Abstract
(1) Background: Exon 20 insertion mutations (ex20ins) in EGFR and HER2 are uncommon driver mutations in non-small-cell lung cancer (NSCLC), with a poor prognosis and few targeted therapy options, and there are limited real-world data. Here, we report the clinicopathologic features and outcomes for patients with ex20ins NSCLC across British Columbia, Canada. (2) Methods: NSCLC patients with ex20ins in EGFR or HER2 were identified via tumour testing between 1 January 2016 and 31 December 2021 (n = 7233). Data were collected by chart review. Survival analyses were performed using the Kaplan–Meier method using the log-rank test. (3) Results: A total of 131 patients were identified. The median age was 66. Thirty-three percent of patients had brain metastases. For the EGFR cohort, the median OS was 18.6 months for patients who received any systemic therapy (ST) vs. 2.6 months for patients who did not (p < 0.001). Median OS was similar for patients treated with ex20ins-specific tyrosine kinase inhibitors (TKIs) vs. other STs (18.6 vs. 15.9 months; p = 0.463). The median first-line PFS was 4.1 vs. 7.4 months for patients treated with a TKI vs. other ST (p = 0.744). For the HER2 cohort, the median OS was 9.0 months for patients who received any ST vs. 4.9 months for patients who did not (p = 0.015). The median OS was 23.0 months for patients treated with an ex20ins TKI vs. 5.6 months for patients who were not (p = 0.019). The median first-line PFS was 5.4 vs. 2.1 months for patients treated with a TKI vs. other ST (p = 0.343). (4) Conclusions: Overall survival was significantly longer among ex20ins patients who received any systemic therapy vs. those who did not. Overall survival was significantly better among HER2 ex20ins patients who received ex20ins-specific TKIs.
1. Introduction
Exon 20 insertion mutations (ex20ins) in epidermal growth factor receptor (EGFR) is the third most common EGFR mutation. They account for approximately 10% of the mutated EGFR population and are found in approximately 2% of all non-small-cell lung cancers (NSCLCs) [1,2]. EGFR exon 20 insertion mutations are associated with known de novo resistance to most first- and second-generation EGFR tyrosine kinase inhibitors (TKIs) [3]. Response rates to third generation TKIs are variable [4,5]. NSCLC patients with EGFR ex20ins have a worse prognosis compared with those with classical EGFR mutations or other uncommon EGFR mutations [6,7]. The most common EGFR ex20ins involve amino acids 766–775 in the adjacent loop after the alpha-C helix [2].
Prior to the development of exon 20-targeted agents, the standard of care for EGFR ex20ins was similar to NSCLC without driver mutations, namely, involving first-line platinum chemotherapy [8]. In patients with poor performance status who are unable to tolerate standard chemotherapy, the second-generation EGFR TKI afatinib was often used; this was based on retrospective data showing objective response rates (ORR) of up to 24% in ex20ins and the post hoc analysis of the original LUX-Lung trials showing afatinib activity in EGFR uncommon mutations [9,10]. Recently, novel agents have been developed, including the bispecific antibody amivantamab, targeting the extracellular domain of EGFR and MET, and the pan-HER TKIs mobocertinib and poziotinib, with high affinity for the loop adjacent to the alpha-C helix [2]. Amivantamab has an ORR of 40% in heavily pretreated patients, and the ORR for mobocertinib in pretreated patients is 28% [11,12]. Both drugs have been approved by the FDA for the treatment of EGFR exon 20 insertion-mutated lung cancers. Poziotinib is less active in the case of EGFR ex20ins compared with its activity in patients with HER2 ex20ins, with ORRs of 14.8% and 27.8% in previously treated and treatment-naïve patients, respectively [13,14]. Preliminary results from the phase II WU-KONG trial of sunvozertinib therapy in EGFR ex20ins lung cancer patients have recently been presented, and these demonstrate a high objective response rate of 73.1% in the first-line setting among 26 evaluable patients [15]. The efficacy data for recently therapeutic agents with exon 20 activity are summarized in Table 1.
Alterations in human epidermal growth factor receptor 2 (ERBB2/HER2) have been identified as oncogenic drivers in NSCLC. Unlike several other HER2-driven cancer types that are primarily due to HER2 copy number gain/amplifications, approximately 90% of HER2 alterations in NSCLC are exon 20 insertions that account for 1.5–2% of all NSCLCs [2]. Compared with EGFR ex20ins, HER2 ex20ins are less heterogeneous and commonly involve insertion–duplication of the amino acids YVMA between amino acids 772–780 [16]. In terms of therapeutic strategies, HER2 ex20ins are less responsive to conventional HER2 TKIs [17]. Response rates to trastuzumab and pertuzumab given alone, together, or in combination with chemotherapy or to trastuzumab–emtansine (TDM1) are low, based on results from early-phase studies [16,18]. More recently, the novel irreversible pan-HER TKI poziotinib has been reported to have clinical activity with an ORR of 28% in pretreated patients [19]. Mobocertinib has been shown to have a robust inhibitory activity against HER2 ex20ins in preclinical studies [20]; clinical trials evaluating its efficacy against this molecular subtype are currently underway. The antibody–drug conjugate trastuzumab–deruxtecan (T-Dxd) conferred a response rate of 55% in heavily pretreated patients in the Destiny-Lung01 trial and is the only FDA-approved targeted anti-HER2 therapy for HER2-mutated NSCLC [21].

Table 1.
Summary of selected recently studied agents with exon 20 activity from clinical trials.
Table 1.
Summary of selected recently studied agents with exon 20 activity from clinical trials.
| Target | Patient Population | Results | |
|---|---|---|---|
| Amivantamab | EGFR | Previously treated advanced NSCLC patients with EGFR ex20ins (N = 81) | ORR 40%, median PFS 8.3 months, median DoR 11.1 months (CHRYSALIS) [11] |
| Mobocertinib | EGFR | Previously treated advanced NSCLC patients with EGFR ex20ins (N = 114) | ORR 28%, median PFS 7.3 months, median DoR 17.5 months, median OS 24 months (EXCLAIM) [12] |
| Poziotinib | EGFR and HER2 | EGFR ex20ins—previously treated advanced NSCLC patients (N = 115) | ORR 14.8%, median PFS 4.2 months, median DoR 7.4 months (ZENITH20-1) [13] |
| EGFR ex20ins—previously untreated advanced NSCLC patients (N = 79) | ORR 27.8%, median PFS 7.2 months, median DoR 6.5 months (ZENITH20-3) [14] | ||
| HER2 ex20ins—previously treated advanced NSCLC patients (N = 90) | ORR 27.8%, median PFS 5.5 months, median DoR 5.1 months (ZENITH20-2) [19] | ||
| HER2 ex20ins—previously untreated advanced NSCLC patients (N = 70) | ORR 41%, median PFS 5.6 months, median DoR 5.7 months (ZENITH20-4) [22] | ||
| Sunvozertinib | EGFR | Previously untreated advanced NSCLC patients with EGFR ex20ins (N = 26 evaluable patients) | ORR 73.1%, median PFS, and DoR not reached (WU-KONG) [15] |
| T-Dxd | HER2 | Previously treated advanced NSCLC patients with HER2 mutations (N = 91 with 78 ex20ins) | ORR 55%, median PFS 8.2 months, median DoR 9.3 months, median OS 17.8 months [21] |
ORR, overall response rate; PFS—progression-free survival; DoR—duration of response; OS—overall survival.
Although considerable knowledge has been gained over the last decade with respect to the clinical behaviour, prognosis, and therapeutic options for the classical oncogenic driver mutations in NSCLC, there are limited real-world data characterizing the clinical course among patients harbouring atypical EGFR and HER2 alterations in the evolving therapeutic landscape. Thus, we aimed to characterize the clinical and histopathological features, treatment patterns, and clinical outcomes of EGFR and HER2 ex20ins patients in this retrospective multi-centre real-world analysis of a Canadian NSCLC population.
2. Materials and Methods
2.1. Study Population
We performed a retrospective chart review of the electronic medical records of patients receiving care across 5 BC Cancer centres across British Columbia, Canada. Eligible patients with a histopathological diagnosis of NSCLC were identified through the Provincial Cancer Genetics Laboratory Database from 1 January 2016 to 31 December 2021 (n = 7233). Patients diagnosed with recurrent or advanced/metastatic NSCLC underwent multi-gene panel-based tumour sequencing via a next-generation sequencing (NGS) technique that included EGFR and HER2 exon 20 mutations.
The inclusion criteria were all of the following: patients identified with exon 20 mutations in EGFR or HER2 during the 6-year study period; patients receiving exon 20-targeted therapies, including those who received therapy through a clinical trial and/or special access programs. Patients with all histology subtypes were included. Exclusion criteria included the following: patients with a T790M mutation; patients with only molecular genetics testing result but no other clinical data (e.g., patients who had tumour testing in British Columbia but who were treated and/or had all diagnostic testing and treatments outside the province. The study protocol was approved by the BC Cancer Research Ethics Board (REB) Accession Number H22-00141.
2.2. Data Collection
Patient characteristics from the time of lung cancer diagnosis were retrospectively collected through chart reviews. Information on their PD-L1 status (by immunohistochemistry, using the antibody Dako 22C3) was collected if available. In this analysis, TKIs with exon 20 activity included poziotinib, mobocertinib, and afatinib. Other palliative systemic therapies (STs) included chemotherapy with or without immunotherapy, immunotherapy alone, and gefitinib or osimertinib for patients with EGFR ex20ins. Follow-up and survival outcomes were calculated using the start date as the date of diagnosis of advanced/metastatic disease. Overall survival (OS) was defined as the time from diagnosis of advanced/metastatic disease to the time of death, whereas progression-free survival (PFS) was defined as the time from starting systemic therapy to the time of clinical or radiographic progression or death. Time to progression was defined as the time from starting treatment to either radiographic or clinical progression, based on the treating physician’s report. Radiographic imaging was conducted at the treating physician’s discretion. Patients who were alive or who were lost to follow-up at the end of the study period were censored. Adverse events were categorized using definitions as per the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
2.3. Statistical Analyses
Statistical analyses were performed using SPSS Version 28.0.0. Patient and treatment characteristics were summarized using the median and range for continuous variables, and the count and percentage for categorical variables. OS and PFS were performed using the Kaplan–Meier method using a log-rank test to assess subgroup differences. p-values and 95% confidence intervals (CI) were calculated for each variable. A p-value of <0.05 was considered as statistically significant.
3. Results
Between 1 January 2016 and 31 December 2021, a total of 136 patients were identified. Of these, 5 patients were excluded, and 131 patients were retained for further analysis (Figure 1). Baseline clinical characteristics and demographic data are summarized in Table 2. Out of the 131 patients, 84 had EGFR ex20ins, and 47 had HER2 ex20ins; the median age was 66 years. In the EGFR group, 51/84 (61%) were female, 41/84 (49%) were Asian, and 63/84 (75%) had never smoked or were light smokers. In the HER2 group, 27/47 (57%) were female, 19/47 (40%) were Asian, and 30/47 (64%) had never smoked or were light smokers. A total of 37% of the EGFR patients and 26% of the HER2 patients had brain metastases. Other common sites of metastases included bone, pleura, liver, and the adrenal gland (Table 2). Most patients were diagnosed with de novo metastatic disease.
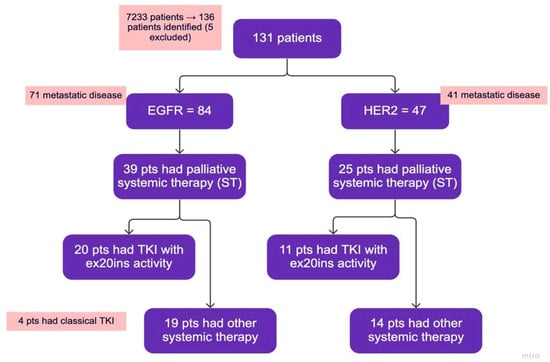
Figure 1.
Study flowchart. TKIs with ex20ins activity: poziotinib, mobocertinib, afatinib. Classical TKI: Osimertinib or gefitinib—grouped together with other systemic therapy. Other systemic therapy: chemotherapy with or without immunotherapy, immunotherapy alone, classical TKIs.

Table 2.
Patient Characteristics.
With respect to histology, 80/84 (95%) of the EGFR patients had adenocarcinoma, 3/84 were adenosquamous and 1/84 had a squamous subtype (this patient was a never-smoker). A total of 46/47 (98%) of the HER2 patients had adenocarcinoma. Concomitant mutations in EGFR or HER2 were rare. Other molecular alterations identified from the NGS panel were uncommon, and these were listed in Table 3A,B. Among EGFR ex20ins patients, 21/84 (25%) had tumours with PD-L1 greater or equal to 50%, 25/84 (30%) had 1–49% PD-L1, and 30/84 (36%) had PD-L1 < 1%. Among HER2 ex20ins patients, 9/47 (19%) had tumours with PD-L1 greater or equal to 50%, 10/47 (11%) had 1–49% PD-L1, and 23/47 (49%) had PD-L1 > 50% (Table 3C).

Table 3.
Molecular Characteristics.
3.1. Treatment Outcomes for Metastatic EGFR ex20ins Patients
Among EGFR ex20ins patients, 71/84 had stage IV disease. The median OS for patients with metastatic disease was 7.1 months (95% CI: 4.73–9.54). A total of 39/71 (55%) patients had palliative systemic therapy, of which 20 (51%) received TKIs with activity against ex20ins (i.e., poziotinib, mobocertinib, or afatinib). Ten patients received poziotinib, five patients received mobocertinib, and seven patients received afatinib. Median OS was 18.6 months (95% CI: 13.40–23.80) for patients who received any palliative ST vs. 2.6 months (95% CI: 1.66–3.54) for patients who did not (p < 0.001) (Figure 2A). Median OS was similar for patients treated with an ex20ins TKI vs. other ST (18.6 months (95% CI: 13.92–23.28) vs. 15.9 months (95% CI: 0.4–31.73); p = 0.463) (Figure 2B). In the first-line setting, median PFS was 7.1 months (95% CI: 2.94–11.26) for all patients; mPFS was 4.1 months (95% CI: 2.52–5.68) vs. 7.4 months (95% CI: 3.64–11.16) for patients treated with a TKI vs. other ST (p = 0.744) (Figure 3A). The best responses achieved with the different ex20ins TKIs for EGFR patients are shown in Figure 4A. Nine out of 10 patients who received poziotinib and all 5 patients who received mobocertinib had either a partial response or stable disease, whereas 3/7 patients who received afatinib had stable disease. Grade 3 toxicity occurred in 6/39 (15.4%) patients who received systemic therapy and 4/20 (20%) patients received TKIs with exon 20 activity (Table 4).
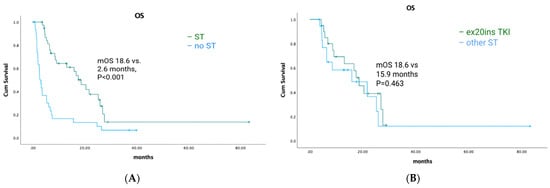
Figure 2.
EGFR-cohort overall survival and exon 20-targeted therapy. (A) Overall survival of patients who received systemic therapy vs. no systemic therapy. (B) Overall survival for exon 20 TKIs vs. other systemic therapy.
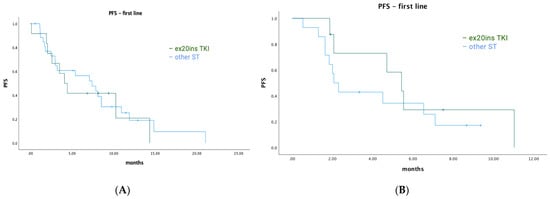
Figure 3.
Progression-free survival for first-line systemic therapy. (A) EGFR cohort—first-line PFS. (B) HER2 cohort—first-line PFS.
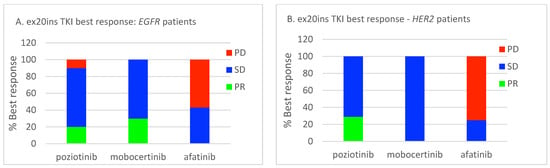
Figure 4.
Relative best responses for ex20ins TKIs. (A) EGFR cohort. (B) HER2 cohort. PR, partial response; SD, stable disease; PD, progressive disease.

Table 4.
Treatment-related adverse events.
Among EGFR ex20ins patients who received other systemic therapy either in the first-line or post-TKI progression setting, four patients received chemo-immunotherapy with platinum doublet plus pembrolizumab. Eleven patients received immunotherapy as monotherapy. For those who received immunotherapy alone, six received first-line pembrolizumab and five received either nivolumab or pembrolizumab in the second-line or beyond setting. A total of 19 patients received chemotherapy alone. Best response outcomes as well as time to progression are summarized in Table 5A.

Table 5.
Treatment responses with other systemic therapy.
Of the 21 EGFR ex20ins patients with tumour PD-L1 expression of 50% or higher, 7 patients had received immunotherapy as monotherapy, 5 had received first-line pembrolizumab, and 2 had received pembrolizumab in the second-line or beyond setting. None received chemo-immunotherapy. The median time to progression was 3 months. Four out of seven of these patients had either stable disease or partial response as their best response, whereas three out of seven had progressive disease (Table 5A).
3.2. Treatment Outcomes for Metastatic HER2 ex20ins Patients
Among HER2 ex20ins patients, 41/47 had stage IV disease. The median OS for patients with metastatic disease was 7.3 months (95% CI: 4.72–9.81). A total of 25/41 (61%) patients had palliative ST, of which 11 (44%) received TKIs with ex20ins activity. Seven patients received poziotinib, one patient received mobocertinib, and four patients received afatinib. The median OS was 9.0 months (95% CI: 6.90–11.16) for patients who received any palliative systemic therapy vs. 4.9 months (95% CI: 1.46–8.40) for patients who did not (p = 0.015) (Figure 5A). The median OS was 23.0 months (95% CI: 3.3–48.51) for patients treated with ex20ins TKI vs. 5.6 months (95% CI: 1.58–9.68) for patients who were not (p = 0.019) (Figure 5B). In the first-line setting, the median PFS was 4.5 months (95% CI: 0.85–8.16) for all patients who had ST; mPFS was 5.4 months (95% CI: 3.58–7.29) vs. 2.1 months (95% CI: 1.52–2.62) for patients treated with a TKI vs. other ST (p = 0.343) (Figure 3B). Best responses achieved with the different ex20ins TKIs for HER2 patients are shown in Figure 4B. Of the seven patients who received poziotinib, two had a partial response and five had stable disease, and the one patient who received mobocertinib had stable disease, whereas one of the four patients who received afatinib had stable disease. Grade 3 toxicity occurred in 4/25 (16%) patients who received systemic therapy, and in 1/11 (9%) patients who received TKI with exon 20 activity (Table 4).
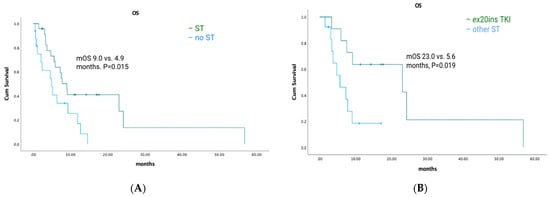
Figure 5.
HER2-cohort overall survival and exon 20-targeted therapy. (A) Overall survival with systemic therapy vs. no systemic therapy. (B) Overall survival with an exon 20 TKI vs. other systemic therapy.
Among HER2 ex20ins patients who received other systemic therapy either in the first-line or post-TKI progression setting, nine patients received chemo-immunotherapy with platinum doublet plus pembrolizumab. Seven patients received immunotherapy alone. Among those who received immunotherapy only, two received first-line pembrolizumab, and five received either nivolumab or pembrolizumab in the second-line and beyond setting. A total of 11 patients received chemotherapy alone. The best response as well as time to progression is summarized in Table 5B.
Of the nine HER2 ex20ins patients with tumour PD-L1 expression of 50% or higher, three patients received immunotherapy as monotherapy: two in first-line and one in third-line therapy. One patient received first-line chemo-immunotherapy. The median time to progression was 2.3 months among those who received immunotherapy as monotherapy. Two out of three patients had progressive disease as their best response and one had stable disease (Table 5B).
4. Discussion
Our study is one of the largest population-based real-world cohorts, with 131 patients with EGFR and HER2 ex20ins-mutated advanced/metastatic NSCLC studied over a 6-year time span. The patients captured in this study were treated in the era before newer targeted agents including amivantamab were available, and those who received exon 20-targeted agents were treated either in a clinical trial setting or through patient special-access programs. This study included patients with ex20ins exclusively confirmed via NGS-based panel testing.
The overall survival was 7.1 months for EGFR+ patients and 7.3 months for the HER2 subgroup, which is shorter than what has been reported in other real-world studies [23,24]. This observation is likely explained by several contributing factors specific to the study cohort. A significant proportion of patients (close to 50% of patients in both groups) did not receive palliative systemic therapy following their diagnosis of advanced/metastatic disease, presumably due to their poor performance status and/or rapid clinical deterioration. Among the patients who did receive systemic therapy, the median OS was 18.6 months in the EGFR cohort and 9.0 months in the HER2 cohort, which is similar to what has been previously reported [19,24,25]. In a recently published retrospective review of Canadian NSCLC patients with the three most common EGFR driver mutations, the ex20ins cohort had 18 patients, of which 72.2% received systemic therapy; the median OS from the initiation of first-line systemic therapy was 10.5 months [26]. In addition, our study has a significant proportion of patients with brain metastases at the diagnosis of stage IV disease that is both prognostic of clinical outcomes and predictive of the response to standard systemic therapy.
In our study population, HER2 exon 20 patients who received TKIs with exon 20 activity lived significantly longer compared with those who received other palliative STs. However, this difference was not reproduced in the EGFR exon 20 cohort. This is not unexpected as most of the patients who received TKIs with exon 20 activity received poziotinib, and this TKI was shown to have an ORR of 27.8% in the first-line setting and an ORR of 14.8% in pretreated patients with EGFR exon 20 mutations [13,14], whereas it was shown to have an ORR of 41% in first-line and 27.8% in pretreated patients with HER2 exon 20 mutations [19,22]. The different activity of poziotinib against EGFR and HER2 ex20ins was also seen in a prospective study in which patients received poziotinib through the expanded access program [25]. In our report, the observed first-line PFS was 5.4 months in HER2 patients who received an exon 20 TKI vs. 2.1 months for those who did not. This is very close to the reported PFS of 5.5 months in ZENITH20-2 (the HER2 cohort). However, given the limitations of the overall small number of patients (N = 11 for those who received exon 20 TKIs), the difference is not statistically significant. Currently, the most promising HER2-targeted therapy for NSCLC is T-Dxd, with an ORR of 55% in pretreated patients and a median OS of 17.8 months in the phase II Destiny-Lung01 trial [21]. T-Dxd is not yet funded in most parts of Canada outside of a clinical trial setting for NSCLC patients.
In contrast to the HER2 cohort, patients with EGFR ex20ins who received TKIs with exon 20 activity had a numerically longer but statistically non-significant OS versus those who received other palliative STs. In a recently reported phase II clinical trial, poziotinib was found to have an ORR of 32% and a median PFS of 5.5 months in 50 patients with EGFR exon 20 alterations, including both insertions and point mutations [27]. In this trial, poziotinib was shown to have more activity in near-loop insertions (amino acids 767–772) compared with far-loop insertions (amino acids 773–775). Mobocertinib and amivantamab have both been shown to have positive clinical outcomes in pretreated patients with EGFR ex20ins and are both approved by the FDA [11,12]. In our patient population, only five patients received mobocertinib and no patients received amivantamab due to lack of access during the study period.
Among the entire study cohort, there were 30 patients with tumour PD-L1 expression levels equal to or greater than 50%, 10 of whom received single-agent immunotherapy; these had a short time to progression, and 5/10 had progressive disease as the best response. These findings are not unexpected as it has been well reported that patients with certain oncogenic driver mutations tend to have a poorer response to immunotherapy in both prospective trials and real-world analyses [28]. In the IMMUNOTARGET registry, 125 patients with sensitizing EGFR mutations had an ORR of 12.2% to immune checkpoint inhibitors and a PFS of 2.1 months, whereas 29 patients with HER2 mutations had an ORR of 7% to immune checkpoint inhibitors and a PFS of 2.5 months [29]. It is unclear whether ex20ins behave similarly to the classic EGFR driver mutations as studies are heterogeneous, and rare molecular subtypes are not specifically analyzed. Retrospective data has shown that NSCLC patients with ex20ins were associated with lower PD-L1 expression and tumour mutational burden and a poor response to immunotherapy compared with NSCLC patients without targetable mutations [30]. However, there is also retrospective evidence showing that patients with ex20ins in EGFR and HER2 may derive greater benefit from immunotherapy compared with those carrying classical sensitizing EGFR mutations [31]. Further studies are needed to investigate the effect of PD-L1 expression status on the response to immunotherapy in ex20ins patients.
Limitations of our study include the retrospective nature of the review. Although it is a multi-center population-based study, patients are from a single province of Canada. Due to the rarity of the mutations, the sample size for the subgroups is small and may not be sufficient to detect any statistically significant difference. The small sample size also limits further subgroup stratification and detailed analysis of homogeneous groups. Similar to other real-world evidence studies, analyses of data are restricted by descriptive information and, sometimes, missing information, which is largely reliant on the treating physicians and details in the documentations.
In conclusion, we report results from a large population-based study evaluating real-world outcomes for 131 NSCLC patients with exon 20 insertion mutations in EGFR and HER2. This study reflects the clinical practice and treatment patterns of physicians and clinical outcomes of patients in an era when exon 20 insertion-targeted therapy was either unavailable or had just become available for select patients. Results from this study confirm that, overall, patients with ex20ins have a poor prognosis. Of note, among the HER2 cohort, overall survival was significantly better for those who received TKIs with exon 20 activity. Our data highlight the ongoing need for improved access to therapeutic agents and clinical trials for lung cancer patients with EGFR/HER2 exon 20 mutations.
Author Contributions
Conceptualization, K.L. and S.S.; Data curation, K.L.; Formal analysis, K.L.; Investigation, K.L.; Methodology, K.L. and S.S.; Resources, I.B., S.Y., C.H., J.L., B.M., Y.W. and S.S.; Software, K.L.; Supervision, S.S.; Validation, K.L. and S.S.; Writing—original draft, K.L.; Writing—review and editing, K.L., C.H., J.L., B.M., Y.W. and S.S.; Funding acquisition—not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Research Ethics Board of University of British Columbia (Access Number H22-00141; 13 March 2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the chart review and was approved by the research ethics board.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to sensitive patient information and is in accordance with institutional policy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Kane, G.M.; Bradbury, P.A.; Feld, R.; Leighl, N.B.; Liu, G.; Pisters, K.M.; Kamel-Reid, S.; Tsao, M.S.; Shepherd, F.A. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer 2017, 109, 137–144. [Google Scholar] [CrossRef]
- Friedlaender, A.; Subbiah, V.; Russo, A.; Banna, G.L.; Malapelle, U.; Rolfo, C.; Addeo, A. EGFR and HER2 exon 20 insertions in solid tumours: From biology to treatment. Nat. Rev. Clin. Oncol. 2022, 19, 51–69. [Google Scholar] [CrossRef]
- Bai, R.; Chen, X.; Song, W.; Tian, H.; Cui, J. Therapeutic exploration of uncommon EGFR exon 20 insertion mutations in advanced non-small cell lung cancer: Breaking through brambles and thorns. J. Cancer Res. Clin. Oncol. 2022, 148, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Ichihara, E.; Sakakibara-Konishi, J.; Zenke, Y.; Takeuchi, S.; Morise, M.; Hotta, K.; Sato, M.; Matsumoto, S.; Tanimoto, Z.; et al. A phase I/II study of osimertinib in EGFR exon 20 insertion mutation-positive non-small cell lung cancer. Lung Cancer 2021, 162, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lim, S.H.; An, H.J.; Kim, K.H.; Park, K.U.; Kang, E.J.; Choi, Y.H.; Ahn, M.S.; Lee, M.H.; Sun, J.; et al. Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: A multicenter, open-label, phase II trial. J. Clin. Oncol. 2020, 38, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Hendriks, L.E.L.; Cardona, A.F.; Besse, B. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat. Rev. 2020, 90, 102105. [Google Scholar] [CrossRef]
- Patil, T.; Mushtaq, R.; Marsh, S.; Azelby, C.; Pujara, M.; Davies, K.D.; Aisner, D.L.; Purcell, W.T.; Schenk, E.L.; Pacheco, J.M.; et al. Clinicopathologic characteristics, treatment outcomes, and acquired resistance patterns of atypical EGFR mutations and HER2 alterations in stage IV non-small-cell lung cancer. Clin. Lung Cancer 2020, 21, e191–e204. [Google Scholar] [CrossRef]
- Ou, S.; Lin, H.M.; Hong, J.; Yin, Y.; Jin, S.; Lin, J.; Mehta, M.; Nguyen, D.; Neal, J.W. Real-world response and outcomes in NSCLC patients with EGFR exon 20 insertion mutations. J. Clin. Oncol. 2021, 39, 9098. [Google Scholar] [CrossRef]
- Yang, J.C.; Schuler, M.; Popat, S.; Miura, S.; Heeke, S.; Park, K.; Marten, A.; Kim, E.S. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: A database of 693 cases. J. Thorac. Oncol. 2020, 15, 803–815. [Google Scholar] [CrossRef]
- Yang, J.C.; Sequist, L.V.; Geater, S.L.; Tsai, C.; Mok, T.S.K.; Schuler, M.; Yamamoto, N.; Yu, C.; Ou, S.I.; Zhou, C.; et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015, 16, 830–838. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.; Kim, S.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: Initial results from the CHRYSALIS Phase I study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Zhou, C.; Ramalingam, S.S.; Kim, T.M.; Kim, S.; Yang, J.C.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Campelo, M.R.G.; Felip, E.; et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: A phase ½ open-label nonrandomized clinical trial. JAMA Oncol. 2021, 7, e214761. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Goldman, J.W.; Clarke, J.M.; Tchekmedyian, N.; Piotrowska, Z.; Chu, D.; Bhat, G.; Lebel, F.M.; Socinski, M.A. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patient. J. Clin. Oncol. 2020, 38, 9514. [Google Scholar] [CrossRef]
- Sacher, A.; Le, X.; Cornelissen, R.; Shum, E.; Suga, J.; Socinski, M.; Molina, J.R.; Haura, E.; Clarke, J.; Bhat, G.; et al. 36MO Safety, tolerability and preliminary efficacy of poziotinib with twice daily strategy in EGFR/HER2 exon 20 mutant non-small cell lung cancer. Ann. Oncol. 2021, 32, S15. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.C.; Chiu, C.; Hsu, P.; Mitchell, P.; Chang, C.I.; John, T.; Bazhenova, L.; Kim, T.M.; Yip, C.W.; et al. Efficacy and safety of sunvozertinib in treatment naïve NSCLC patients with EGFR exon20 insertion mutations. J. Clin. Oncol. 2023, 41, 9073. [Google Scholar] [CrossRef]
- Riudavets, M.; Sullivan, I.; Abdayem, P.; Planchard, D. Targeting HER2 in non-small-cell lung cancer (NSCLC): A glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 2021, 6, 100260. [Google Scholar] [CrossRef] [PubMed]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018, 554, 189–194. [Google Scholar] [CrossRef]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results from a phase II basket trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef]
- Le, X.; Cornelissen, R.; Carassino, M.; Clarke, J.M.; Tchekmedyian, N.; Goldman, J.W.; Leu, S.; Bhat, G.; Lebel, F.; Heymach, J.V.; et al. Poziotinib in non-small-cell lung cancer habouring HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2 Trial. J. Clin. Oncol. 2022, 40, 710–718. [Google Scholar] [CrossRef]
- Han, H.; Li, S.; Chen, T.; Fitzgerald, M.; Liu, S.; Peng, C.; Tang, K.H.; Cao, S.; Chouitar, J.; Wu, J.; et al. Targeting HER2 exon 20 insertion-mutant lung adenocarcinoma with a novel tyrosine kinase inhibitor mobocertinib. Cancer Res. 2021, 81, 5311–5324. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazieres, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-mutant non-small-cell lung cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Prelaj, A.; Baik, C.; Le, X.; Garassino, M.; Wollner, M.; Haura, E.; Piotrowska, Z.; Socinski, M.; Dreiling, L.; et al. 26MO Efficacy and safety of poziotinib in treatment-naïve HER2 exon 20 insertion (ex20ins) mutated non-small cell lung cancer (NSCLC): ZENITH20-4 [abstract]. Ann. Oncol. 2022, 33, S13. [Google Scholar] [CrossRef]
- Mountzios, G.; Planchard, D.; Metro, G.; Tsiouda, D.; Prelaj, A.; Lampaki, S.; Shalata, W.; Riudavets, M.; Christopoulos, P.; Girard, N.; et al. Molecular epidemiology and treatment patterns of patients with EGFR exon 20-mutant NSCLC in the precision oncology Era: The European EXOTIC registry. JTO Clin. Res. Rep. 2022, 4, 100433. [Google Scholar] [CrossRef]
- Ahn, B.; Han, Y.; Kim, H.R.; Hong, M.H.; Cho, B.C.; Lim, S.M. Real world characteristics and clinical outcomes of HER2-mutant non-small cell lung cancer patients detected by next-generation sequencing. Cancer Res. Treat. 2023, 55, 488–497. [Google Scholar] [CrossRef]
- Prelaj, A.; Bottiglieri, A.; Proto, C.; Russo, G.L.; Signorelli, D.; Ferrara, R.; Galli, G.; De Toma, A.; Viscardi, G.; Brambilla, M.; et al. Poziotinib for EGFR and HER2 exon 20 insertion mutation in advanced NSCLC: Results from the expanded access program. Eur. J. Cancer 2021, 149, 235–248. [Google Scholar] [CrossRef]
- O’Sullivan, D.E.; Jarada, T.N.; Yusuf, A.; Hu, L.; Gogna, P.; Brenner, D.R.; Abbie, E.; Rose, J.B.; Eaton, K.; Elia-Pacitti, J.; et al. Prevalence, treatment patterns, and outcomes of individuals with EGFR positive metastatic non-small cell lung cancer in a Canadian real-world setting: A comparison of exon 19 deletion, L858R, and exon 20 insertion EGFR mutation carriers. Curr. Oncol. 2022, 29, 7198–7208. [Google Scholar] [CrossRef]
- Elamin, Y.Y.; Robichaux, J.P.; Carter, B.W.; Altan, M.; Tran, H.; Gibbons, D.L.; Heeke, S.; Fossella, F.V.; Lam, V.K.; Le, X.; et al. Poziotinib for EGFR exon 20-mutant NSCLC: Clinical efficacy, resistance mechanisms, and impact of insertion location on drug sensitivity. Cancer Cell 2022, 40, 754–767. [Google Scholar] [CrossRef]
- Addeo, A.; Passaro, A.; Malapelle, U.; Banna, G.L.; Subbiah, V.; Friedlaender, A. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat. Rev. 2021, 96, 102179. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Schoenfeld, A.J.; Flynn, J.; Falcon, C.J.; Rizvi, H.; Rudin, C.M.; Kris, M.G.; Arcila, M.E.; Heller, G.; Yu, H.A.; et al. Response to standard therapies and comprehensive genomic analysis for patients with lung adenocarcinoma with EGFR exon 20 insertions. Clin. Cancer Res. 2021, 27, 2920–2927. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.C.M.; Fares, A.F.; Le, L.W.; Mackay, K.M.; Soberano, S.; Chan, S.W.; Smith, E.; Ryan, M.; Tsao, M.S.; Bradbury, P.A.; et al. Subtypes of EGFR- and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin. Lung Cancer 2021, 22, 253–259. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).