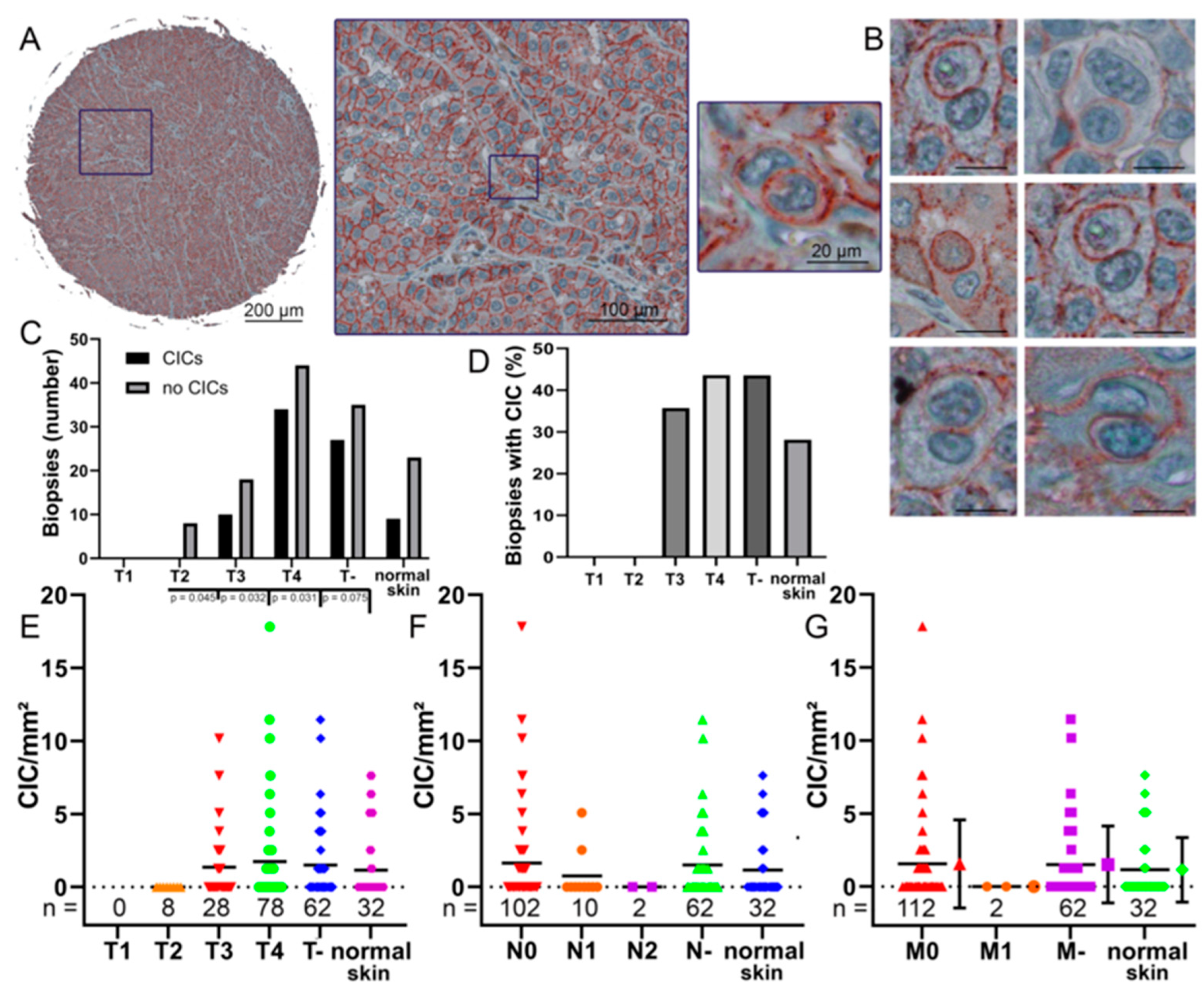
2.6. CIC in Malignant Melanoma Tissues
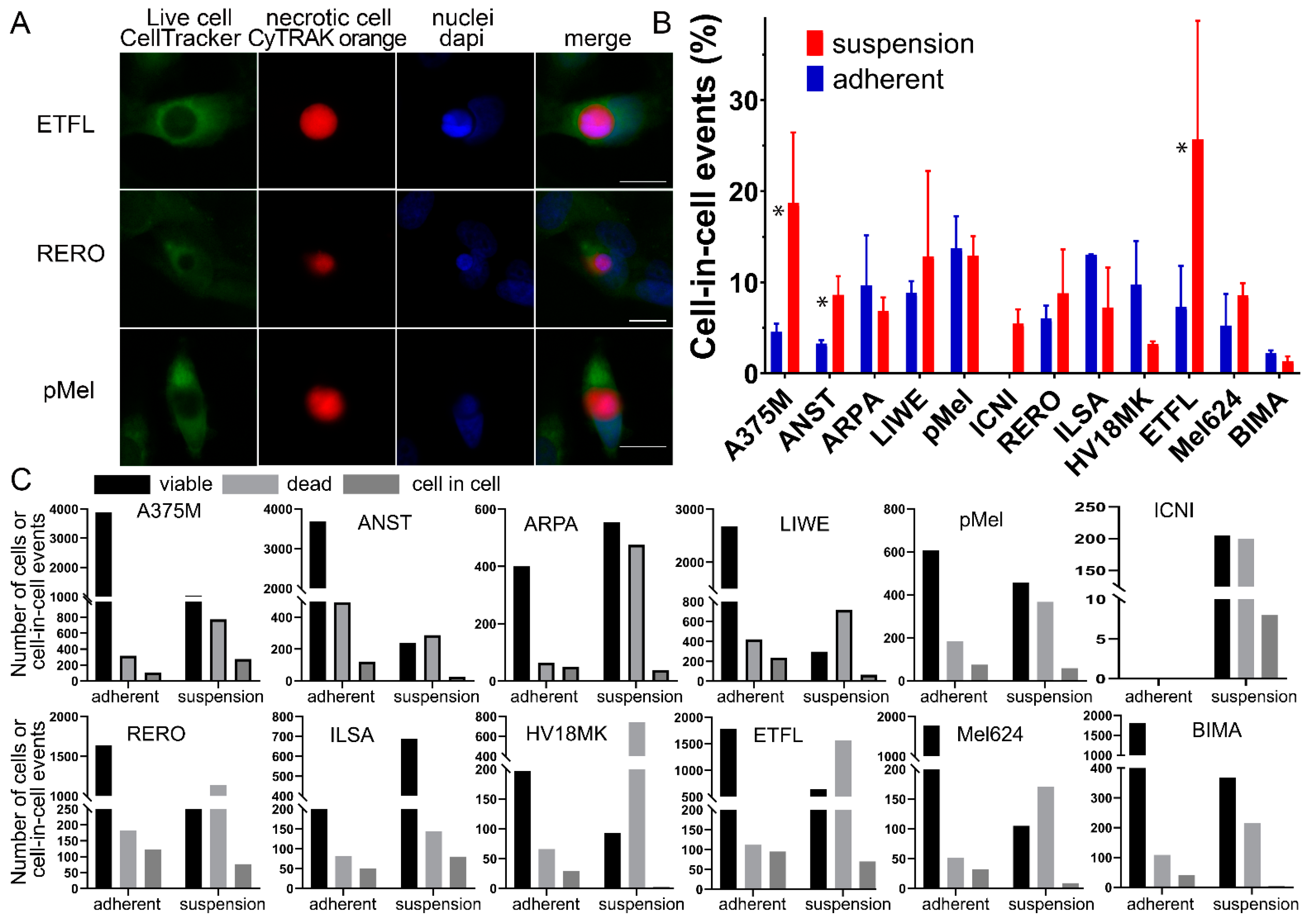
3. Results
3.1. Cell-in-Cell Structures in Cells under Adherent Versus Suspended Cells
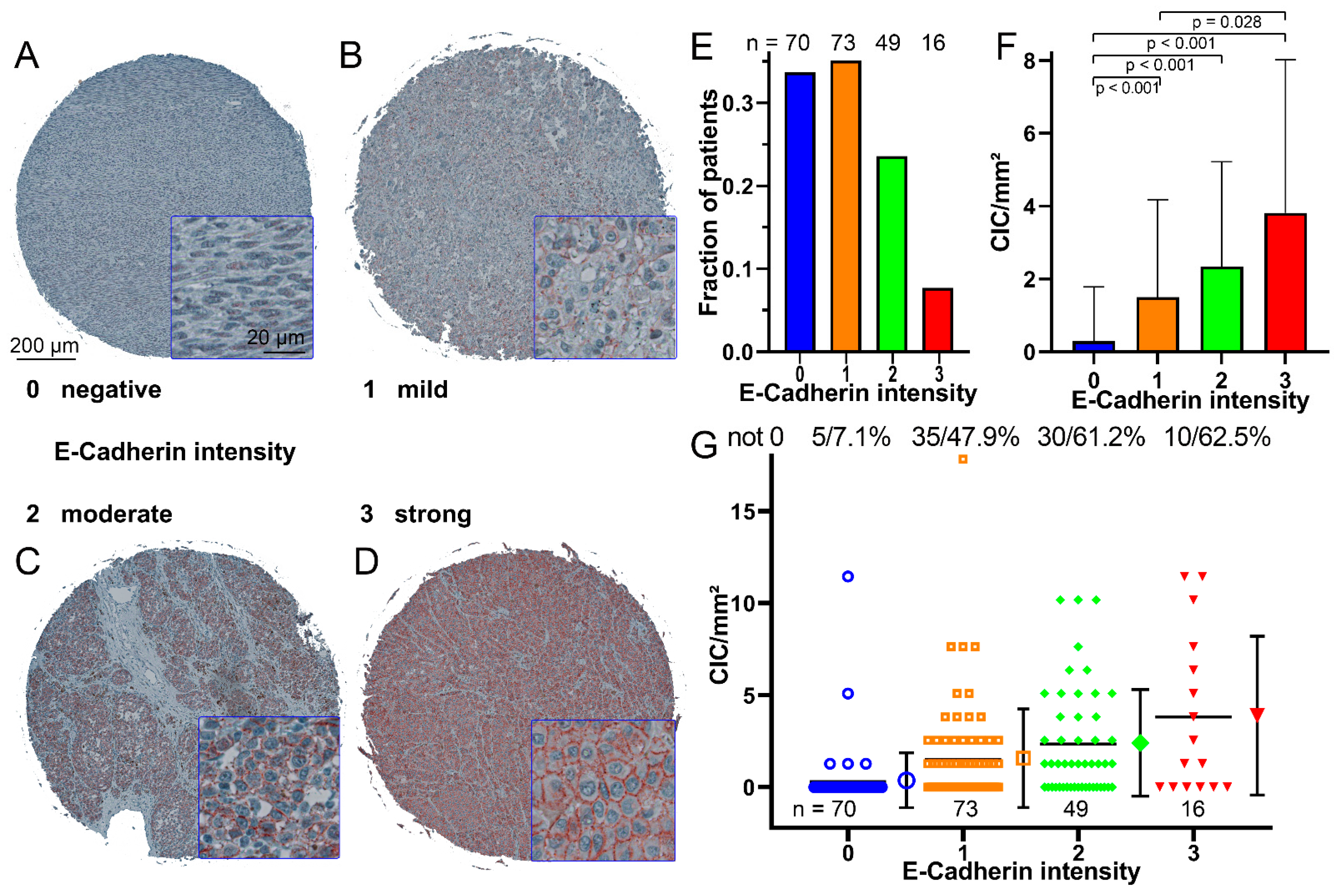
3.2. CIC Structures Depending on the E-Cadherin Expression
3.3. CIC Structures in Melanoma Biopsies Compared to TNM Stage
3.4. CIC Structures in Melanoma Tissues Are Dependent on the E-Cadherin Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinhaus, J. Ueber Carcinom-Einschlüsse. Arch. Pathol. Anat. Physiol. Klin. Med. 1891, 126, 533–541. [Google Scholar] [CrossRef]
- Kinoshita, M.; Matsuda, Y.; Arai, T.; Soejima, Y.; Sawabe, M.; Honma, N. Cytological diagnostic clues in poorly differentiated squamous cell carcinomas of the breast: Streaming arrangement, necrotic background, nucleolar enlargement and cannibalism of cancer cells. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2018, 29, 22–27. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, J.; Li, L.; Zhu, H.; Chen, H.; Kong, R.; Wang, G.; Wang, Y.; Hu, J.; et al. Cell-in-Cell Phenomenon and Its Relationship with Tumor Microenvironment and Tumor Progression: A Review. Front. Cell Dev. Biol. 2019, 7, 311. [Google Scholar] [CrossRef]
- Bauer, M.F.; Hildebrand, L.S.; Rosahl, M.C.; Erber, R.; Schnellhardt, S.; Buttner-Herold, M.; Putz, F.; Ott, O.J.; Hack, C.C.; Fietkau, R.; et al. Cell-In-Cell Structures in Early Breast Cancer Are Prognostically Valuable. Cells 2022, 12, 81. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; He, M. Cell-in-Cell: From Cell Biology to Translational Medicine. BioMed Res. Int. 2022, 2022, 7608521. [Google Scholar] [CrossRef]
- Mackay, H.L.; Muller, P.A.J. Biological relevance of cell-in-cell in cancers. Biochem. Soc. Trans. 2019, 47, 725–732. [Google Scholar] [CrossRef]
- Schwegler, M.; Wirsing, A.M.; Schenker, H.M.; Ott, L.; Ries, J.M.; Buttner-Herold, M.; Fietkau, R.; Putz, F.; Distel, L.V. Prognostic Value of Homotypic Cell Internalization by Nonprofessional Phagocytic Cancer Cells. BioMed Res. Int. 2015, 2015, 359392. [Google Scholar] [CrossRef]
- Fais, S.; Overholtzer, M. Cell-in-cell phenomena in cancer. Nat. Rev. Cancer 2018, 18, 758–766. [Google Scholar] [CrossRef]
- Gupta, N.; Jadhav, K.; Shah, V. Emperipolesis, entosis and cell cannibalism: Demystifying the cloud. J. Oral Maxillofac. Pathol. 2017, 21, 92–98. [Google Scholar] [CrossRef]
- Humble, J.G.; Jayne, W.H.; Pulvertaft, R.J. Biological interaction between lymphocytes and other cells. Br. J. Haematol. 1956, 2, 283–294. [Google Scholar] [CrossRef]
- Overholtzer, M.; Mailleux, A.A.; Mouneimne, G.; Normand, G.; Schnitt, S.J.; King, R.W.; Cibas, E.S.; Brugge, J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 2007, 131, 966–979. [Google Scholar] [CrossRef]
- Fais, S. Cannibalism: A way to feed on metastatic tumors. Cancer Lett. 2007, 258, 155–164. [Google Scholar] [CrossRef]
- Hamann, J.C.; Surcel, A.; Chen, R.; Teragawa, C.; Albeck, J.G.; Robinson, D.N.; Overholtzer, M. Entosis Is Induced by Glucose Starvation. Cell Rep. 2017, 20, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem. Sci. 2012, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Miller, D.D.; Bhawan, J. Cutaneous malignant melanoma: Update on diagnostic and prognostic biomarkers. Am. J. Dermatopathol. 2014, 36, 363–379. [Google Scholar] [CrossRef]
- Ren, J.; Yang, J.; Na, S.; Wang, Y.; Zhang, L.; Wang, J.; Liu, J. Comprehensive characterisation of immunogenic cell death in melanoma revealing the association with prognosis and tumor immune microenvironment. Front. Immunol. 2022, 13, 998653. [Google Scholar] [CrossRef]
- Scheper, J.; Hildebrand, L.S.; Faulhaber, E.M.; Deloch, L.; Gaipl, U.S.; Symank, J.; Fietkau, R.; Distel, L.V.; Hecht, M.; Jost, T. Tumor-specific radiosensitizing effect of the ATM inhibitor AZD0156 in melanoma cells with low toxicity to healthy fibroblasts. Strahlenther. Onkol. 2022. [Google Scholar] [CrossRef]
- Salzmann, M.; Hess, K.; Lang, K.; Enk, A.H.; Jordan, B.; Hassel, J.C. Long-term neurocognitive function after whole-brain radiotherapy in patients with melanoma brain metastases in the era of immunotherapy. Strahlenther. Onkol. 2022, 198, 884–891. [Google Scholar] [CrossRef]
- Lugini, L.; Lozupone, F.; Matarrese, P.; Funaro, C.; Luciani, F.; Malorni, W.; Rivoltini, L.; Castelli, C.; Tinari, A.; Piris, A.; et al. Potent phagocytic activity discriminates metastatic and primary human malignant melanomas: A key role of ezrin. Lab. Investig. 2003, 83, 1555–1567. [Google Scholar] [CrossRef]
- Lugini, L.; Matarrese, P.; Tinari, A.; Lozupone, F.; Federici, C.; Iessi, E.; Gentile, M.; Luciani, F.; Parmiani, G.; Rivoltini, L.; et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 2006, 66, 3629–3638. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y. Suspension state increases reattachment of breast cancer cells by up-regulating lamin A/C. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2272–2282. [Google Scholar] [CrossRef]
- Hazan, R.B.; Qiao, R.; Keren, R.; Badano, I.; Suyama, K. Cadherin switch in tumor progression. Ann. N. Y. Acad. Sci. 2004, 1014, 155–163. [Google Scholar] [CrossRef]
- Sun, Q.; Cibas, E.S.; Huang, H.; Hodgson, L.; Overholtzer, M. Induction of entosis by epithelial cadherin expression. Cell Res. 2014, 24, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, A.; Wang, T.; Wang, M.; Ning, X.; He, M.; Hu, Y.; Yuan, L.; Li, S.; Wang, Q.; et al. Detecting cell-in-cell structures in human tumor samples by E-cadherin/CD68/CD45 triple staining. Oncotarget 2015, 6, 20278–20287. [Google Scholar] [CrossRef] [PubMed]
- Venza, M.; Visalli, M.; Catalano, T.; Biondo, C.; Beninati, C.; Teti, D.; Venza, I. DNA methylation-induced E-cadherin silencing is correlated with the clinicopathological features of melanoma. Oncol. Rep. 2016, 35, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Gharbaran, R. Advances in the molecular functions of syndecan-1 (SDC1/CD138) in the pathogenesis of malignancies. Crit. Rev. Oncol. Hematol. 2015, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Billion, K.; Ibrahim, H.; Mauch, C.; Niessen, C.M. Increased soluble E-cadherin in melanoma patients. Skin. Pharmacol. Physiol. 2006, 19, 65–70. [Google Scholar] [CrossRef]
- Stauffer, T.P.; Guerini, D.; Carafoli, E. Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. A study using specific antibodies. J. Biol. Chem. 1995, 270, 12184–12190. [Google Scholar] [CrossRef]
- Rajasekaran, S.A.; Huynh, T.P.; Wolle, D.G.; Espineda, C.E.; Inge, L.J.; Skay, A.; Lassman, C.; Nicholas, S.B.; Harper, J.F.; Reeves, A.E.; et al. Na,K-ATPase subunits as markers for epithelial-mesenchymal transition in cancer and fibrosis. Mol. Cancer Ther. 2010, 9, 1515–1524. [Google Scholar] [CrossRef]
- Shen, S.; Wolfe, R.; McLean, C.A.; Haskett, M.; Kelly, J.W. Characteristics and associations of high-mitotic-rate melanoma. JAMA Dermatol. 2014, 150, 1048–1055. [Google Scholar] [CrossRef]
- Keung, E.Z.; Gershenwald, J.E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: Implications for melanoma treatment and care. Expert. Rev. Anticancer. Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef]
- Seeberg, J.C.; Loibl, M.; Moser, F.; Schwegler, M.; Buttner-Herold, M.; Daniel, C.; Engel, F.B.; Hartmann, A.; Schlotzer-Schrehardt, U.; Goppelt-Struebe, M.; et al. Non-professional phagocytosis: A general feature of normal tissue cells. Sci. Rep. 2019, 9, 11875. [Google Scholar] [CrossRef]
- Schenker, H.; Buttner-Herold, M.; Fietkau, R.; Distel, L.V. Cell-in-cell structures are more potent predictors of outcome than senescence or apoptosis in head and neck squamous cell carcinomas. Radiat. Oncol. 2017, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Breier, F.; Feldmann, R.; Fellenz, C.; Neuhold, N.; Gschnait, F. Primary invasive signet-ring cell melanoma. J. Cutan. Pathol. 1999, 26, 533–536. [Google Scholar] [CrossRef]
- Cano, C.E.; Sandi, M.J.; Hamidi, T.; Calvo, E.L.; Turrini, O.; Bartholin, L.; Loncle, C.; Secq, V.; Garcia, S.; Lomberk, G.; et al. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol. Med. 2012, 4, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, D.; Putz, F.; Hohmann, N.; Buttner-Herold, M.; Hecht, M.; Fietkau, R.; Distel, L. Role of tumor cell senescence in non-professional phagocytosis and cell-in-cell structure formation. BMC Mol. Cell Biol. 2020, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Schwegler, M.; Wirsing, A.M.; Dollinger, A.J.; Abendroth, B.; Putz, F.; Fietkau, R.; Distel, L.V. Clearance of primary necrotic cells by non-professional phagocytes. Biol. Cell/Under Auspices Eur. Cell Biol. Organ. 2015, 107, 372–387. [Google Scholar] [CrossRef]
- Siquara da Rocha, L.O.; Souza, B.S.F.; Lambert, D.W.; Gurgel Rocha, C.A. Cell-in-Cell Events in Oral Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 931092. [Google Scholar] [CrossRef]
- Hofmann, A.; Putz, F.; Buttner-Herold, M.; Hecht, M.; Fietkau, R.; Distel, L.V. Increase in non-professional phagocytosis during the progression of cell cycle. PLoS ONE 2021, 16, e0246402. [Google Scholar] [CrossRef]
- Ruan, B.; Wang, C.; Chen, A.; Liang, J.; Niu, Z.; Zheng, Y.; Fan, J.; Gao, L.; Huang, H.; Wang, X.; et al. Expression profiling identified IL-8 as a regulator of homotypic cell-in-cell formation. BMB Rep. 2018, 51, 412–417. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zhou, Y.; He, Y.; Wei, Y.; Tao, A. Heterotypic cell-in-cell structures in colon cancer can be regulated by IL-6 and lead to tumor immune escape. Exp. Cell Res. 2019, 382, 111447. [Google Scholar] [CrossRef] [PubMed]
- Gutwillig, A.; Santana-Magal, N.; Farhat-Younis, L.; Rasoulouniriana, D.; Madi, A.; Luxenburg, C.; Cohen, J.; Padmanabhan, K.; Shomron, N.; Shapira, G.; et al. Transient cell-in-cell formation underlies tumor relapse and resistance to immunotherapy. eLife 2022, 11, e80315. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unseld, L.H.; Hildebrand, L.S.; Putz, F.; Büttner-Herold, M.; Daniel, C.; Fietkau, R.; Distel, L.V. Non-Professional Phagocytosis Increases in Melanoma Cells and Tissues with Increasing E-Cadherin Expression. Curr. Oncol. 2023, 30, 7542-7552. https://doi.org/10.3390/curroncol30080547
Unseld LH, Hildebrand LS, Putz F, Büttner-Herold M, Daniel C, Fietkau R, Distel LV. Non-Professional Phagocytosis Increases in Melanoma Cells and Tissues with Increasing E-Cadherin Expression. Current Oncology. 2023; 30(8):7542-7552. https://doi.org/10.3390/curroncol30080547
Chicago/Turabian StyleUnseld, Luzie Helene, Laura S. Hildebrand, Florian Putz, Maike Büttner-Herold, Christoph Daniel, Rainer Fietkau, and Luitpold Valentin Distel. 2023. "Non-Professional Phagocytosis Increases in Melanoma Cells and Tissues with Increasing E-Cadherin Expression" Current Oncology 30, no. 8: 7542-7552. https://doi.org/10.3390/curroncol30080547
APA StyleUnseld, L. H., Hildebrand, L. S., Putz, F., Büttner-Herold, M., Daniel, C., Fietkau, R., & Distel, L. V. (2023). Non-Professional Phagocytosis Increases in Melanoma Cells and Tissues with Increasing E-Cadherin Expression. Current Oncology, 30(8), 7542-7552. https://doi.org/10.3390/curroncol30080547