Safety and Effectiveness of Chemotherapy in Elderly Biliary Tract Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Baseline Characteristics
2.3. Chemotherapy
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
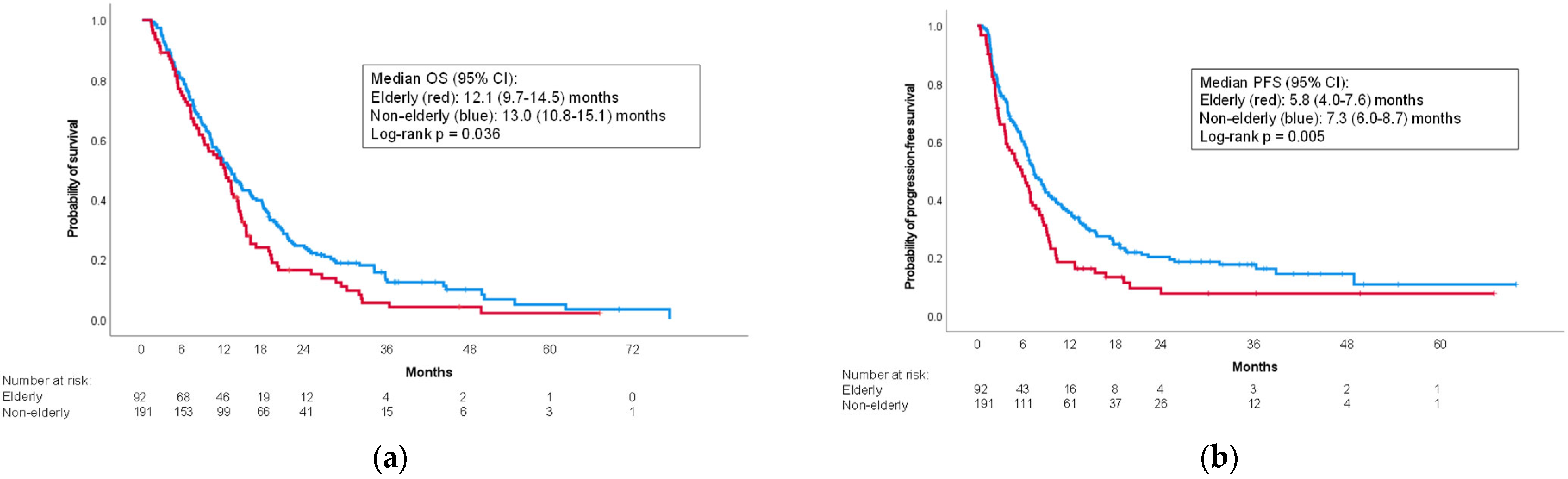
3.1. Patient Characteristics
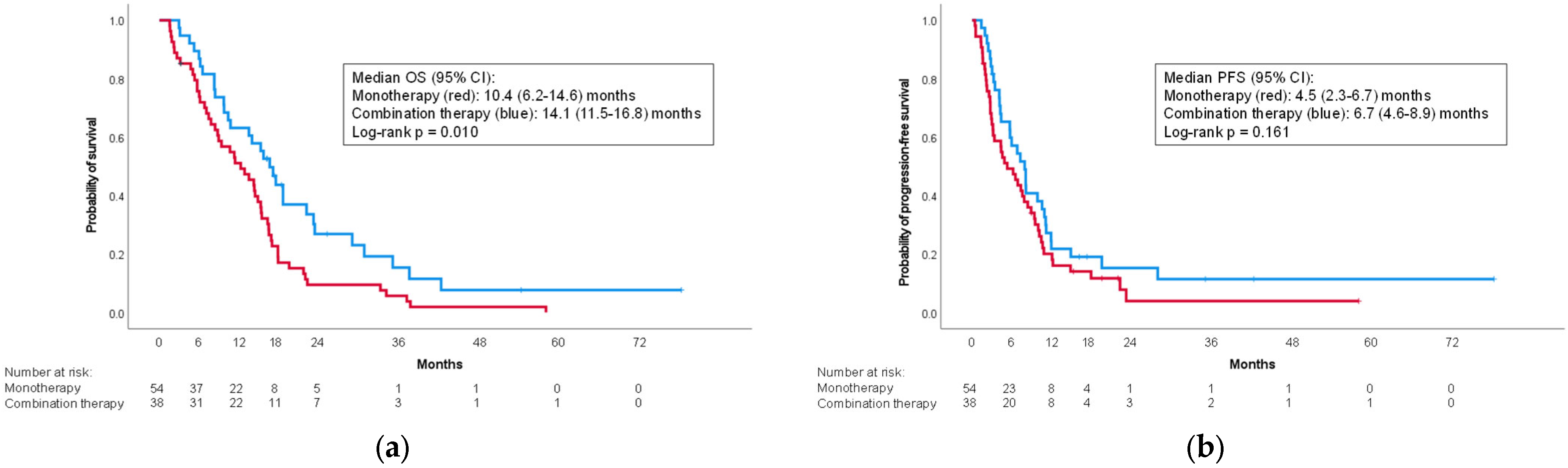
3.2. Treatment-Related Characteristics
3.3. Adverse Events
3.4. Factors Affecting Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Hirano, S.; Yoshitomi, H.; Aoki, T.; Uesaka, K.; Unno, M.; Ebata, T.; Konishi, M.; Sano, K.; Shimada, K.; et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J. Hepatobiliary Pancreat. Sci. 2021, 28, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan). Available online: https://ganjoho.jp/reg_stat/statistics/dl/index.html (accessed on 18 June 2023).
- Cancer Research, U.K. Gallbladder Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-bycancer-type/gallbladder-cancer (accessed on 18 June 2023).
- Pericleous, M.; Khan, S.A. Epidemiology of HPB malignancy in the elderly. Eur. J. Surg. Oncol. 2021, 47, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Horgan, A.M.; Amir, E.; Walter, T.; Knox, J.J. Adjuvant therapy in the treatment of biliary tract cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 1934–1940. [Google Scholar] [CrossRef]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, K.P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; Burris, H.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Ioka, T.; Kanai, M.; Kobayashi, S.; Sakai, D.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J. Hepatobiliary Pancreat. Sci. 2023, 30, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Rakugi, H.; Arai, H.; Akishita, M.; Ito, H.; Toba, K.; Kai, I. Joint Committee of Japan Gerontological Society (JGLS) and Japan Geriatrics Society (JGS) on the definition and classification of the elderly. Redefining the elderly as aged 75 years and older: Proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr. Gerontol. Int. 2017, 17, 1045–1047. [Google Scholar] [CrossRef]
- Yamada, I.; Morizane, C.; Okusaka, T.; Mizusawa, J.; Kataoka, T.; Ueno, M.; Ikeda, M.; Okano, N.; Todaka, A.; Shimizu, S.; et al. The clinical outcomes of combination chemotherapy in elderly patients with advanced biliary tract cancer: An exploratory analysis of JCOG1113. Sci. Rep. 2022, 12, 987. [Google Scholar] [CrossRef]
- Yukisawa, S.; Ishii, H.; Matsuyama, M.; Kuraoka, K.; Takano, K.; Kamei, A.; Ozaka, M. Outcomes and tolerability of systemic chemotherapy for pancreatic or biliary cancer patients aged 75 years or older. Jpn. J. Clin. Oncol. 2011, 41, 76–80. [Google Scholar] [CrossRef]
- Okano, N.; Kasuga, A.; Kawai, K.; Yamauchi, Y.; Kobayashi, T.; Naruge, D.; Nagashima, F.; Furuse, J. The Modified Glasgow Prognostic Score in Patients with Gemcitabine-refractory Biliary Tract Cancer. Anticancer Res. 2018, 38, 1755–1761. [Google Scholar] [CrossRef]
- Tang, H.; Lu, W.; Li, B.; Li, C.; Xu, Y.; Dong, J. Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 36857–36868. [Google Scholar] [CrossRef]
- McNamara, M.G.; Templeton, A.J.; Maganti, M.; Walter, T.; Horgan, A.M.; McKeever, L.; Min, T.; Amir, E.; Knox, J.J. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur. J. Cancer 2014, 50, 1581–1589. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v.4.0. Available online: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 18 June 2023).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Okubo, S.; Nishiuma, S.; Kobayashi, N.; Taketsuna, M.; Taniai, H. Safety and effectiveness of gemcitabine in 260 patients with biliary tract cancer in a Japanese clinical practice based on post-marketing surveillance in Japan. Jpn. J. Clin. Oncol. 2012, 42, 1043–1053. [Google Scholar] [CrossRef]
- Sasaki, T.; Isayama, H.; Nakai, Y.; Togawa, O.; Kogure, H.; Ito, Y.; Yamamoto, K.; Mizuno, S.; Yagioka, H.; Yashima, Y.; et al. Prognostic factors in patients with advanced biliary tract cancer receiving chemotherapy. Cancer Chemother. Pharmacol. 2011, 67, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kan, M.; Kimura, G.; Umemoto, K.; Watanabe, K.; Sasaki, M.; Takahashi, H.; Hashimoto, Y.; Imaoka, H.; Ohno, I.; et al. Predictive factors of the treatment outcome in patients with advanced biliary tract cancer receiving gemcitabine plus cisplatin as first-line chemotherapy. J. Gastroenterol. 2019, 54, 281–290. [Google Scholar] [CrossRef]
- McNamara, M.G.; Bridgewater, J.; Lopes, A.; Wasan, H.; Malka, D.; Jensen, L.H.; Okusaka, T.; Knox, J.J.; Wagner, D.; Cunningham, D.; et al. Systemic therapy in younger and elderly patients with advanced biliary cancer: Sub-analysis of ABC-02 and twelve other prospective trials. BMC Cancer 2017, 17, 262. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.G.; de Liguori Carino, N.; Kapacee, Z.A.; Lamarca, A.; Valle, J.W. Outcomes in older patients with biliary tract cancer. Eur. J. Surg. Oncol. 2021, 47, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.S.; Winther, S.B.; Chen, I.M.; Weber, B.; Ventzel, L.; Liposits, G.; Johansen, J.S.; Detlefsen, S.; Egendal, I.; Shim, S.; et al. A randomized phase II study of full dose gemcitabine versus reduced dose gemcitabine and nab-paclitaxel in vulnerable patients with non-resectable pancreatic cancer (DPCG-01). BMC Cancer 2023, 23, 552. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Takeda, T.; Okamoto, T.; Ozaka, M.; Sasahira, N. Chemotherapy for Biliary Tract Cancer in 2021. J. Clin. Med. 2021, 10, 3108. [Google Scholar] [CrossRef] [PubMed]
| Elderly Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Elderly | Elderly | Monotherapy | Combination | |||||||
| (n = 191) | (n = 92) | p-Value | (n = 54) | (n = 38) | p-Value | |||||
| Age in years, median (range) | 67 | (24–74) | 78.5 | (75–89) | <0.001 | 81 | (75–89) | 77 | (75–82) | <0.001 |
| Male (n, %) | 116 | 60.7% | 55 | 59.8% | 0.878 | 35 | 64.8% | 20 | 52.6% | 0.241 |
| Body mass index, median (range) | 20.8 | (13.6–34.4) | 20.6 | (14.1–29.5) | 0.352 | 20.5 | (14.9–28.9) | 21.7 | (14.1–29.5) | 0.168 |
| Performance status, 0/1/2 | 156/34/1 | 61/29/2 | 0.013 | 30/22/2 | 31/7/0 | 0.027 | ||||
| Primary cancer (n, %) | ||||||||||
| Intrahepatic | 48 | 25.1% | 15 | 16.3% | 0.095 | 9 | 16.7% | 6 | 15.8% | 0.911 |
| Extrahepatic (perihilar) | 51 | 26.7% | 28 | 30.4% | 0.512 | 18 | 33.3% | 10 | 26.3% | 0.471 |
| Extrahepatic (distal) | 24 | 12.6% | 27 | 29.3% | <0.001 | 13 | 24.1% | 14 | 36.8% | 0.185 |
| Gallbladder | 51 | 26.7% | 18 | 19.6% | 0.190 | 13 | 24.1% | 5 | 13.2% | 0.194 |
| Ampulla | 17 | 8.9% | 4 | 4.3% | 0.171 | 1 | 1.9% | 3 | 7.9% | 0.303 |
| Cancer status (n, %) | ||||||||||
| Locally advanced | 33 | 17.3% | 13 | 14.1% | 0.501 | 5 | 9.3% | 8 | 21.1% | 0.110 |
| Metastatic | 96 | 50.3% | 42 | 45.7% | 0.467 | 26 | 48.1% | 16 | 42.1% | 0.567 |
| Recurrent | 62 | 32.5% | 37 | 40.2% | 0.200 | 23 | 42.6% | 14 | 36.8% | 0.580 |
| Location of metastases 1 | ||||||||||
| Liver | 68 | 35.6% | 32 | 34.8% | 0.893 | 21 | 38.9% | 11 | 28.9% | 0.324 |
| Lung | 25 | 13.1% | 13 | 14.1% | 0.810 | 5 | 9.3% | 8 | 21.1% | 0.110 |
| Lymph nodes | 62 | 32.5% | 25 | 27.2% | 0.367 | 15 | 27.8% | 10 | 26.3% | 0.877 |
| Peritoneal dissemination | 52 | 27.2% | 23 | 25.0% | 0.691 | 14 | 25.9% | 9 | 23.7% | 0.807 |
| Bone | 6 | 3.1% | 1 | 1.1% | 0.297 | 1 | 1.9% | 0 | 0.0% | >0.999 |
| Laboratory data | ||||||||||
| Modified Glasgow prognostic score, 0/1/2 | 127/32/32 | 52/17/23 | 0.207 | 27/11/16 | 25/6/7 | 0.308 | ||||
| Neutrophil-to-lymphocyte ratio, median (range) | 2.5 | (0.4–30.7) | 2.7 | (0.7–14.0) | 0.955 | 2.8 | (0.7–14.0) | 2.6 | (1.6–12.3) | 0.943 |
| CEA, ng/mL, median (range) | 3.3 | (0.5–1398) | 4.0 | (1.1–386) | 0.238 | 4.0 | (1.1–386) | 4.0 | (1.2–358) | 0.643 |
| CA19-9, U/mL, median (range) | 159 | (2–50,000) | 177 | (2–50,000) | 0.739 | 159 | (2–50,000) | 177 | (2–50,000) | 0.883 |
| Elderly Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n, %) | Non-Elderly | Elderly | Monotherapy | Combination | ||||||
| (n = 191) | (n = 92) | p-Value | (n = 54) | (n = 38) | p-Value | |||||
| First-line chemotherapy | 191 | 100.0% | 92 | 100.0% | - | 54 | 100.0% | 38 | 100.0% | |
| Combination therapy | 173 | 90.6% | 38 | 41.3% | <0.001 | |||||
| Gemcitabine + cisplatin + S-1 | 19 | 9.9% | 1 | 1.1% | 0.006 | 1 | 2.6% | |||
| Gemcitabine + cisplatin | 150 | 78.5% | 34 | 37.0% | <0.001 | 34 | 89.5% | |||
| Gemcitabine + S-1 | 4 | 2.1% | 3 | 3.3% | 0.686 | 3 | 7.9% | |||
| Monotherapy | 18 | 9.4% | 54 | 58.7% | <0.001 | |||||
| Gemcitabine | 17 | 8.9% | 36 | 39.1% | <0.001 | 36 | 66.7% | |||
| S-1 | 1 | 0.5% | 18 | 19.6% | <0.001 | 18 | 33.3% | |||
| Response to first-line chemotherapy | ||||||||||
| Complete response | 1 | 0.5% | 0 | 0.0% | >0.999 | 0 | 0.0% | 0 | 0.0% | - |
| Partial response | 22 | 11.5% | 6 | 6.5% | 0.187 | 1 | 1.9% | 5 | 13.2% | 0.078 |
| Stable disease | 112 | 58.6% | 48 | 52.2% | 0.304 | 26 | 48.1% | 22 | 57.9% | 0.357 |
| Progressive disease | 40 | 20.9% | 26 | 28.3% | 0.173 | 18 | 33.3% | 8 | 21.1% | 0.198 |
| Not evaluated | 16 | 8.4% | 12 | 13.0% | 0.218 | 9 | 16.7% | 3 | 7.9% | 0.347 |
| Overall response rate | 13.1% | 7.5% | 0.188 | 2.2% | 14.3% | 0.081 | ||||
| Disease control rate | 77.1% | 67.5% | 0.103 | 60.0% | 77.1% | 0.104 | ||||
| Reason for termination of first-line chemotherapy | ||||||||||
| Disease progression | 144 | 75.4% | 77 | 83.7% | 0.114 | 45 | 83.3% | 32 | 84.2% | 0.911 |
| Intolerance | 11 | 5.8% | 6 | 6.5% | 0.800 | 5 | 9.3% | 1 | 2.6% | 0.395 |
| Conversion surgery | 17 | 8.9% | 3 | 3.3% | 0.083 | 0 | 0.0% | 3 | 7.9% | 0.067 |
| Patient refusal | 7 | 3.7% | 1 | 1.1% | 0.220 | 1 | 1.9% | 0 | 0.0% | >0.999 |
| Treatment ongoing | 2 | 1.0% | 1 | 1.1% | >0.999 | 0 | 0.0% | 1 | 2.6% | 0.413 |
| Other | 10 | 5.2% | 4 | 4.3% | 3 | 5.6% | 1 | 2.6% | ||
| Second-line chemotherapy | 77 | 40.3% | 36 | 39.1% | 0.849 | 16 | 29.6% | 20 | 52.6% | 0.026 |
| Gemcitabine + cisplatin | 5 | 2.6% | 2 | 2.2% | 0 | 0.0% | 2 | 5.3% | 0.168 | |
| Gemcitabine + S-1 | 4 | 2.1% | 1 | 1.1% | 0 | 0.0% | 1 | 2.6% | 0.413 | |
| Gemcitabine | 0 | 0.0% | 2 | 2.2% | 2 | 3.7% | 0 | 0.0% | 0.510 | |
| S-1 | 64 | 33.5% | 31 | 33.7% | 14 | 25.9% | 17 | 44.7% | 0.060 | |
| Other | 4 | 2.1% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | - | |
| All Grades | Grades 3–4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n, %) | Non-Elderly | Elderly | Non-Elderly | Elderly | ||||||
| (n = 191) | (n = 92) | p-Value | (n = 191) | (n = 92) | p-Value | |||||
| All adverse events | 191 | 100.0% | 92 | 100.0% | - | 134 | 70.2% | 51 | 55.4% | 0.015 |
| Hematologic adverse events | ||||||||||
| Leukopenia | 141 | 73.8% | 60 | 65.2% | 0.135 | 55 | 28.8% | 12 | 13.0% | 0.004 |
| Neutropenia | 152 | 79.6% | 67 | 72.8% | 0.203 | 103 | 53.9% | 33 | 35.9% | 0.004 |
| Anemia | 186 | 97.4% | 90 | 97.8% | >0.999 | 42 | 22.0% | 19 | 20.7% | 0.798 |
| Thrombocytopenia | 148 | 77.5% | 70 | 76.1% | 0.793 | 14 | 7.3% | 6 | 6.5% | 0.804 |
| Febrile neutropenia | 0 | 0.0% | 0 | 0.0% | - | 0 | 0.0% | 0 | 0.0% | - |
| Non-hematologic adverse events | ||||||||||
| Stomatitis | 45 | 23.6% | 9 | 9.8% | 0.006 | 1 | 0.5% | 0 | 0.0% | >0.999 |
| Decreased appetite | 28 | 14.7% | 21 | 22.8% | 0.089 | 1 | 0.5% | 1 | 1.1% | 0.545 |
| Diarrhea | 32 | 16.8% | 16 | 17.4% | 0.894 | 0 | 0.0% | 0 | 0.0% | - |
| Constipation | 153 | 80.1% | 54 | 58.7% | <0.001 | 0 | 0.0% | 0 | 0.0% | - |
| Nausea/vomiting | 107 | 56.0% | 30 | 32.6% | <0.001 | 1 | 0.5% | 0 | 0.0% | >0.999 |
| Peripheral neuropathy | 64 | 33.5% | 14 | 15.2% | 0.001 | 0 | 0.0% | 0 | 0.0% | - |
| Alopecia | 9 | 4.7% | 5 | 5.4% | 0.776 | 0 | 0.0% | 0 | 0.0% | - |
| Fatigue | 166 | 86.9% | 70 | 76.1% | 0.022 | 1 | 0.5% | 2 | 2.2% | 0.248 |
| Elevated transaminases | 173 | 90.6% | 82 | 89.1% | 0.703 | 25 | 13.1% | 5 | 5.4% | 0.050 |
| Decreased renal function | 35 | 18.3% | 28 | 30.4% | 0.022 | 0 | 0.0% | 1 | 1.1% | 0.325 |
| Interstitial pneumonitis | 0 | 0.0% | 2 | 2.2% | 0.105 | 0 | 0.0% | 2 | 2.2% | 0.105 |
| Rash | 43 | 22.5% | 17 | 18.5% | 0.437 | 1 | 0.5% | 0 | 0.0% | >0.999 |
| All Grades | Grades 3–4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n, %) | Mono- Therapy | Combination | Mono- Therapy | Combination | ||||||
| (n = 54) | (n = 38) | p-Value | (n = 54) | (n = 38) | p-Value | |||||
| All adverse events | 54 | 100.0% | 38 | 100.0% | - | 27 | 50.0% | 24 | 63.2% | 0.211 |
| Hematologic adverse events | ||||||||||
| Leukopenia | 31 | 57.4% | 29 | 76.3% | 0.061 | 6 | 11.1% | 6 | 15.8% | 0.543 |
| Neutropenia | 36 | 66.7% | 31 | 81.6% | 0.113 | 16 | 29.6% | 17 | 44.7% | 0.137 |
| Anemia | 52 | 96.3% | 38 | 100.0% | 0.510 | 9 | 16.7% | 10 | 26.3% | 0.260 |
| Thrombocytopenia | 40 | 74.1% | 30 | 78.9% | 0.589 | 2 | 3.7% | 4 | 10.5% | 0.226 |
| Febrile neutropenia | 0 | 0.0% | 0 | 0.0% | - | 0 | 0.0% | 0 | 0.0% | - |
| Non-hematologic adverse events | ||||||||||
| Stomatitis | 5 | 9.3% | 4 | 10.5% | >0.999 | 0 | 0.0% | 0 | 0.0% | - |
| Decreased appetite | 12 | 22.2% | 9 | 23.7% | 0.869 | 0 | 0.0% | 1 | 2.6% | 0.413 |
| Diarrhea | 6 | 11.1% | 10 | 26.3% | 0.058 | 0 | 0.0% | 0 | 0.0% | - |
| Constipation | 23 | 42.6% | 31 | 81.6% | <0.001 | 0 | 0.0% | 0 | 0.0% | - |
| Nausea/vomiting | 12 | 22.2% | 18 | 47.4% | 0.011 | 0 | 0.0% | 0 | 0.0% | - |
| Peripheral neuropathy | 4 | 7.4% | 10 | 26.3% | 0.013 | 0 | 0.0% | 0 | 0.0% | - |
| Alopecia | 1 | 1.9% | 4 | 10.5% | 0.156 | 0 | 0.0% | 0 | 0.0% | - |
| Fatigue | 34 | 63.0% | 36 | 94.7% | <0.001 | 0 | 0.0% | 2 | 5.3% | 0.168 |
| Elevated transaminases | 47 | 87.0% | 35 | 92.1% | 0.515 | 4 | 7.4% | 1 | 2.6% | 0.400 |
| Decreased renal function | 19 | 35.2% | 9 | 23.7% | 0.238 | 1 | 1.9% | 0 | 0.0% | >0.999 |
| Interstitial pneumonitis | 1 | 1.9% | 1 | 2.6% | >0.999 | 1 | 1.9% | 1 | 2.6% | >0.999 |
| Rash | 9 | 16.7% | 8 | 21.1% | 0.594 | 0 | 0.0% | 0 | 0.0% | - |
| Univariate | Multivariate (Predictors Only) | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Baseline characteristics | ||||||
| Male sex | 0.98 | 0.76–1.27 | 0.904 | |||
| Elderly (75 years or older) | 1.33 | 1.02–1.73 | 0.039 | 1.07 | 0.78–1.48 | 0.669 |
| Performance status (1 or 2) | 1.51 | 1.12–2.02 | 0.008 | 1.03 | 0.74–1.44 | 0.859 |
| Tumor characteristics | ||||||
| Locally advanced (vs. metastatic or recurrence) | 0.58 | 0.40–0.84 | 0.003 | 0.93 | 0.59–1.49 | 0.769 |
| Gallbladder cancer | 1.57 | 1.18–2.09 | 0.002 | 1.30 | 0.94–1.79 | 0.114 |
| Extrahepatic (perihilar) cholangiocarcinoma | 0.80 | 0.60–1.06 | 0.120 | 1.04 | 0.75–1.45 | 0.808 |
| Liver metastasis | 1.33 | 1.03–1.73 | 0.031 | 1.40 | 1.02–1.92 | 0.038 |
| Lung metastasis | 1.34 | 0.94–1.91 | 0.108 | |||
| Lymph node metastasis | 1.37 | 1.05–1.79 | 0.021 | 1.28 | 0.94–1.74 | 0.113 |
| Peritoneal dissemination metastasis | 1.48 | 1.12–1.96 | 0.006 | 1.36 | 0.99–1.86 | 0.057 |
| Bone metastasis | 2.11 | 0.99–4.50 | 0.052 | |||
| Laboratory values | ||||||
| Neutrophil-to-lymphocyte ratio (3 or more) | 1.69 | 1.31–2.17 | <0.001 | 1.57 | 1.20–2.04 | <0.001 |
| mGPS (1 or 2) | 1.87 | 1.45–2.42 | <0.001 | 1.65 | 1.25–2.17 | <0.001 |
| CEA (5 ng/mL or more) | 2.06 | 1.59–2.65 | <0.001 | 1.73 | 1.32–2.27 | <0.001 |
| CA19-9 (500 U/mL or more) | 1.48 | 1.15–1.92 | 0.003 | 1.18 | 0.90–1.55 | 0.243 |
| Treatment | ||||||
| Monotherapy | 1.73 | 1.31–2.28 | <0.001 | 1.39 | 0.97–1.99 | 0.074 |
| First-line GCS | 0.39 | 0.20–0.76 | 0.005 | 0.43 | 0.22–0.85 | 0.016 |
| Any second-line chemotherapy | 0.69 | 0.54–0.89. | 0.005 | |||
| Conversion surgery | 0.21 | 0.11–0.40 | <0.001 | |||
| Univariate | Multivariate (Predictors only) | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Baseline characteristics | ||||||
| Male sex | 1.01 | 0.65–1.57 | 0.980 | |||
| Performance status (1 or 2) | 1.13 | 0.72–1.78 | 0.587 | |||
| Tumor characteristics | ||||||
| Recurrence | 1.21 | 0.78–1.88 | 0.386 | |||
| Extrahepatic (perihilar) cholangiocarcinoma | 0.57 | 0.35–0.94 | 0.021 | 1.01 | 0.62–1.66 | 0.971 |
| Liver metastasis | 1.80 | 1.14–2.84 | 0.140 | |||
| Lung metastasis | 1.42 | 0.76–2.63 | 0.268 | |||
| Lymph node metastasis | 1.19 | 0.74–1.92 | 0.470 | |||
| Peritoneal dissemination metastasis | 1.12 | 0.68–1.82 | 0.667 | |||
| Bone metastasis | 3.41 | 0.46–25.2 | 0.230 | |||
| Laboratory values | ||||||
| Neutrophil-to-lymphocyte ratio (3 or more) | 1.59 | 1.03–2.45 | 0.036 | 1.28 | 0.80–2.04 | 0.303 |
| mGPS (1 or 2) | 2.35 | 1.51–3.67 | <0.001 | 2.24 | 1.42–3.54 | <0.001 |
| CEA (5 ng/mL or more) | 1.87 | 1.20–2.92 | 0.006 | 1.70 | 1.03–2.79 | 0.036 |
| CA19-9 (37 U/mL or more) | 0.90 | 0.56–1.43 | 0.651 | |||
| Treatment | ||||||
| Monotherapy | 1.78 | 1.14–2.78 | 0.012 | 1.48 | 0.93–2.35 | 0.102 |
| Any second-line chemotherapy | 0.62 | 0.40–0.97 | 0.036 | |||
| Conversion surgery | 0.12 | 0.17–0.90 | 0.040 | |||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Baseline characteristics | ||||||
| Male sex | 1.01 | 0.78–1.32 | 0.916 | |||
| Elderly (75 years or older) | 1.47 | 1.12–1.94 | 0.006 | 1.44 | 1.04–1.99 | 0.029 |
| Performance status (1 or 2) | 1.21 | 0.89–1.65 | 0.233 | |||
| Tumor characteristics | ||||||
| Locally advanced (vs. metastatic or recurrence) | 0.47 | 0.31–0.70 | <0.001 | 0.69 | 0.42–1.18 | 0.130 |
| Gallbladder cancer | 1.45 | 1.07–1.92 | 0.017 | 1.21 | 0.87–1.68 | 0.252 |
| Liver metastasis | 1.49 | 1.13–1.96 | 0.005 | 1.47 | 1.06–2.04 | 0.023 |
| Lung metastasis | 1.60 | 1.11–2.29 | 0.016 | 1.69 | 1.15–2.47 | 0.007 |
| Lymph node metastasis | 1.33 | 1.01–1.76 | 0.041 | 1.25 | 0.91–1.70 | 0.167 |
| Peritoneal dissemination metastasis | 1.36 | 1.03–1.82 | 0.034 | 1.28 | 0.93–1.77 | 0.133 |
| Bone metastasis | 1.51 | 0.67–3.39 | 0.325 | |||
| Laboratory values | ||||||
| Neutrophil-to-lymphocyte ratio (3 or more) | 1.69 | 1.30–2.20 | <0.001 | 1.64 | 1.24–2.16 | <0.001 |
| mGPS (1 or 2) | 1.63 | 1.25–2.14 | <0.001 | 1.31 | 0.98–1.75 | 0.072 |
| CEA (5 ng/mL or more) | 1.59 | 1.22–2.07 | <0.001 | 1.27 | 0.96–1.69 | 0.101 |
| CA19-9 (500 ng/mL or more) | 1.55 | 1.18–2.02 | <0.001 | 1.29 | 0.97–1.72 | 0.084 |
| Treatment | ||||||
| Monotherapy | 1.59 | 1.19–2.13 | 0.002 | 1.20 | 0.85–1.69 | 0.313 |
| First-line GCS | 0.52 | 0.29–0.93 | 0.028 | 0.56 | 0.30–1.02 | 0.060 |
| Univariate | Multivariate (Predictors Only) | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Baseline characteristics | ||||||
| Male sex | 1.17 | 0.75–1.83 | 0.494 | |||
| Performance status (1 or 2) | 0.97 | 0.61–1.57 | 0.914 | |||
| Tumor characteristics | ||||||
| Locally advanced (vs. metastatic or recurrence) | 0.40 | 0.20–0.80 | 0.010 | 0.64 | 0.28–1.43 | 0.274 |
| Extrahepatic (perihilar) cholangiocarcinoma | 0.46 | 0.43–0392 | 0.022 | 0.67 | 0.39–1.15 | 0.150 |
| Liver metastasis | 2.61 | 1.63–4.19 | <0.001 | 2.10 | 1.26–3.47 | 0.003 |
| Lung metastasis | 2.40 | 1.27–4.52 | 0.007 | 3.10 | 1.54–6.19 | 0.001 |
| Lymph node metastasis | 1.01 | 0.62–1.65 | 0.977 | |||
| Peritoneal dissemination metastasis | 1.14 | 0.70–1.87 | 0.595 | |||
| Bone metastasis | 2.81 | 0.38–20.7 | 0.310 | |||
| Laboratory values | ||||||
| Neutrophil-to-lymphocyte ratio (3 or more) | 1.58 | 1.02–2.46 | 0.042 | 1.10 | 0.66–1.85 | 0.715 |
| mGPS (1 or 2) | 1.69 | 1.07–2.66 | 0.023 | 2.07 | 1.24–3.45 | 0.006 |
| CEA (5 ng/mL or more) | 2.52 | 1.59–4.01 | <0.001 | 1.87 | 1.05–3.35 | 0.035 |
| CA19-9 (37 ng/mL or more) | 0.97 | 0.60–1.57 | 0.901 | |||
| Treatment | ||||||
| Monotherapy | 1.38 | 0.88–2.16 | 0.163 | |||
| % Aged: | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial Name | Year | Phase | Treatment | Line | n | Upper Age Limit (Inclusion Criteria) | Oldest (Years) | Median Age | ≥65 | ≥70 | ≥75 | PS |
| FUGA-BT [7] | 2019 | III | GC vs. GS | 1 | 354 | 79 | 79 | 67/67 | 64% | 17% | 0–1 | |
| PRODIGE 12-ACCORD 18-UNICANCER GI [9] | 2019 | III | Gemcitabine/Oxaliplatin vs. observation | Adjuvant | 196 | None | 83 | 63/63 | 0–2 | |||
| BILCAP [10] | 2019 | III | Capecitabine vs. observation | Adjuvant | 447 | None | 69 | 62/64 | 0% | 0% | 0–1 | |
| ClarIDHy [11] | 2020 | III | Ivodenib vs. placebo | 2 or 3 | 185 | None | 83 | 61/63 | 0–1 | |||
| FIGHT-202 [12] | 2020 | III | Pemigatinib | 2 | 146 | None | 78 | 59 | 32% | 8% | 0–2 | |
| ABC-06 [13] | 2021 | III | FOLFOX vs. ASC | 2 | 162 | None | 84 | 65/65 | 50% | 0–2 | ||
| NIFTY [14] | 2021 | IIb | 5-FU/LV ± Nanoliposomal irinotacan | 2 | 174 | None | 84 | 63/65 | 48% | 0–1 | ||
| TOPAZ-1 [15] | 2022 | III | GC ± Durvalumab | 1 | 685 | None | 85 | 64/64 | 47% | 0–1 | ||
| KHBO1401-MITSUBA [16] | 2023 | III | GC ± S-1 | 1 | 246 | None | 84 | 68/68 | 0–2 | |||
| KEYNOTE-966 [17] | 2023 | III | GC ± Pembrolizumab | 1 | 1069 | None | 71 | 64/63 | 47% | 0–1 | ||
| (This study) | 2023 | - | GCS, GC, GS, Gemcitabine, S-1 | 1 | 283 | None | 89 | 70 | 74% | 54% | 33% | 0–2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, T.; Takeda, T.; Sasaki, T.; Hamada, T.; Mie, T.; Ishitsuka, T.; Yamada, M.; Nakagawa, H.; Hirai, T.; Furukawa, T.; et al. Safety and Effectiveness of Chemotherapy in Elderly Biliary Tract Cancer Patients. Curr. Oncol. 2023, 30, 7229-7240. https://doi.org/10.3390/curroncol30080524
Okamoto T, Takeda T, Sasaki T, Hamada T, Mie T, Ishitsuka T, Yamada M, Nakagawa H, Hirai T, Furukawa T, et al. Safety and Effectiveness of Chemotherapy in Elderly Biliary Tract Cancer Patients. Current Oncology. 2023; 30(8):7229-7240. https://doi.org/10.3390/curroncol30080524
Chicago/Turabian StyleOkamoto, Takeshi, Tsuyoshi Takeda, Takashi Sasaki, Tsuyoshi Hamada, Takafumi Mie, Takahiro Ishitsuka, Manabu Yamada, Hiroki Nakagawa, Tatsuki Hirai, Takaaki Furukawa, and et al. 2023. "Safety and Effectiveness of Chemotherapy in Elderly Biliary Tract Cancer Patients" Current Oncology 30, no. 8: 7229-7240. https://doi.org/10.3390/curroncol30080524
APA StyleOkamoto, T., Takeda, T., Sasaki, T., Hamada, T., Mie, T., Ishitsuka, T., Yamada, M., Nakagawa, H., Hirai, T., Furukawa, T., Kasuga, A., Ozaka, M., & Sasahira, N. (2023). Safety and Effectiveness of Chemotherapy in Elderly Biliary Tract Cancer Patients. Current Oncology, 30(8), 7229-7240. https://doi.org/10.3390/curroncol30080524





