A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada
Abstract
1. Introduction
2. Systemic Therapy for Unresectable Advanced or Metastatic Biliary Tract Cancer
2.1. Recommendations
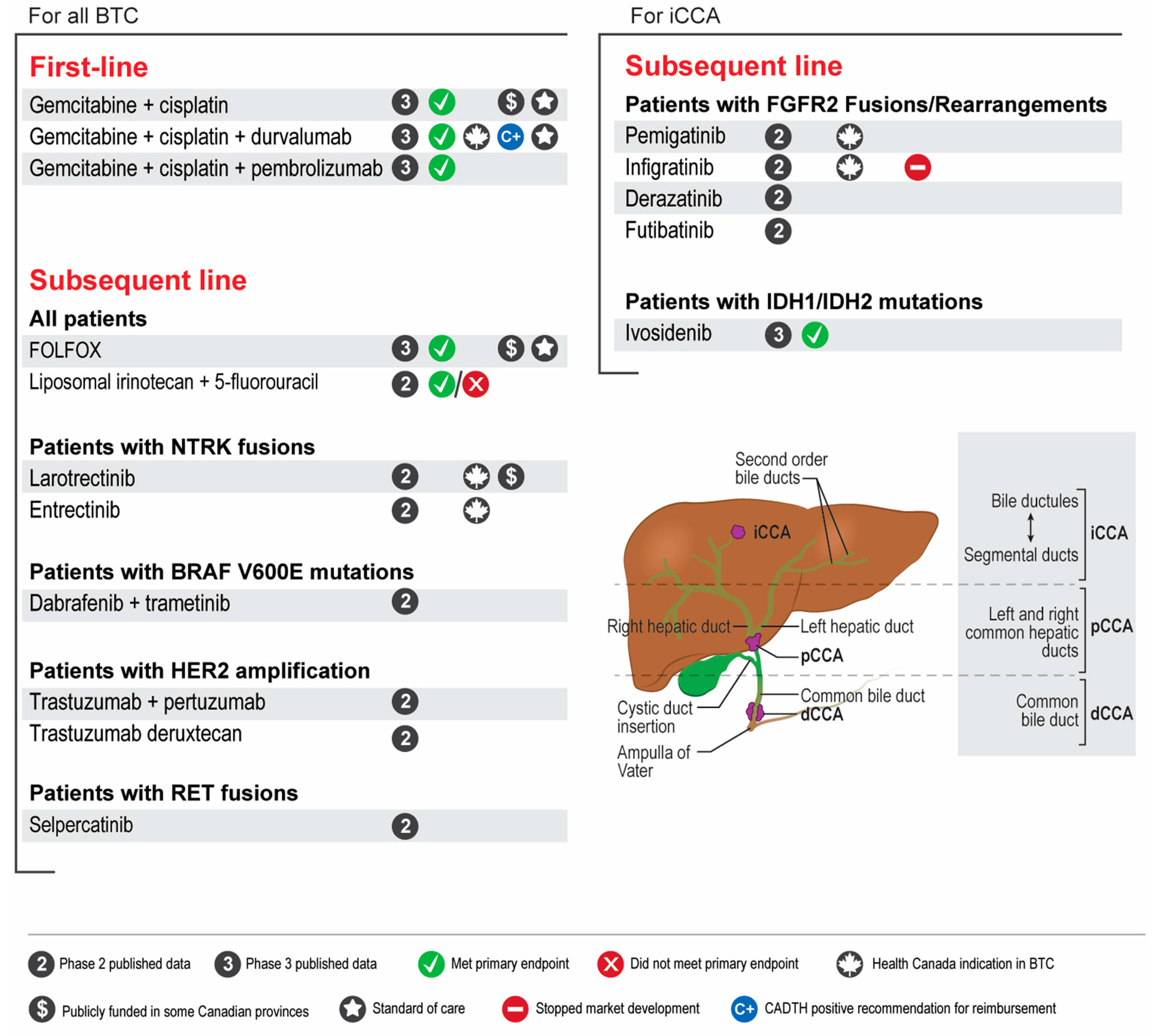
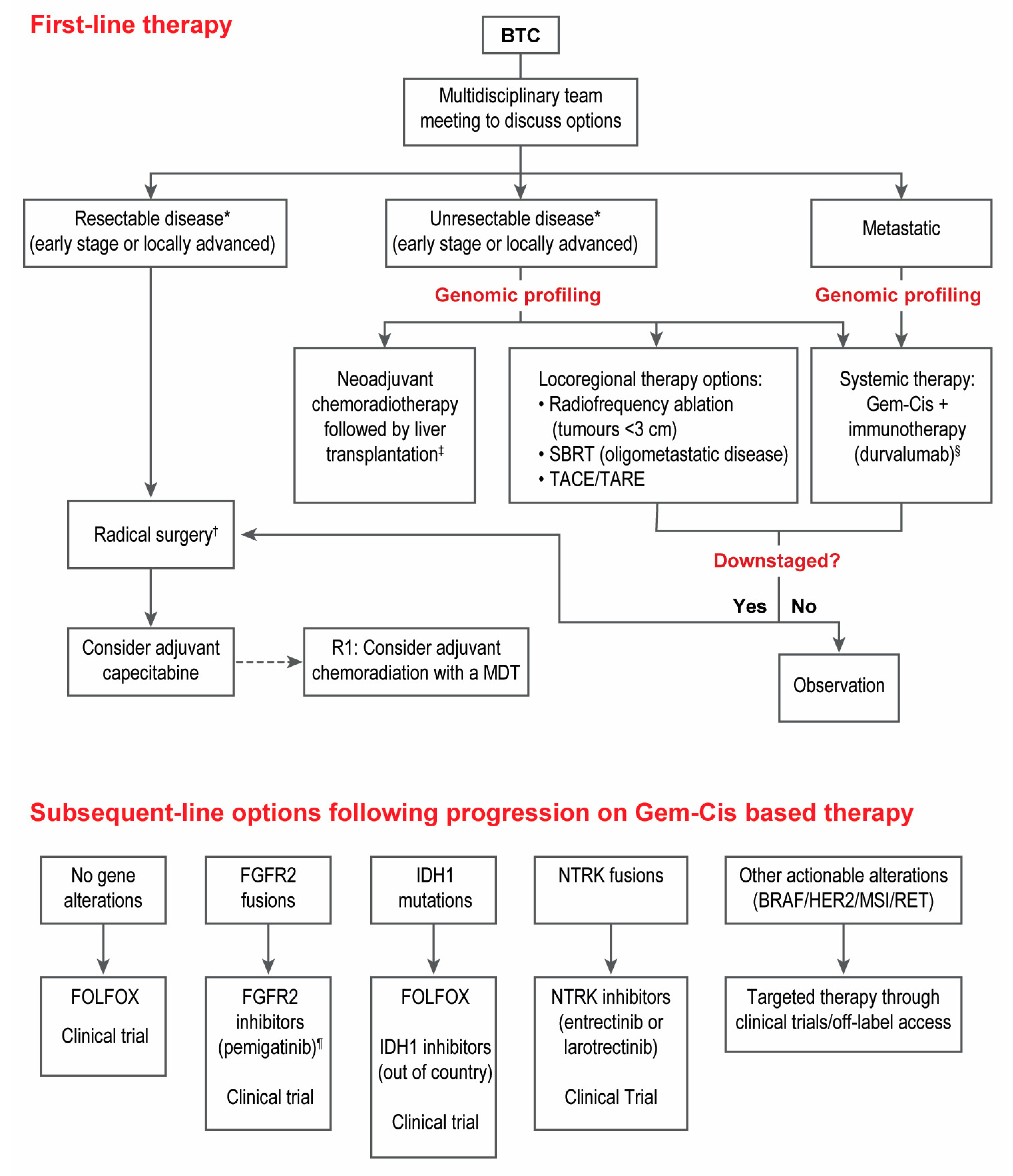
- Patients with advanced unresectable or metastatic BTC should be considered for first-line treatment with gemcitabine-cisplatin plus immunotherapy (durvalumab).
- Genomic profiling of relevant BTC genes by next-generation sequencing is strongly suggested for all patients with advanced unresectable or metastatic BTC that are fit to receive systemic therapy. Profiling is preferred at diagnosis to allow for treatment planning and access to targeted agents in the second line.
- FOLFOX should be considered in the second line setting after progressing on gemcitabine-cisplatin-based therapy for patients with advanced BTC with no actionable genomic alterations.
- Patients with CCA who harbour FGFR2 fusions should be considered for treatment with FGFR2 inhibitors (pemigatinib) after progressing on one prior line of systemic therapy.
- Patients with CCA who harbour IDH1 mutations do not have access to IDH1 inhibitors in Canada. Alternative means of access may be considered but is challenging.
- Patients with BTC who harbour NTRK fusions should be considered for treatment with NTRK inhibitors (entrectinib or larotrectinib) after progressing on one prior line of systemic therapy.
- Patients with BTC who harbour other actionable genomic alterations (e.g., BRAF, HER2, RET, MSI) should be considered for targeted therapy through clinical trials or other means of access.
- Locoregional therapies for palliation should be considered and discussed with multidisciplinary teams.
2.2. Discussion on First-Line Systemic Therapies
2.3. Discussion on Subsequent-Line Systemic Therapies
2.4. Discussion on Locoregional Therapy for Palliation
3. Adjuvant Therapy for Biliary Tract Cancer
3.1. Recommendations
- 9.
- Patients with BTC should be considered for adjuvant chemotherapy with capecitabine following curative-intent resection.
3.2. Discussion on Adjuvant Therapy
4. Neoadjuvant Therapy for Biliary Tract Cancer
4.1. Recommendations
- 10.
- There is no randomized data supporting the routine use of neoadjuvant treatment in surgically resectable BTC. However, cases can be reviewed in a multi-disciplinary fashion where downstaging may be warranted in borderline cases.
4.2. Discussion on Neoadjuvant Therapy
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef] [PubMed]
- Baria, K.; De Toni, E.N.; Yu, B.; Jiang, Z.; Kabadi, S.M.; Malvezzi, M. Worldwide Incidence and Mortality of Biliary Tract Cancer. Gastro Hep Adv. 2022, 1, 618–626. [Google Scholar] [CrossRef]
- Van Dyke, A.L.; Shiels, M.S.; Jones, G.S.; Pfeiffer, R.M.; Petrick, J.L.; Beebe-Dimmer, J.L.; Koshiol, J. Biliary tract cancer incidence and trends in the United States by demographic group, 1999–2013. Cancer 2019, 125, 1489–1498. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Piñeros, M.; Ferreccio, C.; Adsay, V.; Soerjomataram, I.; Bray, F.; Koshiol, J. Gallbladder and extrahepatic bile duct cancers in the Americas: Incidence and mortality patterns and trends. Int. J. Cancer 2020, 147, 978–989. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, L.; Li, F.; Li, Q.; Yuan, S.; Huang, S.; Fu, Y.; Yan, X.; Chen, J.; Li, H.; et al. The epidemiological trends of biliary tract cancers in the United States of America. BMC Gastroenterol. 2022, 22, 546. [Google Scholar] [CrossRef]
- Xiao, Y.; Cattelan, L.; Lagacé, F.; Ghazawi, F.M.; Alakel, A.; Grose, E.; Le, M.; Nechaev, V.; Sasseville, D.; Waschke, K.; et al. Epidemiologic trends and geographic distribution of patients with gallbladder and extrahepatic biliary tract cancers in Canada. HPB 2021, 23, 1541–1549. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.C.; Patel, T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J. Hepatol. 2012, 57, 69–76. [Google Scholar] [CrossRef]
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.-M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. Can. Med. Assoc. J. 2022, 194, E601–E607. [Google Scholar] [CrossRef]
- Beaulieu, C.; Lui, A.; Yusuf, D.; Abdelaziz, Z.; Randolph, B.; Batuyong, E.; Ghosh, S.; Bathe, O.F.; Tam, V.; Spratlin, J.L. A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada. Curr. Oncol. 2021, 28, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Yu, B.; Kabadi, S.M.; Baria, K.; Shroff, R.T. Epidemiologic patterns of biliary tract cancer in the United States: 2001–2015. BMC Cancer 2022, 22, 1178. [Google Scholar] [CrossRef]
- Kang, M.J.; Lim, J.; Han, S.-S.; Park, H.M.; Kim, S.-W.; Lee, W.J.; Woo, S.M.; Kim, T.H.; Won, Y.-J.; Park, S.-J. Distinct prognosis of biliary tract cancer according to tumor location, stage, and treatment: A population-based study. Sci. Rep. 2022, 12, 10206. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Survival Rates for Bile Duct Cancer. Available online: https://www.cancer.org/cancer/bile-duct-cancer/detection-diagnosis-staging/survival-by-stage.html (accessed on 4 May 2023).
- American Cancer Society. Survival Rates for Gallbladder Cancer. Available online: https://www.cancer.org/cancer/types/gallbladder-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 4 May 2023).
- Horgan, A.; Knox, J.; Aneja, P.; Le, L.; McKeever, E.; McNamara, M. Patterns of care and treatment outcomes in older patients with biliary tract cancer. Oncotarget 2015, 6, 44995. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Moore, M.J.; Cox, T.F.; Valle, J.W.; Palmer, D.H.; McDonald, A.C.; Carter, R.; Tebbutt, N.C.; Dervenis, C.; Smith, D.; et al. Effect of Adjuvant Chemotherapy with Fluorouracil Plus Folinic Acid or Gemcitabine vs Observation on Survival in Patients with Resected Periampullary Adenocarcinoma. JAMA 2012, 308, 147. [Google Scholar] [CrossRef]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma. JAMA Surg. 2014, 149, 565. [Google Scholar] [CrossRef]
- Duignan, S.; Maguire, D.; Ravichand, C.S.; Geoghegan, J.; Hoti, E.; Fennelly, D.; Armstrong, J.; Rock, K.; Mohan, H.; Traynor, O. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: A single-centre national experience. HPB 2014, 16, 91–98. [Google Scholar] [CrossRef]
- Loveday, B.P.T.; Knox, J.J.; Dawson, L.A.; Metser, U.; Brade, A.; Horgan, A.M.; Gallinger, S.; Greig, P.D.; Moulton, C.-A. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J. Surg. Oncol. 2018, 117, 213–219. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.R.; Javle, M.; Kodali, S.; Saharia, A.; Mobley, C.; Heyne, K.; Hobeika, M.J.; Lunsford, K.E.; Victor, D.W.; Shetty, A.; et al. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am. J. Transplant. 2022, 22, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Butler, J.R.; Noguchi, D.; Ha, M.; Aziz, A.; Agopian, V.G.; Dinorcia, J.; Yersiz, H.; Farmer, D.G.; Busuttil, R.W.; et al. A 3-Decade, Single-Center Experience of Liver Transplantation for Cholangiocarcinoma: Impact of Era, Tumor Size, Location, and Neoadjuvant Therapy. Liver Transpl. 2022, 28, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Mauro, E.; Ferrer-Fàbrega, J.; Sauri, T.; Soler, A.; Cobo, A.; Burrel, M.; Iserte, G.; Forner, A. New Challenges in the Management of Cholangiocarcinoma: The Role of Liver Transplantation, Locoregional Therapies, and Systemic Therapy. Cancers 2023, 15, 1244. [Google Scholar] [CrossRef]
- Glimelius, B.; Hoffman, K.; Sjödén, P.O.; Jacobsson, G.; Sellström, H.; Enander, L.K.; Linné, T.; Svensson, C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann. Oncol. 1996, 7, 593–600. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Tam, V.C.; Ramjeesingh, R.; Burkes, R.; Yoshida, E.M.; Doucette, S.; Lim, H.J. Emerging Systemic Therapies in Advanced Unresectable Biliary Tract Cancer: Review and Canadian Perspective. Curr. Oncol. 2022, 29, 7072–7085. [Google Scholar] [CrossRef]
- Gennari, A.; Stockler, M.; Puntoni, M.; Sormani, M.; Nanni, O.; Amadori, D.; Wilcken, N.; D’Amico, M.; Decensi, A.; Bruzzi, P. Duration of Chemotherapy for Metastatic Breast Cancer: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Oncol. 2011, 29, 2144–2149. [Google Scholar] [CrossRef]
- Doherty, M.K.; McNamara, M.G.; Aneja, P.; McInerney, E.; Moignard, S.; Horgan, A.M.; Jiang, H.; Panzarella, T.; Jang, R.; Dhani, N.; et al. Long term responders to palliative chemotherapy for advanced biliary tract cancer. J. Gastrointest. Oncol. 2017, 8, 352–360. [Google Scholar] [CrossRef]
- Hyung, J.; Kim, B.; Yoo, C.; Kim, K.-P.; Jeong, J.H.; Chang, H.-M.; Ryoo, B.-Y. Clinical Benefit of Maintenance Therapy for Advanced Biliary Tract Cancer Patients Showing No Progression after First-Line Gemcitabine Plus Cisplatin. Cancer Res. Treat. 2019, 51, 901–909. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kalyan Mohanti, B.; Pal Chaudhary, S.; Sreenivas, V.; Kumar Sahoo, R.; Kumar Shukla, N.; Thulkar, S.; Pal, S.; Deo, S.V.; Pathy, S.; et al. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: Results of a phase III randomised controlled trial. Eur. J. Cancer 2019, 123, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Phelip, J.M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients with Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J. Clin. Oncol. 2022, 40, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, T.H.; Lee, I.S.; You, Y.K.; Lee, M.A. Comparison of the Efficacy between Gemcitabine-Cisplatin and Capecitabine-Cisplatin Combination Chemotherapy for Advanced Biliary Tract Cancer. Cancer Res. Treat. 2014, 47, 259–265. [Google Scholar] [CrossRef][Green Version]
- Markussen, A.; Jensen, L.H.; Diness, L.V.; Larsen, F.O. Treatment of Patients with Advanced Biliary Tract Cancer with Either Oxaliplatin, Gemcitabine, and Capecitabine or Cisplatin and Gemcitabine—A Randomized Phase II Trial. Cancers 2020, 12, 1975. [Google Scholar] [CrossRef]
- Shroff, R.T.; Guthrie, K.A.; Scott, A.J.; Borad, M.J.; Goff, L.W.; Matin, K.; Mahipal, A.; Kalyan, A.; Javle, M.M.; Aghajanian, C.; et al. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J. Clin. Oncol. 2023, 41, LBA490. [Google Scholar]
- Oh, D.; He, A.R.; Qin, S.; Chen, L.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. 56P -Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). Ann. Oncol. 2022, 33 (Suppl. S7), S19–S26. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Valle, J.W.; Wasan, H.; Lopes, A.; Backen, A.C.; Palmer, D.H.; Morris, K.; Duggan, M.; Cunningham, D.; Anthoney, D.A.; Corrie, P.; et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): A randomised phase 2 trial. Lancet Oncol. 2015, 16, 967–978. [Google Scholar] [CrossRef]
- Valle, J.W.; Vogel, A.; Denlinger, C.S.; He, A.R.; Bai, L.Y.; Orlova, R.; Van Cutsem, E.; Adeva, J.; Chen, L.T.; Obermannova, R.; et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: A randomised, double-blind, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 1468–1482. [Google Scholar] [CrossRef]
- Burris III, H.A.; Okusaka, T.; Vogel, A.; Lee, M.A.; Takahashi, H.; Breder, V.V.; Blanc, J.-F.; Li, J.; Watras, M.; Xiong, J.; et al. Patient-reported outcomes for the phase 3 TOPAZ-1 study of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. J. Clin. Oncol. 2022, 40, 4070. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Biliary Tract Cancers (Version 1.2023). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1438 (accessed on 5 May 2023).
- Lamarca, A.; Hubner, R.A.; David Ryder, W.; Valle, J.W. Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann. Oncol. 2014, 25, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Kim, I.H. 81P Final results from the NIFTY trial, a phase IIb, randomized, open-label study of liposomal irinotecan (nal-IRI) plus fluorouracil (5-FU)/leucovorin (LV) in patients (pts) with previously treated metastatic biliary tract cancer (BTC). Ann. Oncol. 2022, 33, S1464. [Google Scholar] [CrossRef]
- Vogel, A.; Wenzel, P.; Folprecht, G.; Schütt, P.; Wege, H.; Kretzschmar, A.; Jacobasch, L.; Ziegenhagen, N.; Boeck, S.; Kanzler, S.; et al. Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabine-based therapies (NALIRICC–AIO-HEP-0116). Ann. Oncol. 2022, 33 (Suppl. S7), S19–S26. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Kelley, R.K.; Roychowdhury, S.; Weiss, K.H.; Abou-Alfa, G.K.; Macarulla, T.; Sadeghi, S.; Waldschmidt, D.; Zhu, A.X.; Goyal, L.; et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann. Oncol. 2018, 29, viii720. [Google Scholar] [CrossRef]
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, S.; Borad, M.; Gallinson, D.; Murphy, A.; et al. Pemigatinib for previously treated locally advanced or metastatic cholangiocarcinoma: Final results from FIGHT-202. In Proceedings of the 16th International Liver Cancer Association Annual Conference, Madrid, Spain, 1–4 September 2022. [Google Scholar]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Droz dit Busset, M.; Shaib, W.L.; Mody, K.; Personeni, N.; Damjanov, N.; Harris, W.P.; Bergamo, F.; Brandi, G.; Masi, G.; Halfdanarson, T.; et al. Derazantinib for patients with intrahepatic cholangiocarcinoma harboring FGFR2 fusions/rearrangements: Primary results from the phase II study FIDES-01. Ann. Oncol. 2021, 32, S376–S381. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Morizane, C.; Valle, J.W.; Karasic, T.B.; Abrams, T.A.; Kelley, R.K.; Cassier, P.A.; Furuse, J.; et al. Updated results of the FOENIX-CCA2 trial: Efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/rearrangements. J. Clin. Oncol. 2022, 40, 4009. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma With IDH1 Mutation. JAMA Oncology. 2021, 7, 1669–1677. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Kawamoto, Y.; Komatsu, Y.; Ueno, M.; Kobayashi, S.; Ikeda, M.; Sasaki, M.; Furuse, J.; Okano, N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). J. Clin. Oncol. 2022, 40, 4006. [Google Scholar] [CrossRef]
- Canadian Agency for Drugs and Technology in Health. CADTH Reimbursement Recommendation Pemigatinib (Pemazyre). Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0252%20Pemazyre%20-%20CADTH%20Final%20Rec.pdf (accessed on 9 June 2022).
- Drilon, A.; Laetsch, T.W.; Kummar, S.; Dubois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib inTRKFusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion–positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.-I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients with Tumors with BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef]
- Okawa, Y.; Ebata, N.; Kim, N.K.D.; Fujita, M.; Maejima, K.; Sasagawa, S.; Nakamura, T.; Park, W.-Y.; Hirano, S.; Nakagawa, H. Actionability evaluation of biliary tract cancer by genome transcriptome analysis and Asian cancer knowledgebase. Oncotarget 2021, 12, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.; Jain, P.; El-Refai, S.M.; Azad, N.S.; Zabransky, D.J.; Baretti, M.; Shroff, R.T.; Kelley, R.K.; El-Khouiery, A.B.; Hockenberry, A.J.; et al. Clinical, Genomic, and Transcriptomic Data Profiling of Biliary Tract Cancer Reveals Subtype-Specific Immune Signatures. JCO Precis. Oncol. 2022, 6, e2100510. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.-R.; Kim, J.-W.; Cha, Y.; Ha, H.; Park, J.E.; Bang, J.-H.; Jin, M.H.; Lee, K.-H.; Kim, T.-Y.; Han, S.-W.; et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget 2016, 7, 58007–58021. [Google Scholar] [CrossRef]
- Han, K.; Ko, H.K.; Kim, K.W.; Won, H.J.; Shin, Y.M.; Kim, P.N. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. J. Vasc. Interv. Radiol. 2015, 26, 943–948. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Franzese, C.; Bonu, M.L.; Comito, T.; Clerici, E.; Loi, M.; Navarria, P.; Franceschini, D.; Pressiani, T.; Rimassa, L.; Scorsetti, M. Stereotactic body radiotherapy in the management of oligometastatic and recurrent biliary tract cancer: Single-institution analysis of outcome and toxicity. J. Cancer Res. Clin. Oncol. 2020, 146, 2289–2297. [Google Scholar] [CrossRef]
- Sebastian, N.T.; Tan, Y.; Miller, E.D.; Williams, T.M.; Alexandra Diaz, D. Stereotactic body radiation therapy is associated with improved overall survival compared to chemoradiation or radioembolization in the treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Transl. Radiat. Oncol. 2019, 19, 66–71. [Google Scholar] [CrossRef]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Groot Koerkamp, B.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef]
- Schartz, D.A.; Porter, M.; Schartz, E.; Kallas, J.; Gupta, A.; Butani, D.; Cantos, A. Transarterial Yttrium-90 Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2022, 33, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.G., 2nd; Simo, K.A.; Hansen, P.; Rocha, F.; Philips, P.; McMasters, K.M.; Tatum, C.M.; Kelly, L.R.; Driscoll, M.; Sharma, V.R.; et al. Drug-Eluting Bead, Irinotecan Therapy of Unresectable Intrahepatic Cholangiocarcinoma (DELTIC) with Concomitant Systemic Gemcitabine and Cisplatin. Ann. Surg. Oncol. 2022, 29, 5462–5473. [Google Scholar] [CrossRef]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A.; Fairchild, A.M.; Dennis, K.; Mahmud, A.; Stuckless, T.L.; Vincent, F.; Roberge, D.; Follwell, M.; Wong, R.K.W.; Jonker, D.J.; et al. Canadian Cancer Trials Group HE.1: A phase III study of palliative radiotherapy for symptomatic hepatocellular carcinoma and liver metastases. J. Clin. Oncol. 2023, 41, LBA492. [Google Scholar] [CrossRef]
- Gillespie, E.F.; Mathis, N.J.; Marine, C.; Zhang, Z.; Barker, C.A.; Guttmann, D.M.; Kotecha, R.; McIntosh, A.F.; Vaynrub, M.; Bartelstein, M.; et al. Prophylactic Radiation Therapy vs. Standard-of-Care for Patients with High-Risk, Asymptomatic Bone Metastases: A Multicenter, Randomized Phase II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 1059. [Google Scholar] [CrossRef]
- Mocan, T.; Horhat, A.; Mois, E.; Graur, F.; Tefas, C.; Craciun, R.; Nenu, I.; Spârchez, M.; Sparchez, Z. Endoscopic or percutaneous biliary drainage in hilar cholangiocarcinoma: When and how? World. J. Gastrointest. Oncol. 2021, 13, 2050–2063. [Google Scholar] [CrossRef]
- Queen, T.; Adler, D.G. Stent placement in perihilar cholangiocarcinoma. Clin. Liver Dis. 2014, 3, 74–78. [Google Scholar] [CrossRef]
- Taggar, A.S.; Mann, P.; Folkert, M.R.; Aliakbari, S.; Myrehaug, S.D.; Dawson, L.A. A systematic review of intraluminal high dose rate brachytherapy in the management of malignant biliary tract obstruction and cholangiocarcinoma. Radiother. Oncol. 2021, 165, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Khizar, H.; Hu, Y.; Wu, Y.; Ali, K.; Iqbal, J.; Zulqarnain, M.; Yang, J. Efficacy and Safety of Radiofrequency Ablation Plus Stent Versus Stent-alone Treatments for Malignant Biliary Strictures: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2023, 57, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sandru, V.; Ungureanu, B.S.; Stan-Ilie, M.; Oprita, R.; Balan, G.G.; Plotogea, O.-M.; Rinja, E.; Butuc, A.; Panaitescu, A.; Constantinescu, A.; et al. Efficacy of Endobiliary Radiofrequency Ablation in Preserving Survival, Performance Status and Chemotherapy Eligibility of Patients with Unresectable Distal Cholangiocarcinoma: A Case-Control Study. Diagnostics 2022, 12, 1804. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Naitoh, I.; Kitano, R.; Ibusuki, M.; Kobayashi, Y.; Sumida, Y.; Nakade, Y.; Ito, K.; Yoneda, M. Endobiliary Radiofrequency Ablation Combined with Gemcitabine and Cisplatin in Patients with Unresectable Extrahepatic Cholangiocarcinoma. Curr. Oncol. 2022, 29, 2240–2251. [Google Scholar] [CrossRef]
- Allen, M.J.; Knox, J.J. A review of current adjuvant and neoadjuvant systemic treatments for cholangiocarcinoma and gallbladder carcinoma. Hepatoma Res. 2021, 7, 73. [Google Scholar] [CrossRef]
- Yoo, C.; Jeong, H.; Kim, K.-P.; Hwang, D.W.; Lee, J.-H.; Kim, K.-H.; Moon, D.B.; Lee, M.A.; Park, S.J.; Chon, H.J.; et al. Adjuvant gemcitabine plus cisplatin (GemCis) versus capecitabine (CAP) in patients (pts) with resected lymph node (LN)-positive extrahepatic cholangiocarcinoma (CCA): A multicenter, open-label, randomized, phase 2 study (STAMP). J. Clin. Oncol. 2022, 40, 4019. [Google Scholar] [CrossRef]
- Ben-Josef, E.; Guthrie, K.A.; El-Khoueiry, A.B.; Corless, C.L.; Zalupski, M.M.; Lowy, A.M.; Thomas, C.R.; Alberts, S.R.; Dawson, L.A.; Micetich, K.C.; et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J. Clin. Oncol. 2015, 33, 2617–2622. [Google Scholar] [CrossRef]
- Horgan, A.M.; Amir, E.; Walter, T.; Knox, J.J. Adjuvant Therapy in the Treatment of Biliary Tract Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2012, 30, 1934–1940. [Google Scholar] [CrossRef]
- Yadav, S.; Xie, H.; Bin-Riaz, I.; Sharma, P.; Durani, U.; Goyal, G.; Borah, B.; Borad, M.J.; Smoot, R.L.; Roberts, L.R.; et al. Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur. J. Surg. Oncol. 2019, 45, 1432–1438. [Google Scholar] [CrossRef]
- Chaudhari, V.A.; Ostwal, V.; Patkar, S.; Sahu, A.; Toshniwal, A.; Ramaswamy, A.; Shetty, N.S.; Shrikhande, S.V.; Goel, M. Outcome of neoadjuvant chemotherapy in “locally advanced/borderline resectable” gallbladder cancer: The need to define indications. HPB 2018, 20, 841–847. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tomokuni, A.; Gotoh, K.; Takahashi, H.; Akita, H.; Marubashi, S.; Yamada, T.; Teshima, T.; Fukui, K.; Fujiwara, Y.; et al. A retrospective analysis of the clinical effects of neoadjuvant combination therapy with full-dose gemcitabine and radiation therapy in patients with biliary tract cancer. Eur. J. Surg. Oncol. 2017, 43, 763–771. [Google Scholar] [CrossRef]
- De Vreede, I.; Steers, J.L.; Burch, P.A.; Rosen, C.B.; Gunderson, L.L.; Haddock, M.G.; Burgart, L.; Gores, G.J. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000, 6, 309–316. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Gores, G.J.; Haddock, M.G.; Alberts, S.R.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, A.; Heneghan, H.M.; Fiore, B.; Stafford, A.; Gallagher, T.; Geoghegan, J.; Maguire, D.; Hoti, E. Neoadjuvant Chemoradiotherapy and Liver Transplantation for Unresectable Hilar Cholangiocarcinoma: The Irish Experience of the Mayo Protocol. Transplantation 2020, 104, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Ivanics, T.; Heimbach, J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: Ready for Prime Time? Hepatology 2022, 75, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, K.E.; Javle, M.; Heyne, K.; Shroff, R.T.; Abdel-Wahab, R.; Gupta, N.; Mobley, C.M.; Saharia, A.; Victor, D.W.; Nguyen, D.T.; et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: A prospective case-series. Lancet Gastroenterol. Hepatol. 2018, 3, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.C.; Jones, C.M.; Duffy, J.P.; Petrowsky, H.; Farmer, D.G.; French, S.; Finn, R.; Durazo, F.A.; Saab, S.; Tong, M.J.; et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: A 24-year experience in a single center. Arch. Surg. 2011, 146, 683–689. [Google Scholar] [CrossRef] [PubMed]
| Trial Name/Phase | Population | Treatment Arms | Response Rate | PFS | OS | |||
|---|---|---|---|---|---|---|---|---|
| Median (months | HR (95% CI); p-Value | Median (Months | HR (95% CI); p-Value | 1 or 2-yr Rate | ||||
| ABC-02 Trial [28] Phase III | Advanced BTC N = 410 | Arm A: Gem-Cis | A: 26.1% | A: 8.0 | 0.64 (0.51–0.77); p < 0.001 | A: 11.7 | 0.64 (0.52–0.80); p < 0.001 | Not reported |
| Arm B: Gem | B: 15.5% | B: 5.0 | B: 8.1 | |||||
| Sharma et al., 2019 [34] Phase III | Unresectable GBC N = 243 | Arm A: GEMOX | A: 25.2% | A: 5.0 | Not reported; p = 0.047 | A: 9.0 | 0.78 (0.60−1.02); p = 0.057 | A: 32% at 1 yr |
| Arm B: Gem-Cis | B: 23.4% | B: 4.0 | B: 8.3 | B: 24% at 1 yr | ||||
| PRODIGE 38 [35] Phase II/III | Advanced BTC N = 190 | Arm A: mFOLFIRI-NOX | A: 25.0% | A: 6.2 | 0.78 (0.57–1.05); p = 0.11 | A: 11.7 | Not reported | A: 48% at 1 yr |
| Arm B: Gem-Cis | B: 19.4% | B: 7.4 | B: 13.8 | B: 56% at 1 yr | ||||
| Lee et al., 2014 [36] Phase II | Advanced BTC N = 93 | Arm A: Cape-Cis | A: 27.3% | A: 5.2 | Not reported; p = 0.016 | A: 10.7 | Not reported; p = 0.365 | Not reported |
| Arm B: Gem-Cis | B: 6.1% | B: 3.6 | B: 8.6 | |||||
| Markussen et al., 2020 [37] Phase II | Advanced BTC N = 96 | Arm A: GEMOX-Cape | A: 17% | A: 5.7 | 0.721 (not reported); p = 0.1 | A: 8.7 | 0.731 (not reported); p = 0.1 | Not reported |
| Arm B: Gem-Cis | B: 16% | B: 7.3 | B: 12.0 | |||||
| SWOG 1815 [38] Phase III | Advanced BTC N = 441 | Arm A: GAP | A: 34% | A: 8.2 | 0.92 (0.72–1.16); p = 0.47 | A: 14 | 0.93 (0.74–1.19); p = 0.58 | Not reported |
| Arm B: Gem-Cis | B: 25% | B: 6.4 | B: 12.7 | |||||
| TOPAZ-1 [39,40] Phase III | Advanced/unresectable BTC N = 685 | Arm A: Durva-lumab + Gem-Cis | A: 26.7% | A: 7.2 | 0.75 (0.63−0.89); p = 0.001 | A: 12.9 | 0.76 (0.64−0.91); p, not reported | A: 23.6% at 2 yr |
| Arm B: Placebo + Gem-Cis | B: 18.7% | B: 5.7 | B: 11.3 | B: 11.5% at 2 yr | ||||
| KEYNOTE-966 [41] Phase III | Advanced/unresectable BTC N = 1069 | Arm A: Pembrolizumab + Gem-Cis | A: 28.7% | A: 6.5 | 0.86 (0.75–1.00); p = 0.023 * | A: 12.7 | 0.76 (0.64−0.91); p, not reported | A: 25% at 2 yr |
| Arm B: Placebo + Gem-Cis | B: 28.5% | B: 5.6 | B: 10.9 | B: 18% at 2 yr | ||||
| Trial Name/Phase | Population | Treatment Arms | Target/Biomarker | Response Rate | Median PFS (Months) | Median OS (Months) |
|---|---|---|---|---|---|---|
| FIGHT-202 [52] Phase II | Chemotherapy refractory advanced iCCA N = 107 | Pemigatinib | FGFR 1–3 FGFR2 fusions | 37% * | 7.0 | 17.5 |
| Javle et al., 2021 [53] Phase II | Chemotherapy refractory advanced iCCA N = 108 | Infigratinib | FGFR 1–4 FGFR fusions | 23.1% * | 7.3 | 12.2 |
| FIDES-01 [54] Phase II | Chemotherapy refractory advanced iCCA N = 103 | Derazantinib | FGFR 1–3 FGFR2 fusions | 21.4% * | 8.0 | 15.9 |
| FOENIX-CCA2 [55] Phase II | Chemotherapy refractory advanced iCCA N = 103 | Futibatinib | FGFR 1–4 FGFR fusions | 41.7% * | 8.9 | 20.0 |
| ClarIDHy [56,57] Phase III | Advanced/ metastatic CCA Second line N = 187 | Arm A: Ivosidenib | IDH1 IDH1 mutations | 2% | 2.7 | 10.3 |
| Arm B: Placebo | 0% | 1.4 | 7.5 | |||
| HR(ivo vs. plb) 0.37 (95% CI 0.25–0.54) p < 0.0001 | HR(ivo vs. plb) 0.79 (95% CI 0.56–1.12) p = 0.09 | |||||
| ROAR [58] Phase II | Advanced/metastatic CCA Second line N = 43 | Dabrafenib + trametinib | BRAF + MEK BRAF V600E | 51% | 9 | 14 |
| MyPathway [59] Phase II | Previously treated metastatic BTC N = 39 | Pertuzumab + trastuzumab | HER2 HER2 amplification/overexpression | 23% | 4 | 10.9 |
| HERB [60] Phase II | Previously treated metastatic BTC N = 22 | Trastuzumab deruxtecan | HER2 HER2 amplification/overexpression | 36.4% | 4.4 | 7.1 |
| Trial Name/Phase | Population | Treatment Arms | Median RFS/DFS (Months) | HR for RFS/DFS (95% CI); p-Value | Median OS (Months) | HR for OS (95% CI); p-Value |
|---|---|---|---|---|---|---|
| ESPAC-3 trial [18] Phase III | eCCA, AC N = 428 | Arm A: 5FU + FA | A: 23.0 | HR A vs. C 0.69 (0.51–0.95); p = 0.02 HR B vs. C 0.68 (0.50−0.95); p = 0.02 | A: 38.9 | HR A vs. C 0.95; (0.71–1.28); p = 0.74 HR B vs. C 0.77 (0.57−1.05); p = 0.10 |
| Arm B: Gem | B: 29.1 | B: 45.7 | ||||
| Arm C: Surgery alone | C: 19.5 | C: 35.2 | ||||
| BCAT trial [19] Phase III | eCCA N = 225 | Arm A: Gem | A: 36.0 | 0.93 (0.66−1.32); p = 0.693 | A: 62.3 | 1.01 (0.70−1.45); p = 0.964 |
| Arm B: Surgery alone | B: 39.9 | B: 63.8 | ||||
| PRODIGE 12-ACCORD 18 [20] Phase III | iCCA, eCCA GBC N = 194 | Arm A: GEMOX | A: 30.4 | 0.88 (0.62−1.25); p = 0.48 | A: 75.8 | 1.08 (0.70−1.66); p = 0.74 |
| Arm B: Surgery alone | B: 18.5 | B: 50.8 | ||||
| BILCAP [17] * Phase III | iCCA, eCCA GBC N = 447 | Arm A: Cape | A: 25.9 | 0.70 (0.54–0.92); p = 0.0093 | A: 53.0 | 0.75 (0.58–0.97); p = 0.028 |
| Arm B: Surgery alone | B: 17.4 | B: 36.0 | ||||
| STAMP [87] Phase II | eCCA N = 101 | Arm A: Gem-Cis | A: 14.3 | 0.96 (0.71–1.30); p = 0.86 | A: 35.7 | 1.08 (0.72–1.64); p = 0.81 |
| Arm B: Cape | B: 11.1 | B: 35.7 |
| Recommendation | Level of Evidence * |
| Systemic therapy for unresectable advanced or metastatic BTC | |
| I |
| V |
| I |
| III |
| I |
| III |
| V |
| V |
| Adjuvant therapy | |
| II |
| Neoadjuvant therapy | |
| V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramjeesingh, R.; Chaudhury, P.; Tam, V.C.; Roberge, D.; Lim, H.J.; Knox, J.J.; Asselah, J.; Doucette, S.; Chhiber, N.; Goodwin, R. A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada. Curr. Oncol. 2023, 30, 7132-7150. https://doi.org/10.3390/curroncol30080517
Ramjeesingh R, Chaudhury P, Tam VC, Roberge D, Lim HJ, Knox JJ, Asselah J, Doucette S, Chhiber N, Goodwin R. A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada. Current Oncology. 2023; 30(8):7132-7150. https://doi.org/10.3390/curroncol30080517
Chicago/Turabian StyleRamjeesingh, Ravi, Prosanto Chaudhury, Vincent C. Tam, David Roberge, Howard J. Lim, Jennifer J. Knox, Jamil Asselah, Sarah Doucette, Nirlep Chhiber, and Rachel Goodwin. 2023. "A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada" Current Oncology 30, no. 8: 7132-7150. https://doi.org/10.3390/curroncol30080517
APA StyleRamjeesingh, R., Chaudhury, P., Tam, V. C., Roberge, D., Lim, H. J., Knox, J. J., Asselah, J., Doucette, S., Chhiber, N., & Goodwin, R. (2023). A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada. Current Oncology, 30(8), 7132-7150. https://doi.org/10.3390/curroncol30080517







