Radiation-Induced Oropharyngeal Squamous Cell Carcinoma: Case Report and Review of the Literature
Abstract
1. Introduction
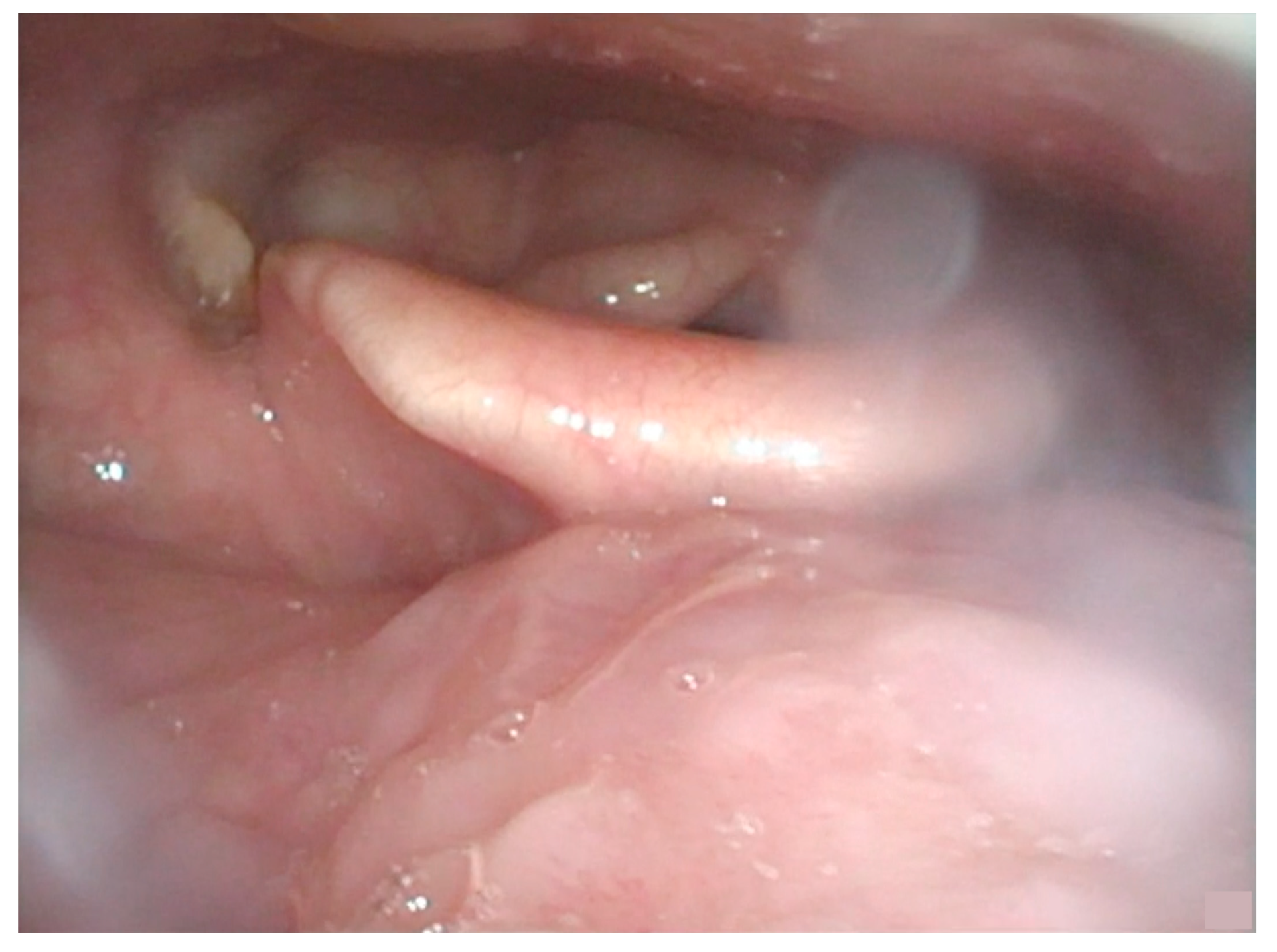
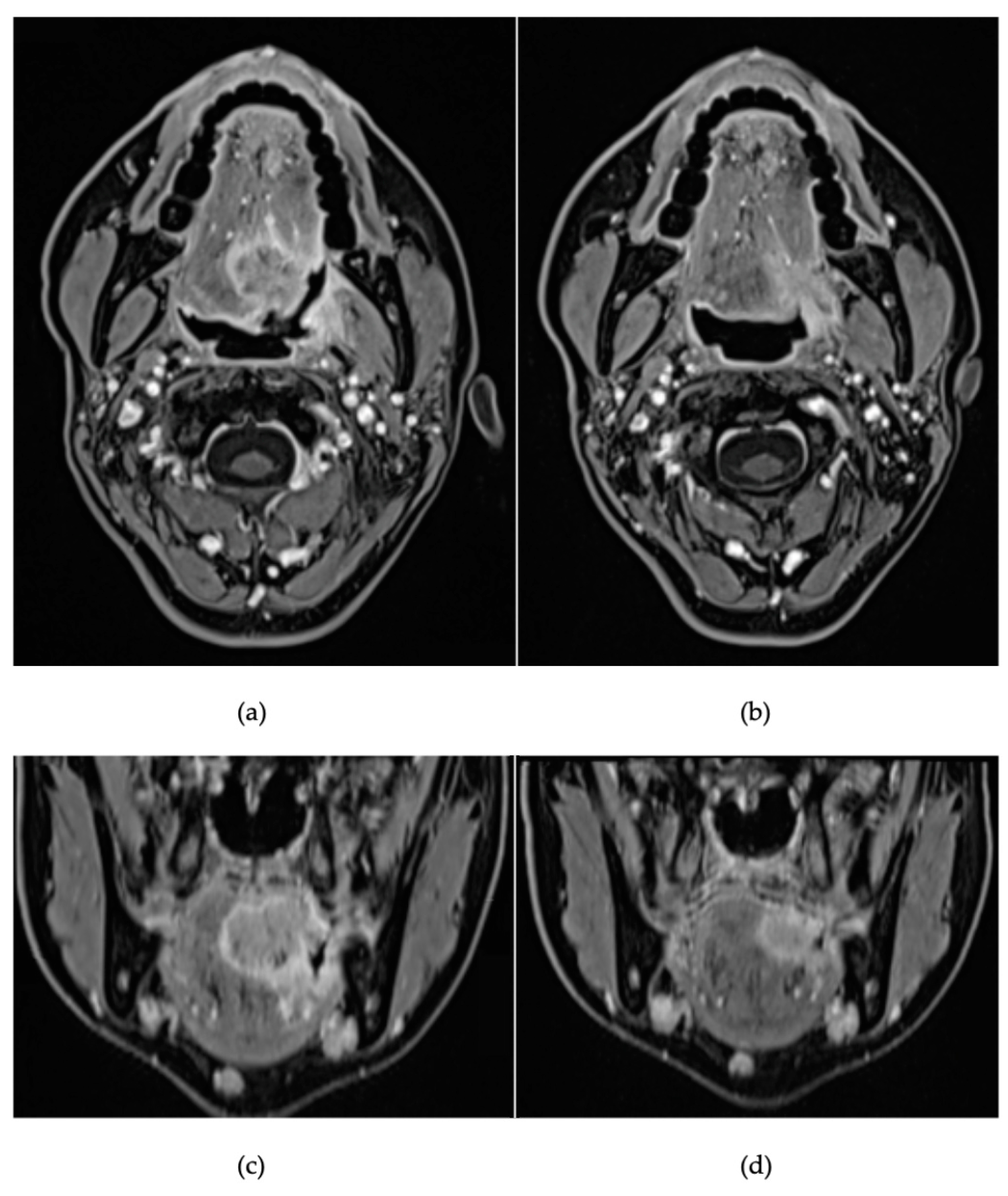
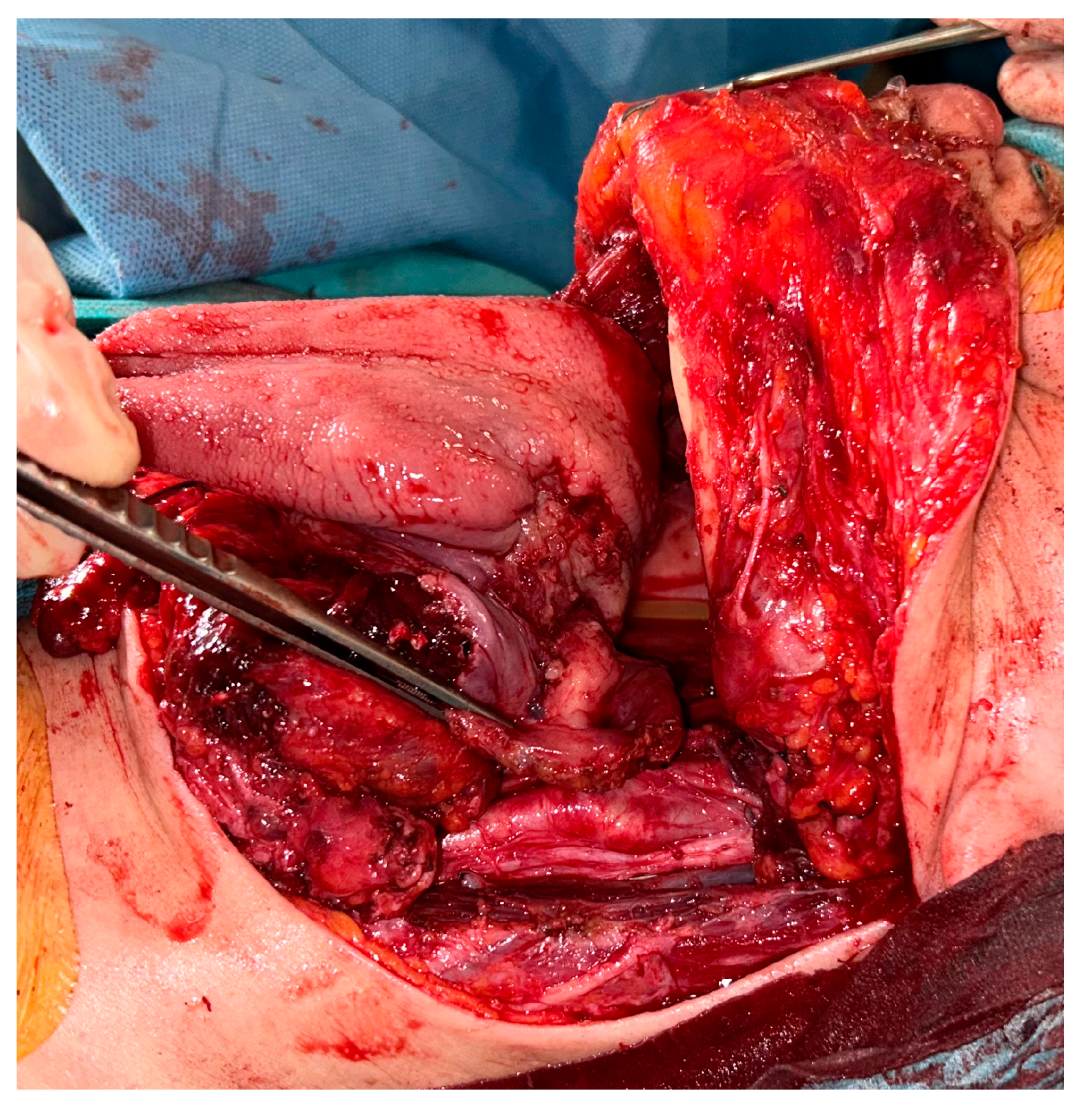
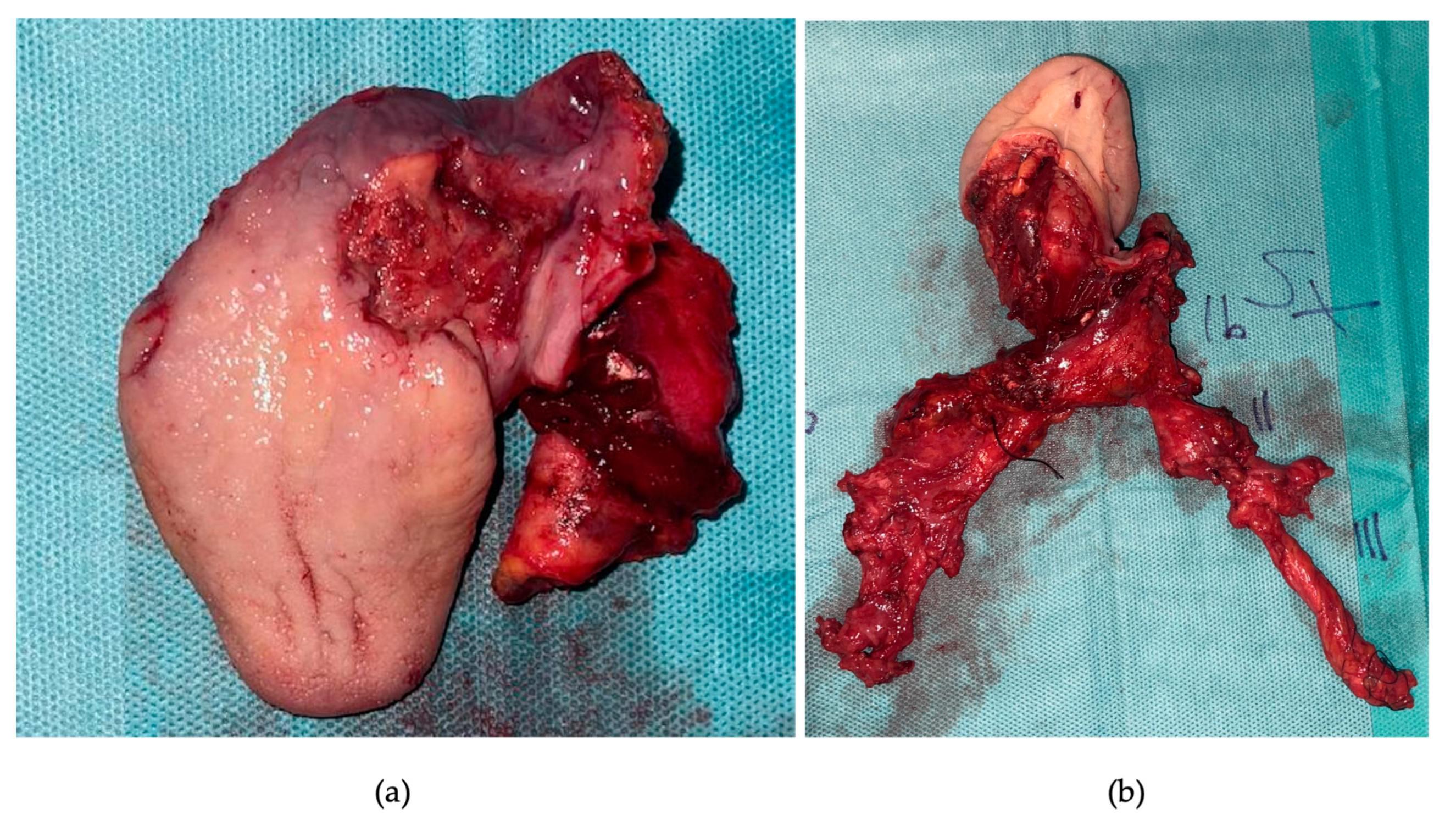
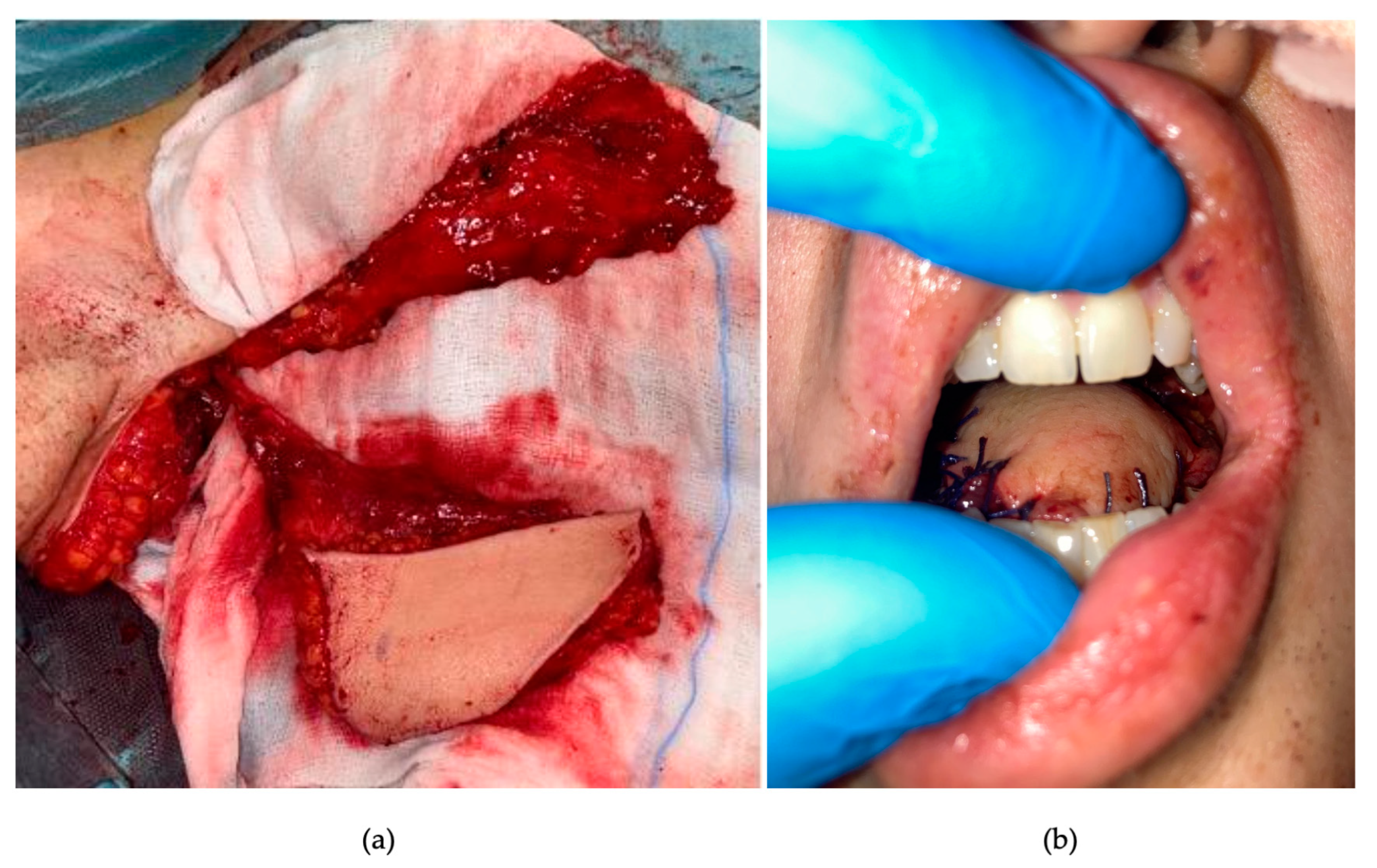
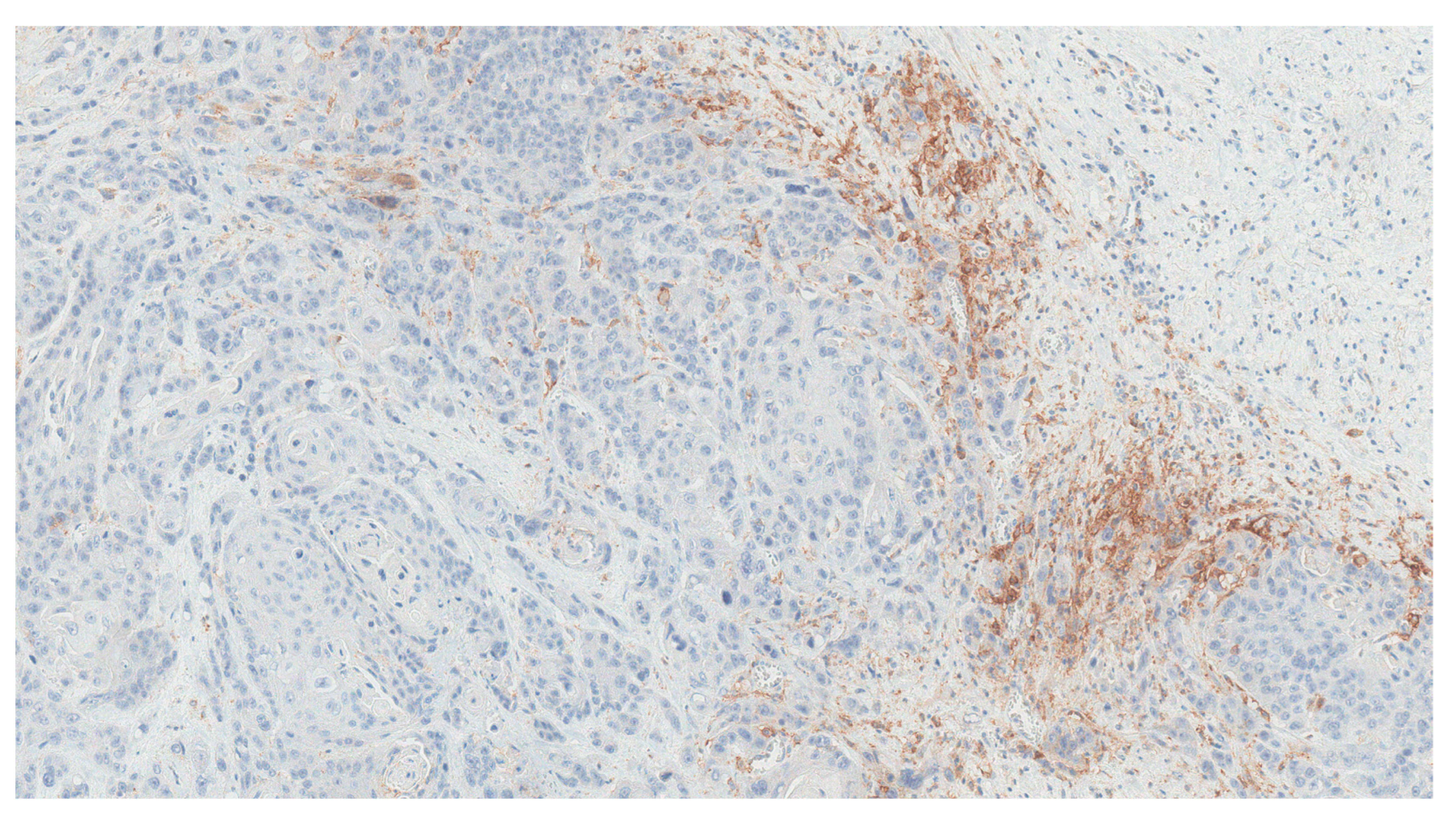
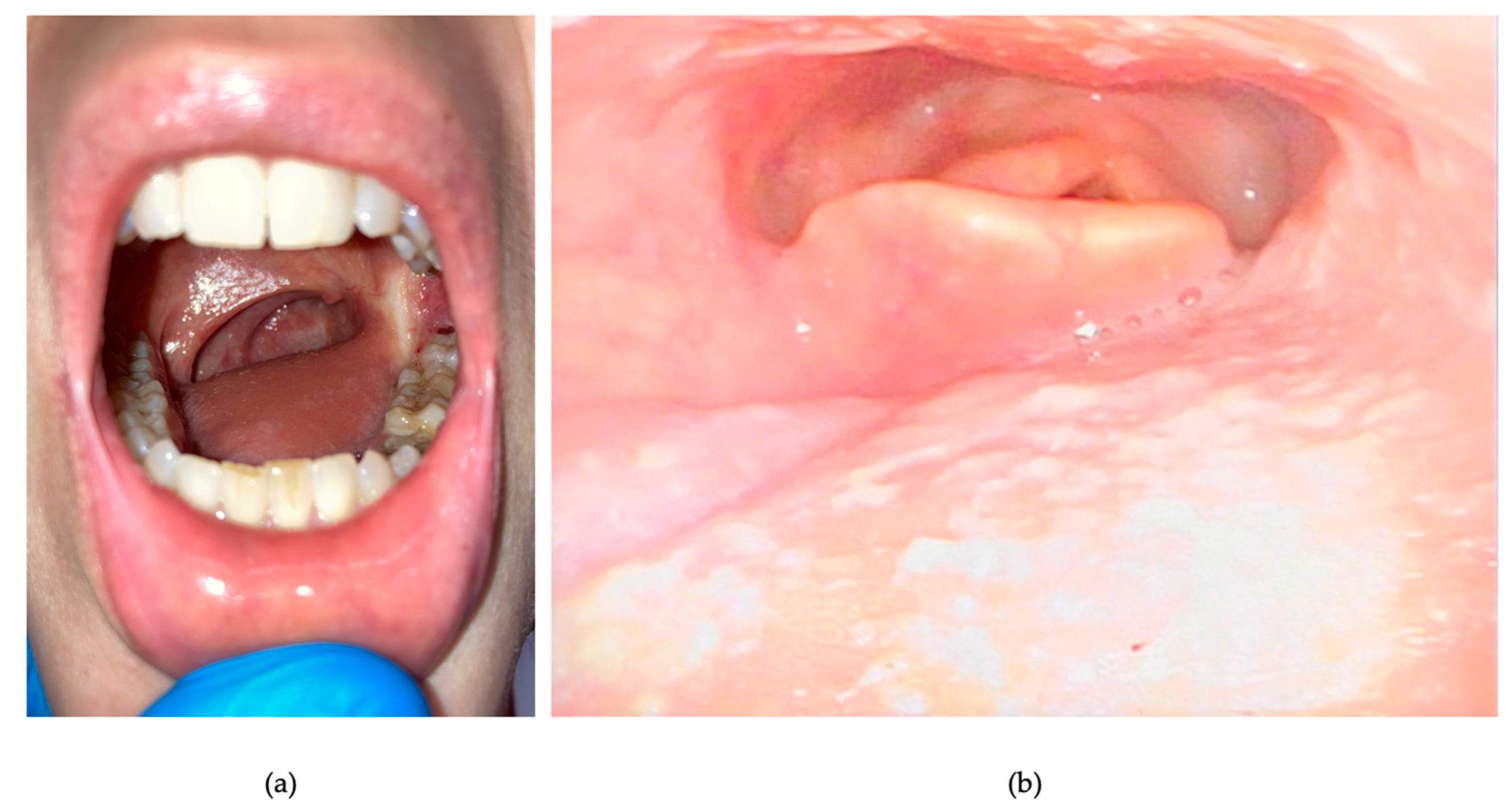
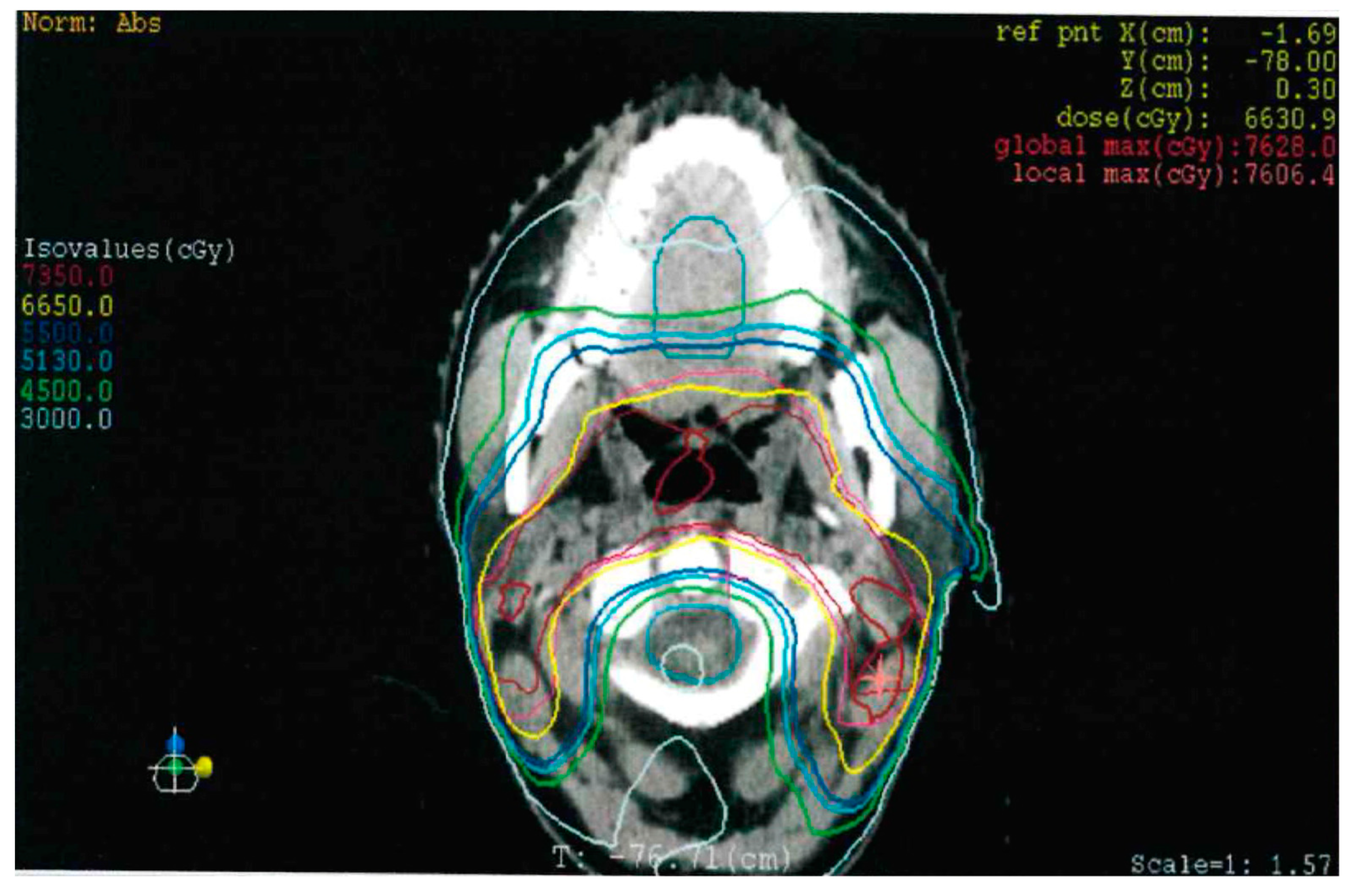
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 26, 92. [Google Scholar] [CrossRef] [PubMed]
- Meireles Da Costa, N.; Mendes, F.A.; Pontes, B.; Nasciutti, L.E.; Ribeiro Pinto, L.F.; Palumbo Júnior, A. Potential Therapeutic Significance of Laminin in Head and Neck Squamous Carcinomas. Cancers 2021, 15, 1890. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Hutcheson, K.A.; Eisbruch, A.; Beitler, J.J.; Langendijk, J.A.; Lee, A.W.M.; Corry, J.; Mendenhall, W.M.; Smee, R.; Rinaldo, A.; et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017, 59, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Li, X.; Zhao, B.; Zhang, C.; Rong, X.; Qin, C.; Wen, G.; Wu, W.; Wang, H.; Lu, K.; et al. Radiotherapy-Related Neurologic Complications in Patients with Nasopharyngeal Carcinoma: A Multicenter Epidemiologic Study in Southern China. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.M.; Aass, H.C.D.; Falk, R.S.; Astrup, G.L.; Helland, Å.; Bjøro, T.; Bjordal, K.; Dale, E.; Hellebust, T.P.; Herlofson, B.B.; et al. Associations between patient-reported late effects and systemic cytokines in long-term survivors of head and neck cancer treated with radiotherapy. J. Cancer Surviv. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Cahan, W.G.; Woodard, H.Q. Sarcoma arising in irradiated bone; report of 11 cases. Cancer 1948, 1, 3–29. [Google Scholar] [CrossRef]
- Murray, E.M.; Werner, D.; Greeff, E.A.; Taylor, D.A. Postradiation sarcomas: 20 cases and a literature review. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 951–961. [Google Scholar] [CrossRef]
- Deganello, A.; Rampinelli, V.; Gualtieri, T.; Piazza, C. Versatility of the subscapular system of flaps in head and neck oncologic reconstruction. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 161–167. [Google Scholar] [CrossRef]
- Curtis, R.E.; Freedman, D.M.; Ron, E.; Ries, L.A.G.; Hacker, D.G.; Edwards, B.K.; Tucker, M.A.; Fraumeni, J.F., Jr. (Eds.) New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000; National Cancer Institute (NIH): Bethesda, MD, USA, 2006; pp. 36–37. [Google Scholar]
- Xiang, M.; Chang, D.T.; Pollom, E.L. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 2020, 126, 3560–3568. [Google Scholar] [CrossRef]
- Liu, C.; Liao, L.; Wu, G.; Yan, H.; Chen, X.; Wang, C.; Zheng, X.; Zeng, Z.; Zhao, Z.; Wu, D.; et al. Radiation-induced second primary squamous cell carcinoma of the oral cavity after radiotherapy for nasopharyngeal carcinoma. Oral Oncol. 2020, 109, 104863. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Rubin, P.; Tupchong, L.; Brady, L.W.; Leibel, S.A.; Laramore, G.E.; Marcial, V.A.; Davis, L.W.; Cox, J.D.; et al. Second malignancies in patients who have head and neck cancer: Incidence, effect on survival and implications based on the RTOG experience. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Lu, J.J.; Hu, C.; Guo, X.; Wu, Y.; Zhang, Y. The risk of second primary tumors in patients with nasopharyngeal carcinoma after definitive radiotherapy. Cancer 2006, 107, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, S.W.; Weng, S.F.; Lin, Y.S. Risk of second primary malignancies after nasopharyngeal carcinoma: A population-based cohort study in Taiwan. Head Neck 2014, 36, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Giannini, L.; Incandela, F.; Fiore, M.; Gronchi, A.; Stacchiotti, S.; Sangalli, C.; Piazza, C. Radiation-Induced Sarcoma of the Head and Neck: A Review of the Literature. Front. Oncol. 2018, 8, 449. [Google Scholar] [CrossRef]
- Sale, K.A.; Wallace, D.I.; Girod, D.A.; Tsue, T.T. Radiation-induced malignancy of the head and neck. Otolaryngol. Head Neck Surg. 2004, 131, 643–645. [Google Scholar] [CrossRef]
- Chung, J.; Lee, V.; Tsang, R.; Chan, J.; Kwong, D.L.; Lam, K.O.; Sze, H.C.; Leung, T.W. Treatment outcomes of postradiation second head and neck malignancies managed by a multidisciplinary approach. Head Neck 2015, 37, 815–822. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, T.; Zeng, M.; He, Y.; Cai, Y.; Huang, Z. Age-specific characteristics of head and neck second primary malignancies in patients treated for nasopharyngeal carcinoma: A retrospective study. Int. J. Oral. Maxillofac. Surg. 2023, 20, S0901-502700108-X. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zhang, J.; Peng, J.; Cheng, B.; Li, H.; Hu, Q. Radiotherapy upregulated immune checkpoints contribute to the development of second primary OSCC. Oral Dis. 2023, 22, 14621. [Google Scholar] [CrossRef]
- Chen, M.C.; Feng, I.J.; Lu, C.H.; Chen, C.C.; Lin, J.T.; Huang, S.H.; Lee, K.D. The incidence and risk of second primary cancers in patients with nasopharyngeal carcinoma: A population-based study in Taiwan over a 25-year period (1979–2003). Ann. Oncol. 2008, 19, 1180–1186. [Google Scholar] [CrossRef]
- Ng, S.P.; Pollard, C., III; Kamal, M.; Ayoub, Z.; Garden, A.S.; Bahig, H.; Gunn, G.B.; Frank, S.J.; Skinner, H.D.; Phan, J.; et al. Risk of second primary malignancies in head and neck cancer patients treated with definitive radiotherapy. NPJ Precis. Onc. 2019, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Ardenfors, O.; Josefsson, D.; Dasu, A. Are IMRT treatments in the head and neck region increasing the risk of secondary cancers? Acta Oncol. 2014, 53, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Tay, G.C.; Iyer, N.G.; Ong, W.S.; Tai, D.; Ang, M.K.; Ha, T.C.; Soo, K.C.; Tan, H.K. Outcomes and Prognostic Factors of Radiation-Induced and De Novo Head and Neck Squamous Cell Carcinomas. Otolaryngol. Head Neck Surg. 2016, 154, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Rosko, A.J.; Birkeland, A.C.; Chinn, S.B.; Shuman, A.G.; Prince, M.E.; Patel, R.M.; McHugh, J.B.; Spector, M.E. Survival and margin status in head and neck radiation-induced sarcomas and de novo sarcomas. Otolaryngol. Head Neck Surg. 2017, 157, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kasperts, N.; Slotman, B.; Leemans, C.R.; Langendijk, J.A. A review on re-irradiation for recurrent and second primary head and neck cancer. Oral Oncol. 2005, 41, 225–243. [Google Scholar] [CrossRef]
- Liao, L.-Q.; Yan, H.-H.; Mai, J.-H.; Liu, W.-W.; Li, H.; Guo, Z.-M.; Zeng, Z.-Y.; Liu, X.-K. Radiation-induced osteosarcoma oof the maxilla and mandible after radiotherapy for nasopharyngeal carcinoma. Chin J. Cancer 2016, 35, 89. [Google Scholar] [CrossRef]
- Sun, C.; Wang, X.; Zhong, Z.; Wang, W.; Luo, J.; Chen, X.; Chu, H.; Tao, X.; Yang, A. Differences in Clinicopathological Characteristics and Prognosis Between Primary and Postirradiation Tongue Squamous Cell Carcinoma. J. Oral Maxillofac. Surg. 2017, 75, 2235–2241. [Google Scholar] [CrossRef]
- Merlino, D.J.; Johnson, J.M.; Tuluc, M.; Gargano, S.; Stapp, R.; Harshyne, L., Jr.; Leiby, B.E.; Flanders, A.; Zinner, R.; Axelrod, R.; et al. Discordant Responses Between Primary Head and Neck Tumors and Nodal Metastases Treated with Neoadjuvant Nivolumab: Correlation of Radiographic and Pathologic Treatment Effect. Front. Oncol. 2020, 10, 566315. [Google Scholar] [CrossRef]
- Vos, J.L.; Elbers, J.B.W.; Krijgsman, O.; Traets, J.J.H.; Qiao, X.; van der Leun, A.M.; Lubeck, Y.; Seignette, I.M.; Smit, L.A.; Willems, S.M.; et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat. Commun. 2021, 12, 7348. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.E.; Lipson, E.J.; Cottrell, T.R.; Forde, P.M.; Anders, R.A.; Cimino-Mathews, A.; Thompson, E.D.; Allaf, M.E.; Yarchoan, M.; Feliciano, J.; et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin. Cancer Res. 2020, 26, 545–551. [Google Scholar] [CrossRef]
- Pignon, J.P.; Le Maitre, A.; Maillard, E.; Bourhis, J.; Mach-Nc Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother. Oncol. 2021, 156, 281–293. [Google Scholar] [CrossRef]
- Petit, C.; Lacas, B.; Pignon, J.P.; Le, Q.T.; Grégoiere, V.; Grau, C.; Hackshaw, A.; Zackrisson, B.; Parmar, M.K.B.; Lee, J.-W.; et al. Chemotherapy and radiotherapy in locally advanced head and neck cancer: An individual patient data network meta-analysis. Lancet Oncol. 2021, 22, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Lo Vullo, S.; Guzzo, M.; Mariani, L.; Granata, R.; Orlandi, E.; Locati, L.; Scaramellini, G.; Fallai, C.; Licitra, L. Preoperative chemotherapy in advanced resectable OCSCC: Long-term results of a randomized phase III trial. Ann. Oncol. 2014, 25, 462–466. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.B.; Marta, G.N.; de Castro Junior, G.; Kowalski, L.P. Induction Chemotherapy for Advanced Oral Cavity Cancer. Curr Oncol Rep 2021, 23, 129. [Google Scholar] [CrossRef]
- Uppaluri, R.; Campbell, K.M.; Egloff, A.M.; Zolkind, P.; Skidmore, Z.L.; Nussenbaum, B.; Paniello, R.C.; Rich, J.T.; Jackson, R.; Pipkorn, P.; et al. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase II Trial. Clin. Cancer Res. 2020, 26, 5140–5152. [Google Scholar] [CrossRef]
- Amin, N.; Maroun, C.A.; El Asmar, M.; Alkhatib, H.H.; Guller, M.; Herberg, M.E.; Zhu, G.; Seiwert, T.Y.; Pardoll, D.; Eisele, D.W.; et al. Neoadjuvant immunotherapy prior to surgery for mucosal head and neck squamous cell carcinoma: Systematic review. Head Neck 2022, 44, 562–571. [Google Scholar] [CrossRef]
- Khadela, A.; Shah, Y.; Mistry, P.; Bodiwala, K.; Cb, A. Immunomodulatory Therapy in Head and Neck Squamous Cell Carcinoma: Recent Advances and Clinical Prospects. Technol. Cancer Res. Treat. 2023, 22, 15330338221150559. [Google Scholar] [CrossRef]
- Egloff, A.M.; Uppaluri, R. Preoperative immunotherapy for head and neck cancers: State of art. Curr. Opin. Oncol. 2022, 1, 185–195. [Google Scholar] [CrossRef]
- Luginbuhl, A.J.; Johnson, J.M.; Harshyne, L.A.; Linnenbach, A.J.; Shukla, S.K.; Alnemri, A.; Kumar, G.; Cognetti, D.M.; Curry, J.M.; Kotlov, N.; et al. Tadalafil Enhances Immune Signatures in Response to Neoadjuvant Nivolumab in Resectable Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2022, 28, 915–927. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannini, L.; Alliata, A.; Cristofaro, V.; Incandela, F.; Pompilio, M.; Ottini, A.; Cavalieri, S.; Nuzzolese, I.; Iacovelli, N.A.; Franceschini, M.; et al. Radiation-Induced Oropharyngeal Squamous Cell Carcinoma: Case Report and Review of the Literature. Curr. Oncol. 2023, 30, 6708-6719. https://doi.org/10.3390/curroncol30070492
Giannini L, Alliata A, Cristofaro V, Incandela F, Pompilio M, Ottini A, Cavalieri S, Nuzzolese I, Iacovelli NA, Franceschini M, et al. Radiation-Induced Oropharyngeal Squamous Cell Carcinoma: Case Report and Review of the Literature. Current Oncology. 2023; 30(7):6708-6719. https://doi.org/10.3390/curroncol30070492
Chicago/Turabian StyleGiannini, Lorenzo, Andrea Alliata, Valentina Cristofaro, Fabiola Incandela, Madia Pompilio, Arianna Ottini, Stefano Cavalieri, Imperia Nuzzolese, Nicola Alessandro Iacovelli, Marzia Franceschini, and et al. 2023. "Radiation-Induced Oropharyngeal Squamous Cell Carcinoma: Case Report and Review of the Literature" Current Oncology 30, no. 7: 6708-6719. https://doi.org/10.3390/curroncol30070492
APA StyleGiannini, L., Alliata, A., Cristofaro, V., Incandela, F., Pompilio, M., Ottini, A., Cavalieri, S., Nuzzolese, I., Iacovelli, N. A., Franceschini, M., & Deganello, A. (2023). Radiation-Induced Oropharyngeal Squamous Cell Carcinoma: Case Report and Review of the Literature. Current Oncology, 30(7), 6708-6719. https://doi.org/10.3390/curroncol30070492




