Nanoparticle-Based Treatment Approaches for Skin Cancer: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
3. Results
3.1. Literature Search Results
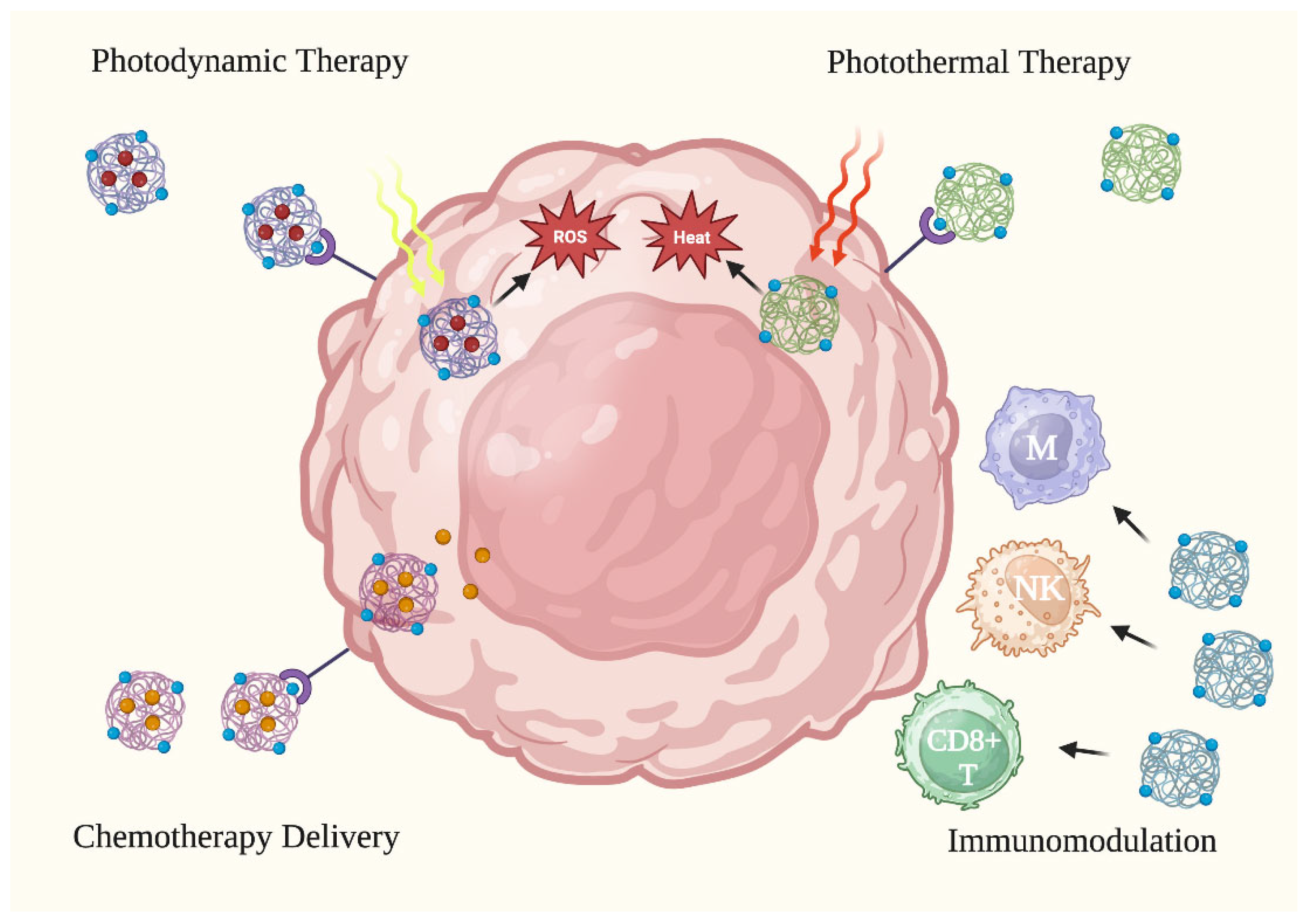
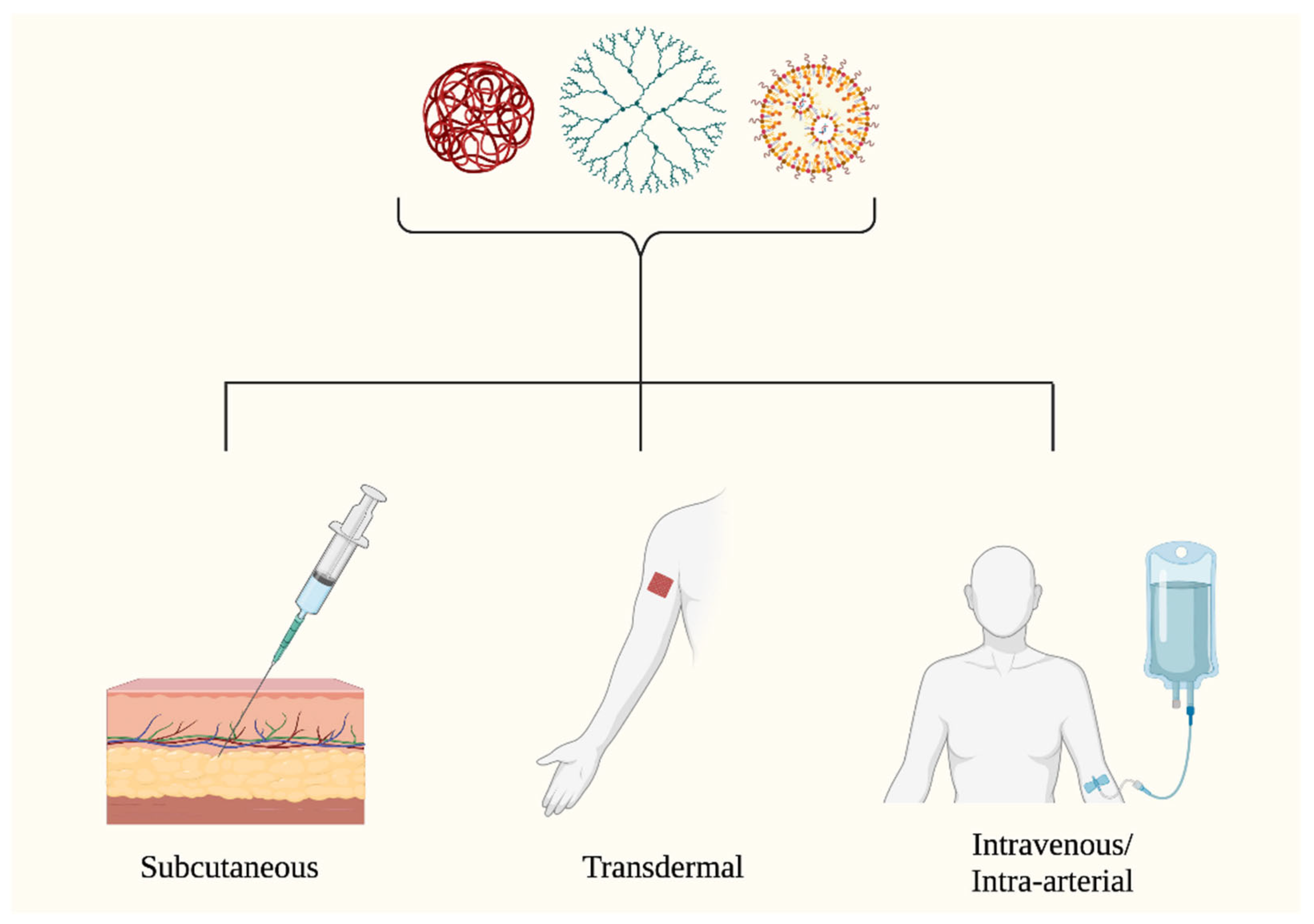
3.2. Primer on Nanoparticle Utility for Skin Cancer Treatment
3.3. Inorganic Nanoparticles
3.3.1. Gold Nanoparticles (AuNPs)
3.3.2. Silver Nanoparticles (AgNPs)
3.3.3. Silica Nanoparticles (SiNPs)
3.4. Organic Nanoparticles
3.4.1. Lipid Nanoparticles (LNPs)
3.4.2. Polymeric Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Melanoma Skin Cancer Statistics. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html (accessed on 14 April 2023).
- Guy, G.P.; Machlin, S.R.; Ekwueme, D.U.; Yabroff, K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015, 48, 183–187. [Google Scholar] [CrossRef]
- Kao, S.Y.Z.; Ekwueme, D.U.; Holman, D.M.; Rim, S.H.; Thomas, C.C.; Saraiya, M. Economic burden of skin cancer treatment in the USA: An analysis of the Medical Expenditure Panel Survey Data, 2012–2018. Cancer Causes Control 2023, 34, 205–212. [Google Scholar] [CrossRef]
- Mofidi, A.; Tompa, E.; Spencer, J.; Kalcevich, C.; Peters, C.E.; Kim, J.; Song, C.; Mortazavi, S.B.; Demers, P.A. The economic burden of occupational non-melanoma skin cancer due to solar radiation. J. Occup. Environ. Hyg. 2018, 15, 481–491. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Zeng, F.; Meng, Y.; Du, S.; Deng, G. Melanoma survivors are at increased risk for second primary keratinocyte carcinoma. Int. J. Dermatol. 2022, 61, 1397–1404. [Google Scholar] [CrossRef]
- Espinosa, P.; Pfeiffer, R.M.; García-Casado, Z.; Requena, C.; Landi, M.T.; Kumar, R.; Nagore, E. Risk factors for keratinocyte skin cancer in patients diagnosed with melanoma, a large retrospective study. Eur. J. Cancer 2016, 53, 115–124. [Google Scholar] [CrossRef]
- Diaz, M.J.; Mark, I.; Rodriguez, D.; Gelman, B.; Tran, J.T.; Kleinberg, G.; Levin, A.; Beneke, A.; Root, K.T.; Tran, A.X.V.; et al. Melanoma Brain Metastases: A Systematic Review of Opportunities for Earlier Detection, Diagnosis, and Treatment. Life 2023, 13, 828. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Liu, J.P.; Wang, T.T.; Wang, D.G.; Dong, A.J.; Li, Y.P.; Yu, H.J. Smart nanoparticles improve therapy for drug-resistant tumors by overcoming pathophysiological barriers. Acta Pharmacol. Sin. 2017, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Elsherbeny, A.; Pittalà, V.; Consoli, V.; Alghamdi, M.A.; Hussain, Z.; Khoder, G.; Greish, K. Nanomedicine Strategies for Management of Drug Resistance in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 1853. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: https://www.covidence.org (accessed on 10 March 2023).
- Toderascu, L.I.; Sima, L.E.; Orobeti, S.; Florian, P.E.; Icriverzi, M.; Maraloiu, V.A.; Comanescu, C.; Iacob, N.; Kuncser, V.; Antohe, I.; et al. Synthesis and Anti-Melanoma Activity of L-Cysteine-Coated Iron Oxide Nanoparticles Loaded with Doxorubicin. Nanomaterials 2023, 13, 621. [Google Scholar] [CrossRef]
- Guo, H.; Li, F.; Qiu, H.; Liu, J.; Qin, S.; Hou, Y.; Wang, C. Preparation and Characterization of Chitosan Nanoparticles for Chemotherapy of Melanoma Through Enhancing Tumor Penetration. Front. Pharmacol. 2020, 11, 317. [Google Scholar] [CrossRef]
- Xue, J.; Zhu, Y.; Bai, S.; He, C.; Du, G.; Zhang, Y.; Zhong, Y.; Chen, W.; Wang, H.; Sun, X. Nanoparticles with rough surface improve the therapeutic effect of photothermal immunotherapy against melanoma. Acta Pharm. Sin. B 2022, 12, 2934–2949. [Google Scholar] [CrossRef]
- Mello, V.C.; Araújo, V.H.S.; de Paiva, K.L.R.; Simões, M.M.; Marques, D.C.; da Silva Costa, N.R.; de Souza, I.F.; da Silva, P.B.; Santos, I.; Almeida, R.; et al. Development of New Natural Lipid-Based Nanoparticles Loaded with Aluminum-Phthalocyanine for Photodynamic Therapy against Melanoma. Nanomaterials 2022, 12, 3547. [Google Scholar] [CrossRef]
- Dayan, A.; Fleminger, G.; Ashur-Fabian, O. RGD-modified dihydrolipoamide dehydrogenase conjugated to titanium dioxide nanoparticles—Switchable integrin-targeted photodynamic treatment of melanoma cells. RSC Adv. 2018, 8, 9112–9119. [Google Scholar] [CrossRef]
- Bilkan, M.T.; Çiçek, Z.; Kurşun, A.G.C.; Özler, M.; Eşmekaya, M.A. Investigations on effects of titanium dioxide (TiO2) nanoparticle in combination with UV radiation on breast and skin cancer cells. Med. Oncol. 2022, 40, 60. [Google Scholar] [CrossRef]
- Dam, D.H.M.; Zhao, L.; Jelsma, S.A.; Zhao, Y.; Paller, A.S. Folic acid functionalized hollow nanoparticles for selective photodynamic therapy of cutaneous squamous cell carcinoma. Mater. Chem. Front. 2019, 3, 1113–1122. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, L.; Pan, Y. Photothermal ablation of murine melanomas by Fe3O4 nanoparticle clusters. Beilstein J. Nanotechnol. 2022, 13, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qin, M.; Chen, X.; Wang, Q.; Zhang, Z.; Sun, X. Combining photothermal therapy and immunotherapy against melanoma by polydopamine-coated Al2O3 nanoparticles. Theranostics 2018, 8, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Behnam, M.A.; Emami, F.; Sobhani, Z.; Koohi-Hosseinabadi, O.; Dehghanian, A.R.; Zebarjad, S.M.; Moghim, M.H.; Oryan, A. Novel Combination of Silver Nanoparticles and Carbon Nanotubes for Plasmonic Photo Thermal Therapy in Melanoma Cancer Model. Adv. Pharm. Bull. 2018, 8, 49–55. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Yin, L.; Hou, Y.; Zhao, S. Chitosan-Poly(Acrylic Acid) Nanoparticles Loaded with R848 and MnCl2 Inhibit Melanoma via Regulating Macrophage Polarization and Dendritic Cell Maturation. Int. J. Nanomed. 2021, 16, 5675–5692. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Heath, M.; Kramer, M.F.; Velazquez, T.C.; Bullimore, A.; Skinner, M.A.; Speiser, D.E.; Bachmann, M.F. In situ delivery of nanoparticles formulated with micron-sized crystals protects from murine melanoma. J. Immunother. Cancer 2022, 10, e004643. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef]
- Neek, M.; Tucker, J.A.; Butkovich, N.; Nelson, E.L.; Wang, S.W. An Antigen-Delivery Protein Nanoparticle Combined with Anti-PD-1 Checkpoint Inhibitor Has Curative Efficacy in an Aggressive Melanoma Model. Adv. Ther. 2020, 3, 2000122. [Google Scholar] [CrossRef]
- Kuang, X.; Wang, Z.; Luo, Z.; He, Z.; Liang, L.; Gao, Q.; Li, Y.; Xia, K.; Xie, Z.; Chang, R.; et al. Ag nanoparticles enhance immune checkpoint blockade efficacy by promoting of immune surveillance in melanoma. J. Colloid Interface Sci. 2022, 616, 189–200. [Google Scholar] [CrossRef]
- Mioc, M.; Pavel, I.Z.; Ghiulai, R.; Coricovac, D.E.; Farcaş, C.; Mihali, C.V.; Oprean, C.; Serafim, V.; Popovici, R.A.; Dehelean, C.A.; et al. The Cytotoxic Effects of Betulin-Conjugated Gold Nanoparticles as Stable Formulations in Normal and Melanoma Cells. Front. Pharmacol. 2018, 9, 429. [Google Scholar] [CrossRef]
- Suarasan, S.; Campu, A.; Vulpoi, A.; Banciu, M.; Astilean, S. Assessing the Efficiency of Triangular Gold Nanoparticles as NIR Photothermal Agents In Vitro and Melanoma Tumor Model. Int. J. Mol. Sci. 2022, 23, 13724. [Google Scholar] [CrossRef] [PubMed]
- Bonamy, C.; Pesnel, S.; Ben Haddada, M.; Gorgette, O.; Schmitt, C.; Morel, A.L.; Sauvonnet, N. Impact of Green Gold Nanoparticle Coating on Internalization, Trafficking, and Efficiency for Photothermal Therapy of Skin Cancer. ACS Omega 2023, 8, 4092–4105. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 8720. [Google Scholar] [CrossRef]
- Li, X.; Zhong, S.; Zhang, C.; Li, P.; Ran, H.; Wang, Z. MAGE-Targeted Gold Nanoparticles for Ultrasound Imaging-Guided Phototherapy in Melanoma. Biomed. Res. Int. 2020, 2020, 6863231. [Google Scholar] [CrossRef]
- Chi, Y.F.; Qin, J.J.; Li, Z.; Ge, Q.; Zeng, W.H. Enhanced anti-tumor efficacy of 5-aminolevulinic acid-gold nanoparticles-mediated photodynamic therapy in cutaneous squamous cell carcinoma cells. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui Med. E Biol. 2020, 53, e8457. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, H.; Li, J. Red Blood Cell Membrane-Camouflaged Gold Nanoparticles for Treatment of Melanoma. J. Oncol. 2022, 2022, 3514984. [Google Scholar] [CrossRef]
- Jeon, H.J.; Choi, B.B.R.; Park, K.H.; Hwang, D.S.; Kim, U.K.; Kim, G.C. Induction of Melanoma Cell-Selective Apoptosis Using Anti-HER2 Antibody-Conjugated Gold Nanoparticles. Yonsei Med. J. 2019, 60, 509–516. [Google Scholar] [CrossRef]
- Malindi, Z.; Barth, S.; Abrahamse, H. The Potential of Antibody Technology and Silver Nanoparticles for Enhancing Photodynamic Therapy for Melanoma. Biomedicines 2022, 10, 2158. [Google Scholar] [CrossRef]
- Himalini, S.; Uma Maheshwari Nallal, V.; Razia, M.; Chinnapan, S.; Chandrasekaran, M.; Ranganathan, V.; Gatasheh, M.K.; Hatamleh, A.A.; Al-Khattaf, F.S.; Kanimozhi, S. Antimicrobial, anti-melanogenesis and anti-tyrosinase potential of myco-synthesized silver nanoparticles on human skin melanoma SK-MEL-3 cells. J. King Saud Univ.—Sci. 2022, 34, 101882. [Google Scholar] [CrossRef]
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-Silver Nanoparticles: A Potential Multimodal Therapeutics for Conventional and Photothermal Treatment of Skin Cancer. Pharmaceutics 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Salas, L.M.; Girón-Vázquez, N.G.; García-Ramos, J.C.; Torres-Bugarín, O.; Gómez, C.; Pestryakov, A.; Villarreal-Gómez, L.J.; Toledano-Magaña, Y.; Bogdanchikova, N. Antiproliferative and Antitumour Effect of Nongenotoxic Silver Nanoparticles on Melanoma Models. Oxid. Med. Cell Longev. 2019, 2019, 4528241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, F.; Turker, M.Z.; Ma, K.; Zanzonico, P.; Gallazzi, F.; Shah, M.A.; Prater, A.R.; Wiesner, U.; Bradbury, M.S.; et al. Targeted melanoma radiotherapy using ultrasmall 177Lu-labeled α-melanocyte stimulating hormone-functionalized core-shell silica nanoparticles. Biomaterials 2020, 241, 119858. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, X.; Ma, K.; Madajewski, B.; Benezra, M.; Zhang, L.; Phillips, E.; Turker, M.Z.; Gallazzi, F.; Penate-Medina, O.; et al. Melanocortin-1 Receptor-Targeting Ultrasmall Silica Nanoparticles for Dual-Modality Human Melanoma Imaging. ACS Appl. Mater. Interfaces 2018, 10, 4379–4393. [Google Scholar] [CrossRef]
- Clemente, N.; Miletto, I.; Gianotti, E.; Sabbatini, M.; Invernizzi, M.; Marchese, L.; Dianzani, U.; Renò, F. Verteporfin-Loaded Mesoporous Silica Nanoparticles’ Topical Applications Inhibit Mouse Melanoma Lymphangiogenesis and Micrometastasis In Vivo. Int. J. Mol. Sci. 2021, 22, 13443. [Google Scholar] [CrossRef] [PubMed]
- Marinheiro, D.; Ferreira, B.J.M.L.; Oskoei, P.; Oliveira, H.; Daniel-da-Silva, A.L. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef] [PubMed]
- Drača, D.; Edeler, D.; Saoud, M.; Dojčinović, B.; Dunđerović, D.; Đmura, G.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Antitumor potential of cisplatin loaded into SBA-15 mesoporous silica nanoparticles against B16F1 melanoma cells: In vitro and in vivo studies. J. Inorg. Biochem. 2021, 217, 111383. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Alipanah, H.; Roozitalab, G.; Ebrahimi, M.; Osanloo, M. Anticancer Effect of Solid-Lipid Nanoparticles Containing Mentha longifolia and Mentha pulegium Essential Oils: In Vitro Study on Human Melanoma and Breast Cancer Cell Lines. Biointerface Res. Appl. Chem. 2021, 12, 2128–2137. [Google Scholar] [CrossRef]
- Valizadeh, A.; Khaleghi, A.A.; Roozitalab, G.; Osanloo, M. High anticancer efficacy of solid lipid nanoparticles containing Zataria multiflora essential oil against breast cancer and melanoma cell lines. BMC Pharmacol. Toxicol. 2021, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, F.; Gu, W.; Soares, C.P.; Xu, Z.P. Encapsulating Anti-Parasite Benzimidazole Drugs into Lipid-Coated Calcium Phosphate Nanoparticles to Efficiently Induce Skin Cancer Cell Apoptosis. Front. Nanotechnol. 2021, 3, 693837. [Google Scholar] [CrossRef]
- Sakpakdeejaroen, I.; Somani, S.; Laskar, P.; Mullin, M.; Dufès, C. Regression of Melanoma Following Intravenous Injection of Plumbagin Entrapped in Transferrin-Conjugated, Lipid-Polymer Hybrid Nanoparticles. Int. J. Nanomed. 2021, 16, 2615–2631. [Google Scholar] [CrossRef] [PubMed]
- Clemente, N.; Ferrara, B.; Gigliotti, C.L.; Boggio, E.; Capucchio, M.T.; Biasibetti, E.; Schiffer, D.; Mellai, M.; Annovazzi, L.; Cangemi, L.; et al. Solid Lipid Nanoparticles Carrying Temozolomide for Melanoma Treatment. Preliminary In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.B.; Yu, Z.C.; He, Y.N.; Zhang, T.; Du, L.B.; Dong, Y.M.; Chen, H.W.; Zhang, Y.Y.; Wang, W.Q. Salinomycin-loaded lipid-polymer nanoparticles with anti-CD20 aptamers selectively suppress human CD20+ melanoma stem cells. Acta Pharmacol. Sin. 2018, 39, 261–274. [Google Scholar] [CrossRef]
- Fattore, L.; Cafaro, G.; Di Martile, M.; Campani, V.; Sacconi, A.; Liguoro, D.; Marra, E.; Bruschini, S.; Stoppoloni, D.; Cirombella, R.; et al. Oncosuppressive miRNAs loaded in lipid nanoparticles potentiate targeted therapies in BRAF-mutant melanoma by inhibiting core escape pathways of resistance. Oncogene 2023, 42, 293–307. [Google Scholar] [CrossRef]
- Fattore, L.; Campani, V.; Ruggiero, C.F.; Salvati, V.; Liguoro, D.; Scotti, L.; Botti, G.; Ascierto, P.A.; Mancini, R.; De Rosa, G.; et al. In Vitro Biophysical and Biological Characterization of Lipid Nanoparticles Co-Encapsulating Oncosuppressors miR-199b-5p and miR-204-5p as Potentiators of Target Therapy in Metastatic Melanoma. Int. J. Mol. Sci. 2020, 21, 1930. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Neamatallah, T.; Alshehri, S.; Mujtaba, M.A.; Riadi, Y.; Radhakrishnan, A.K.; Khalilullah, H.; Gupta, M.; Akhter, M.H. Development, Characterization, and Evaluation of α-Mangostin-Loaded Polymeric Nanoparticle Gel for Topical Therapy in Skin Cancer. Gels 2021, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, L.S.; Watashi, C.M.; Colturato-Kido, C.; Pelegrino, M.T.; Paredes-Gamero, E.J.; Weller, R.B.; Seabra, A.B.; Rodrigues, T. Antitumor Potential of S-Nitrosothiol-Containing Polymeric Nanoparticles against Melanoma. Mol. Pharm. 2018, 15, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Guo, Z.; Zeng, B.; Wang, T.; Zeng, X.; Cao, W.; Lian, D. Dacarbazine-Loaded Targeted Polymeric Nanoparticles for Enhancing Malignant Melanoma Therapy. Front. Bioeng. Biotechnol. 2022, 10, 847901. [Google Scholar] [CrossRef] [PubMed]
- Chepenik, K.P.; George, M. Hydrolysis of phospholipids by embryonic palate mesenchyme cells in vitro. Prog. Clin. Biol. Res. 1985, 163B, 369–376. [Google Scholar] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef]
- Silva, C.O.; Rijo, P.; Molpeceres, J.; Figueiredo, I.V.; Ascensão, L.; Fernandes, A.S.; Roberto, A.; Reis, C.P. Polymeric nanoparticles modified with fatty acids encapsulating betamethasone for anti-inflammatory treatment. Int. J. Pharm. 2015, 493, 271–284. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]


| Author, Year | Study Design | Key Findings |
|---|---|---|
| Toderascu, 2023 [14] | Controlled trial, mouse (B16F10) and human (A375) metastatic melanoma cells |
|
| Guo, 2020 [15] | Controlled trial, Mice melanoma B16F10 and B16F1 cells |
|
| Xue, 2022 [16] | Controlled trial, Mice with B16F10 melanoma |
|
| Mello, 2022 [17] | Controlled trial, Mice with B16F10 melanoma |
|
| Dayan, 2018 [18] | Controlled trial, Mice melanoma B16F10 cancer cells |
|
| Bilkan, 2023 [19] | Controlled trial, Human melanoma cell line |
|
| Dam, 2019 [20] | Controlled trial, Human cutaneous SCC lines |
|
| Wang, 2022 [21] | Controlled trial, BALB/c mice |
|
| Chen, 2018 [22] | Controlled trial, Mice melanoma B16F10 cancer cells |
|
| Behnam, 2018 [23] | Controlled trial, Mice with B16F10 melanoma |
|
| Liu, 2021 [24] | Controlled trial, Mice with B16F10 melanoma |
|
| Mohsen, 2022 [25] | Controlled trial, Mice with B16F10 melanoma |
|
| Cao, 2019 [26] | Controlled trial, Mice with B16F10 melanoma |
|
| Neek, 2020 [27] | Controlled trial, Mice with B16F10 melanoma |
|
| Kuang, 2022 [28] | Controlled trial, mouse (B16F10) and human (A375) metastatic melanoma cells |
|
| Author, Year | Study Design | Key Findings |
|---|---|---|
| Mioc, 2018 [29] | Case-control, Human melanoma cell line A375 |
|
| Suarasan, 2022 [30] | Case-control, Agarose-based skin biological phantoms and B16:F10 melanoma cells |
|
| Bonamy, 2023 [31] | Case-control, Human melanoma cell line SK-MEL-28 |
|
| Zhang, 2018 [32] | Case-control, Murine melanoma cell line B16-BL6 |
|
| Li, 2020 [33] | Case-control, B16 mouse melanoma cells |
|
| Chi, 2020 [34] | Case-control, A431 cells and HaCat cells |
|
| Zhao, 2022 [35] | Case-control, B16-F10 melanoma cells |
|
| Jeon, 2019 [36] | Case-control, B16-F10 melanoma cells |
|
| Malindi, 2022 [37] | Systematic review |
|
| Himalini, 2022 [38] | Case-control, Human skin melanoma SK-MEL-3 cells |
|
| Kim, 2021 [39] | Case-control, B16F10 murine melanoma cells |
|
| Behnam, 2018 [23] | Case-control, B16/F10 melanoma cell lines injected into mice |
|
| Valenzuela-Salas, 2019 [40] | Case-control, B16-F10 murine skin melanoma cells from C57BL/6J mice |
|
| Zhang, 2020 [41] | Case-control, B16F10 murine melanoma cells |
|
| Chen, 2018 [42] | Case-control, B16F10 melanoma bearing mice |
|
| Clemente, 2021 [43] | Case-control, B16-F10 melanoma bearing mice |
|
| Marinheiro, 2021 [44] | Case-control, Human A375 and MNT-1 melanoma cell cultures |
|
| Drača, 2021 [45] | Case-control, B16F1 melanoma cell lines and B16F1 melanoma bearing mice |
|
| Author, Year | Study Design | Key Findings |
|---|---|---|
| Mello, 2022 [17] | In vitro study with murine B16-F10 melanoma cells |
|
| Kelidari, 2022 [46] | In vitro study |
|
| Valizadeh, 2021 [47] | In vitro study |
|
| Movahedi, 2021 [48] | In vitro study |
|
| Sakpakdeejaroen, 2021 [49] | In vivo murine study (n = 25 total, n = 5 each group) |
|
| Clemente, 2018 [50] | In vivo study with B16-F10 melanoma in C57/BL6 mice |
|
| Zeng, 2018 [51] | In vivo study and murine in vivo study |
|
| Fattore, 2020 [52] | In vivo murine study (n = 7 A375; n = 10 M14) |
|
| Fattore, 2020 [53] | In vitro study |
|
| Md, 2021 [54] | In vitro and in vivo rat study |
|
| Ferraz, 2018 [55] | In vivo murine study |
|
| Xiong, 2022 [56] | In vitro study |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz, M.J.; Natarelli, N.; Aflatooni, S.; Aleman, S.J.; Neelam, S.; Tran, J.T.; Taneja, K.; Lucke-Wold, B.; Forouzandeh, M. Nanoparticle-Based Treatment Approaches for Skin Cancer: A Systematic Review. Curr. Oncol. 2023, 30, 7112-7131. https://doi.org/10.3390/curroncol30080516
Diaz MJ, Natarelli N, Aflatooni S, Aleman SJ, Neelam S, Tran JT, Taneja K, Lucke-Wold B, Forouzandeh M. Nanoparticle-Based Treatment Approaches for Skin Cancer: A Systematic Review. Current Oncology. 2023; 30(8):7112-7131. https://doi.org/10.3390/curroncol30080516
Chicago/Turabian StyleDiaz, Michael Joseph, Nicole Natarelli, Shaliz Aflatooni, Sarah J. Aleman, Sphurti Neelam, Jasmine Thuy Tran, Kamil Taneja, Brandon Lucke-Wold, and Mahtab Forouzandeh. 2023. "Nanoparticle-Based Treatment Approaches for Skin Cancer: A Systematic Review" Current Oncology 30, no. 8: 7112-7131. https://doi.org/10.3390/curroncol30080516
APA StyleDiaz, M. J., Natarelli, N., Aflatooni, S., Aleman, S. J., Neelam, S., Tran, J. T., Taneja, K., Lucke-Wold, B., & Forouzandeh, M. (2023). Nanoparticle-Based Treatment Approaches for Skin Cancer: A Systematic Review. Current Oncology, 30(8), 7112-7131. https://doi.org/10.3390/curroncol30080516






