Association between COPD and Stage of Lung Cancer Diagnosis: A Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Sources
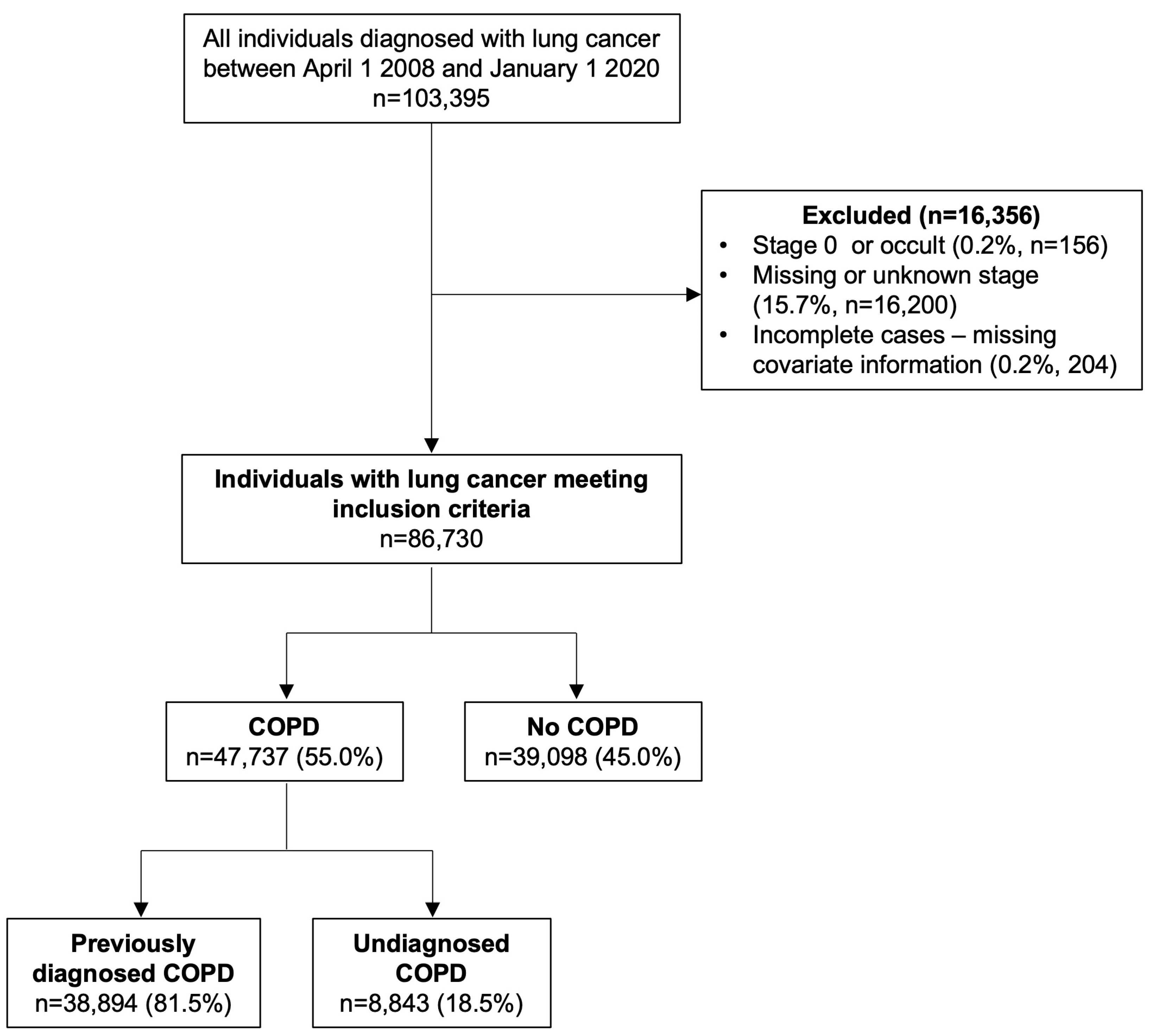
2.3. Study Population
2.4. Primary Outcome
2.5. Exposures
2.6. Covariates
2.7. Statistical Analyses
2.8. Sensitivity Analyses
3. Results
3.1. COPD and Stage of Lung Cancer
3.2. Impact of Prior Imaging
3.3. Other Factors Associated with Stage of Lung Cancer
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, C.; Sun, S.-W.; Xiong, X.-Z. From COPD to Lung Cancer: Mechanisms Linking, Diagnosis, Treatment, and Prognosis. Int. J. Chron. Obstruct. Pulmon. Dis. 2022, 17, 2603–2621. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous Lung Diseases and Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2011, 6, e17479. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.J.; Ellerton, L.; Goldstein, R.S.; Brooks, D. Prevalence of Lung Cancer in Chronic Obstructive Pulmonary Disease: A Systematic Review. Respir. Med. X 2019, 1, 100003. [Google Scholar] [CrossRef]
- Park, H.Y.; Kang, D.; Shin, S.H.; Yoo, K.H.; Rhee, C.K.; Suh, G.Y.; Kim, H.; Shim, Y.M.; Guallar, E.; Cho, J.; et al. Chronic Obstructive Pulmonary Disease and Lung Cancer Incidence in Never Smokers: A Cohort Study. Thorax 2020, 75, 506–509. [Google Scholar] [CrossRef]
- Gao, Y.; Guan, W.; Liu, Q.; Wang, H.; Zhu, Y.; Chen, R.; Zhang, G. Impact of COPD and Emphysema on Survival of Patients with Lung Cancer: A Meta-Analysis of Observational Studies. Respirology 2016, 21, 269–279. [Google Scholar] [CrossRef]
- Goffin, J.R.; Corriveau, S.; Tang, G.H.; Pond, G.R. Management and Outcomes of Patients with Chronic Obstructive Lung Disease and Lung Cancer in a Public Healthcare System. PLoS ONE 2021, 16, e0251886. [Google Scholar] [CrossRef]
- Shah, S.; Blanchette, C.M.; Coyle, J.C.; Kowalkowski, M.; Arthur, S.T.; Howden, R. Healthcare Utilization and Costs Associated with COPD among SEER-Medicare Beneficiaries with NSCLC. J. Med. Econ. 2018, 21, 861–868. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Society; Canadian Cancer Statistics Advisory Committee: Toronto, ON, Canada, 2020. [Google Scholar]
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics [Internet]; Surveillance Research Program at the National Cancer Institute, United States of America: 2021. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html (accessed on 4 August 2021).
- Cassim, S.; Chepulis, L.; Keenan, R.; Kidd, J.; Firth, M.; Lawrenson, R. Patient and Carer Perceived Barriers to Early Presentation and Diagnosis of Lung Cancer: A Systematic Review. BMC Cancer 2019, 19, 25. [Google Scholar] [CrossRef]
- Sætre, L.M.S.; Rasmussen, S.; Balasubramaniam, K.; Søndergaard, J.; Jarbøl, D.E. A Population-Based Study on Social Inequality and Barriers to Healthcare-Seeking with Lung Cancer Symptoms. NPJ Prim. Care Respir. Med. 2022, 32, 48. [Google Scholar] [CrossRef]
- Cunningham, Y.; Wyke, S.; Blyth, K.G.; Rigg, D.; Macdonald, S.; Macleod, U.; Harrow, S.; Robb, K.A.; Whitaker, K.L. Lung Cancer Symptom Appraisal among People with Chronic Obstructive Pulmonary Disease: A Qualitative Interview Study. Psychooncology 2019, 28, 718–725. [Google Scholar] [CrossRef]
- Bjerager, M.; Palshof, T.; Dahl, R.; Vedsted, P.; Olesen, F. Delay in Diagnosis of Lung Cancer in General Practice. Br. J. Gen. Pract. 2006, 56, 863–868. [Google Scholar] [PubMed]
- Birt, L.; Hall, N.; Emery, J.; Banks, J.; Mills, K.; Johnson, M.; Hamilton, W.; Walter, F.M. Responding to Symptoms Suggestive of Lung Cancer: A Qualitative Interview Study. BMJ Open Respir. Res. 2014, 1, e000067. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, B.; Soriano, J.B.; Studnicka, M.; Kaiser, B.; Vanfleteren, L.E.; Gnatiuc, L.; Burney, P.; Miravitlles, M.; Garcîa-Rio, F.; Akbari, K.; et al. Determinants of Underdiagnosis of COPD in National and International Surveys. Chest 2015, 148, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Carter-harris, L. Lung Cancer Stigma as a Barrier to Medical Help-Seeking Behavior: Practice Implications. J. Am. Assoc. Nurse Pract. 2015, 27, 240–245. [Google Scholar] [CrossRef]
- Scott, N.; Crane, M.; Lafontaine, M.; Seale, H.; Currow, D. Stigma as a Barrier to Diagnosis of Lung Cancer: Patient and General Practitioner Perspectives. Prim. Health Care Res. Dev. 2015, 16, 618–622. [Google Scholar] [CrossRef]
- Friedemann Smith, C.; Whitaker, K.L.; Winstanley, K.; Wardle, J. Smokers Are Less Likely than Non-Smokers to Seek Help for a Lung Cancer “alarm” Symptom. Thorax 2016, 71, 659–661. [Google Scholar] [CrossRef]
- ICES Data Dictionary. Available online: https://datadictionary.ices.on.ca/Applications/DataDictionary/Default.aspx (accessed on 30 May 2023).
- Gershon, A.S.; Wang, C.; Guan, J.; Vasilevska-Ristovska, J.; Cicutto, L.; To, T. Identifying Individuals with Physcian Diagnosed COPD in Health Administrative Databases. J. Chronic Obstr. Pulm. Dis. 2009, 6, 388–394. [Google Scholar] [CrossRef]
- Gershon, A.S.; Warner, L.; Cascagnette, P.; Victor, J.C.; To, T. Lifetime Risk of Developing Chronic Obstructive Pulmonary Disease: A Longitudinal Population Study. Lancet 2011, 378, 991–996. [Google Scholar] [CrossRef]
- Cho, E.E.; Mecredy, G.C.; Wong, H.H.; Stanbrook, M.B.; Gershon, A.S. Which Physicians Are Taking Care of People With COPD? Chest 2019, 155, 771–777. [Google Scholar] [CrossRef]
- Gershon, A.S.; Wang, C.; Guan, J.; Vasilevska-Ristovska, J.; Cicutto, L.; To, T. Identifying Patients with Physician-Diagnosed Asthma in Health Administrative Databases. Can. Respir. J. 2009, 16, 183–188. [Google Scholar] [CrossRef]
- Jaakkimainen, R.L.; Bronskill, S.E.; Tierney, M.C.; Herrmann, N.; Green, D.; Young, J.; Ivers, N.; Butt, D.; Widdifield, J.; Tu, K. Identification of Physician-Diagnosed Alzheimer’s Disease and Related Dementias in Population-Based Administrative Data: A Validation Study Using Family Physicians’ Electronic Medical Records. J. Alzheimer’s Dis. 2016, 54, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Lipscombe, L.L.; Hwee, J.; Webster, L.; Shah, B.R.; Booth, G.L.; Tu, K. Identifying Diabetes Cases from Administrative Data: A Population-Based Validation Study. BMC Health Serv. Res. 2018, 18, 316. [Google Scholar] [CrossRef]
- Schultz, S.E.; Rothwell, D.M.; Chen, Z.; Tu, K. Identifying Cases of Congestive Heart Failure from Administrative Data: A Validation Study Using Primary Care Patient Records. Chronic Dis. Inj. Can. 2013, 33, 160–166. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards Best Practice When Using Inverse Probability of Treatment Weighting (IPTW) Using the Propensity Score to Estimate Causal Treatment Effects in Observational Studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Xu, S.; Ross, C.; Raebel, M.A.; Shetterly, S.; Blanchette, C.; Smith, D. Use of Stabilized Inverse Propensity Scores as Weights to Directly Estimate Relative Risk and Its Confidence Intervals Stanley. Value Health 2010, 13, 273–277. [Google Scholar] [CrossRef]
- Ridgeway, G.; McCaffrey, D.; Morral, A.; Cefalu, M.; Burgette, L.; Pane, J.; Griffin, B.A. Toolkit for Weighting and Analysis of Nonequivalent Groups: A Guide to the Twang Package. Available online: https://cran.r-project.org/web/packages/twang/vignettes/twang.pdf (accessed on 4 August 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 4 August 2021).
- Lofters, A.K.; Gatov, E.; Lu, H.; Baxter, N.N.; Guilcher, S.J.T.; Kopp, A.; Vahabi, M.; Datta, G.D. Lung Cancer Inequalities in Stage of Diagnosis in Ontario, Canada. Curr. Oncol. 2021, 28, 1946–1956. [Google Scholar] [CrossRef]
- Lofters, A.K.; Gatov, E.; Lu, H.; Baxter, N.N.; Corrado, A.M.; Guilcher, S.J.T.; Kopp, A.; Vahabi, M.; Datta, G.D. Stage of Colorectal Cancer Diagnosis for Immigrants: A Population-Based Retrospective Cohort Study in Ontario, Canada. Cancer Causes Control 2021, 32, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Saab, M.M.; Fitzgerald, S.; Noonan, B.; Kilty, C.; Collins, A.; Lyng, Á.; Kennedy, U.; O’brien, M.; Hegarty, J. Promoting Lung Cancer Awareness, Help-Seeking and Early Detection: A Systematic Review of Interventions. Health Promot. Int. 2021, 36, 1656–1671. [Google Scholar] [CrossRef] [PubMed]
- Ruparel, M.; Quaife, S.L.; Dickson, J.L.; Horst, C.; Tisi, S.; Hall, H.; Taylor, M.N.; Ahmed, A.; Shaw, P.J.; Burke, S.; et al. Prevalence, Symptom Burden, and Underdiagnosis of Chronic Obstructive Pulmonary Disease in a Lung Cancer Screening Cohort. Ann. Am. Thorac. Soc. 2020, 17, 869–878. [Google Scholar] [CrossRef]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Vestbo, J.; Lange, P. Prognosis of Asymptomatic and Symptomatic, Undiagnosed COPD in the General Population in Denmark: A Prospective Cohort Study. Lancet Respir. Med. 2017, 5, 426–434. [Google Scholar] [CrossRef]
- Johnson, K.; Bryan, S.; Ghanbarian, S.; Sin, D.; Sadatsafavi, M. Characterising Undiagnosed Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Respir. Res. 2018, 26, 184986. [Google Scholar] [CrossRef]
- Dai, J.; He, Y.; Maneenil, K.; Liu, H.; Liu, M.; Guo, Q.; Bennett, A.C.; Stoddard, S.M.; Wampfler, J.A.; Jiang, G.; et al. Timing of Chronic Obstructive Pulmonary Disease Diagnosis in Lung Cancer Prognosis: A Clinical and Genomic-Based Study. Transl. Lung Cancer Res. 2021, 10, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Lipscombe, L.L.; Fischer, H.D.; Austin, P.C.; Fu, L.; Jaakkimainen, R.L.; Ginsburg, O.; Rochon, P.A.; Narod, S.; Paszat, L. The Association between Diabetes and Breast Cancer Stage at Diagnosis: A Population-Based Study. Breast Cancer Res. Treat. 2015, 150, 613–620. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Danese, M.D.; Gleeson, M.L.; Valderas, J.M. Epidemiology and Outcomes of Previously Undiagnosed Diabetes in Older Women with Breast Cancer: An Observational Cohort Study Based on SEER-Medicare. BMC Cancer 2012, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R.J. Chronic Obstructive Pulmonary Disease (COPD) and Lung Cancer Screening. Transl. Lung Cancer Res. 2018, 7, 347–360. [Google Scholar] [CrossRef]
- Young, R.P.; Hopkins, R. The Potential Impact of Chronic Obstructive Pulmonary Disease in Lung Cancer Screening: Implications for the Screening Clinic. Expert Rev. Respir. Med. 2019, 13, 699–707. [Google Scholar] [CrossRef] [PubMed]
- The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]

| COPD | ||||
|---|---|---|---|---|
| Characteristics | Total COPD | Previously Diagnosed COPD | Undiagnosed COPD | No COPD |
| (n = 47,737) | (n = 38,894) | (n = 8843) | (n = 39,098) | |
| Lung Cancer Stage | ||||
| Early | 31.1% | 32.0% | 27.2% | 23.9% |
| Advanced | 68.9% | 68.0% | 72.8% | 76.1% |
| Type of Lung Cancer | ||||
| NSCLC: Adenocarcinoma | 36.7% | 35.5% | 42.2% | 49.4% |
| NSCLC: Squamous cell carcinoma | 21.7% | 21.9% | 20.5% | 13.9% |
| NSCLC: Large cell carcinoma | 2.5% | 2.4% | 2.6% | 2.7% |
| NSCLC: Adenocarcinoma | 0.6% | 0.5% | 0.7% | 0.6% |
| NSCLC: Not otherwise specified | 14.1% | 14.2% | 13.6% | 14.2% |
| SCLC | 12.5% | 12.7% | 11.7% | 10.4% |
| Unspecified | 12.0% | 12.7% | 8.8% | 8.8% |
| Method of Confirmation | ||||
| Clinical | 1.3% | 1.4% | 1.0% | 0.8% |
| Histology/Cytology | 87.1% | 85.9% | 92.2% | 91.1% |
| Imaging | 4.4% | 5.0% | 2.0% | 2.7% |
| Unknown | 7.2% | 7.8% | 4.8% | 5.4% |
| Age, mean (SD) | 71.3 (9.6) | 71.9 (9.4) | 68.7 (9.9) | 68.9 (11.3) |
| Sex, % male | 52.1% | 51.5% | 54.7% | 51.0% |
| Rurality/Income Quintile | ||||
| Rural | 16.3% | 16.5% | 15.5% | 13.8% |
| Urban 1 (lowest) | 22.9% | 23.3% | 21.3% | 18.2% |
| Urban 2 | 19.7% | 19.6% | 20.3% | 18.8% |
| Urban 3 | 15.8% | 15.8% | 15.7% | 17.1% |
| Urban 4 | 13.6% | 13.4% | 14.4% | 16.5% |
| Urban 5 (highest) | 11.6% | 11.4% | 12.8% | 15.6% |
| Immigration Category | ||||
| ≤10 years | 0.6% | 0.4% | 1.5% | 2.2% |
| Long-term resident (>10 years) | 2.9% | 2.7% | 4.2% | 6.6% |
| Nonimmigrant | 96.5% | 97.0% | 94.4% | 91.2% |
| Comorbidities | ||||
| Asthma | 23.1% | 26.1% | 9.9% | 7.9% |
| Congestive heart failure | 16.2% | 17.7% | 9.7% | 7.5% |
| Dementia | 5.0% | 5.5% | 2.8% | 3.7% |
| Diabetes | 28.3% | 29.4% | 23.3% | 24.8% |
| Previous pneumonia | 30.9% | 32.5% | 24.3% | 18.5% |
| Cancer in the previous 5 years | 8.3% | 8.7% | 6.6% | 9.1% |
| Rate of Primary Care Visits | ||||
| ≥5 per year | 47.0% | 50.6% | 31.3% | 34.0% |
| >2 and <5 per year | 35.4% | 35.1% | 37.0% | 38.1% |
| >0 and ≤2 per year | 14.9% | 12.6% | 24.9% | 22.3% |
| 0 | 2.6% | 1.7% | 6.7% | 5.6% |
| Specialist care | 46.9% | 49.7% | 34.7% | 36.4% |
| Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|
| Characteristics | COPD | No COPD | ||||
| (n = 47,737) | (n = 39,098) | SMD | COPD | No COPD | SMD | |
| Age, mean (SD) | 71.3 (9.6) | 68.9 (11.3) | 0.229 | 70.4 (9.7) | 70.3 (11.3) | 0.003 |
| Sex, % male | 52.10% | 51.00% | 0.022 | 51.90% | 51.90% | 0 |
| Rurality/Income Quintile | ||||||
| Rural | 16.3% | 13.8% | 0.072 | 15.2% | 15.1% | 0.003 |
| Urban 1 (lowest) | 22.9% | 18.2% | 0.115 | 20.8% | 20.6% | 0.004 |
| Urban 2 | 19.7% | 18.8% | 0.023 | 19.4% | 19.5% | 0.002 |
| Urban 3 | 15.8% | 17.1% | 0.034 | 16.4% | 16.4% | 0.001 |
| Urban 4 | 13.6% | 16.5% | 0.082 | 14.8% | 14.9% | 0.003 |
| Urban 5 (highest) | 11.6% | 15.6% | 0.117 | 13.5% | 13.5% | 0.003 |
| Immigration Category | ||||||
| ≤10 years | 0.6% | 2.2% | 0.140 | 1.3% | 1.3% | 0.004 |
| Long-term resident (>10 years) | 2.9% | 6.6% | 0.177 | 4.6% | 4.6% | 0.003 |
| Non-immigrant | 96.5% | 91.2% | 0.224 | 94.2% | 94.1% | 0.005 |
| Type of Lung Cancer | ||||||
| NSCLC: Adenocarcinoma | 36.7% | 49.4% | 0.256 | 42.5% | 42.9% | 0.008 |
| NSCLC: Squamous cell carcinoma | 21.7% | 13.9% | 0.202 | 18.2% | 18.1% | 0.001 |
| NSCLC: Large cell carcinoma | 2.5% | 2.7% | 0.015 | 2.6% | 2.6% | 0.002 |
| NSCLC: Adenosquamous | 0.6% | 0.6% | 0.006 | 0.6% | 0.6% | 0 |
| NSCLC: Not otherwise specified | 14.1% | 14.2% | 0.005 | 14.1% | 14.0% | 0.003 |
| SCLC | 12.5% | 10.4% | 0.066 | 11.5% | 11.4% | 0.004 |
| Unspecified | 12.0% | 8.8% | 0.104 | 10.5% | 10.3% | 0.006 |
| Comorbidities | ||||||
| Asthma | 23.1% | 7.9% | 0.412 | 16.1% | 15.4% | 0.021 |
| Congestive heart failure | 16.2% | 7.5% | 0.267 | 12.3% | 12.0% | 0.008 |
| Dementia | 5.0% | 3.7% | 0.064 | 4.4% | 4.40% | 0.002 |
| Diabetes | 28.3% | 24.8% | 0.080 | 26.8% | 26.5% | 0.006 |
| Previous pneumonia | 30.9% | 18.5% | 0.287 | 25.2% | 24.8% | 0.009 |
| Cancer in the previous 5 years | 8.3% | 9.1% | 0.026 | 8.7% | 8.7% | 0 |
| Rate of Primary Care Visits | ||||||
| ≥5 per year | 47.0% | 34.0% | 0.266 | 41.1% | 40.7% | 0.008 |
| >2 and <5 per year | 35.4% | 38.1% | 0.056 | 36.7% | 37.0% | 0.006 |
| >0 and ≤2 per year | 14.9% | 22.3% | 0.192 | 18.3% | 18.3% | 0.002 |
| 0 | 2.6% | 5.6% | 0.152 | 3.9% | 4.0% | 0.003 |
| Specialist care | 46.9% | 36.4% | 0.214 | 42.2% | 41.9% | 0.006 |
| Odds of Advanced-Stage Lung Cancer Diagnosis Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Exposure Groups | Unweighted Univariable Analysis | Weighted Univariable Analysis | Weighted Multivariable Analysis | Weighted Multivariable Analysis Adjusted for Prior Imaging | |
| Overall analysis | COPD | 0.70 (0.68 to 0.72) | 0.72 (0.70 to 0.75) | 0.70 (0.68 to 0.72) | 0.77 (0.75 to 0.80) |
| No COPD | Reference | Reference | Reference | Reference | |
| Prior chest CT scan | – | – | – | 0.36 (0.35 to 0.38) | |
| No prior chest CT scan | Reference | ||||
| Subgroup analysis: Previously diagnosed COPD vs. no COPD | Previously diagnosed COPD | 0.67 (0.65 to 0.69) | 0.70 (0.68 to 0.73) | 0.68 (0.66 to 0.70) | 0.77 (0.75 to 0.80) |
| No COPD | Reference | Reference | Reference | Reference | |
| Prior chest CT scan | – | – | – | 0.36 (0.35 to 0.38) | |
| No prior chest CT scan | Reference | ||||
| Subgroup analysis: Undiagnosed COPD vs. no COPD | Undiagnosed COPD | 0.84 (0.80 to 0.89) | 0.80 (0.76 to 0.84) | 0.77 (0.73 to 0.82) | 0.77 (0.73 to 0.81) |
| No COPD | Reference | Reference | Reference | Reference | |
| Prior chest CT scan | – | – | – | 0.31 (0.30 to 0.33) | |
| No prior chest CT scan | Reference | ||||
| Subgroup analysis: Previously diagnosed COPD vs. Undiagnosed COPD | Undiagnosed COPD | 1.26 (1.19 to 1.32) | 1.16 (1.10 to 1.22) | 1.18 (1.12 to 1.24) | 1.05 (1.00 to 1.11) |
| Previously diagnosed COPD | Reference | Reference | Reference | Reference | |
| Prior chest CT scan | – | – | – | 0.40 (0.39 to 0.42) | |
| No prior chest CT scan | Reference | ||||
| Odds of Advanced-Stage Lung Cancer Diagnosis Odds Ratio (95% CI) | ||||
|---|---|---|---|---|
| Subgroup Analysis | Unweighted Univariable Analysis | Weighted Univariable Analysis | Weighted Multivariable Analysis | Weighted Multivariable Analysis Adjusted for Prior Imaging |
| COPD * | 0.65 (0.63 to 0.67) | 0.66 (0.64 to 0.68) | 0.64 (0.62 to 0.66) | 0.74 (0.72 to 0.77) |
| No COPD | Reference | Reference | Reference | Reference |
| Prior chest CT scan | – | – | – | 0.37 (0.36 to 0.38) |
| No prior chest CT scan | Reference | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler, S.J.; Louie, A.V.; Sutradhar, R.; Paszat, L.; Brooks, D.; Gershon, A.S. Association between COPD and Stage of Lung Cancer Diagnosis: A Population-Based Study. Curr. Oncol. 2023, 30, 6397-6410. https://doi.org/10.3390/curroncol30070471
Butler SJ, Louie AV, Sutradhar R, Paszat L, Brooks D, Gershon AS. Association between COPD and Stage of Lung Cancer Diagnosis: A Population-Based Study. Current Oncology. 2023; 30(7):6397-6410. https://doi.org/10.3390/curroncol30070471
Chicago/Turabian StyleButler, Stacey J., Alexander V. Louie, Rinku Sutradhar, Lawrence Paszat, Dina Brooks, and Andrea S. Gershon. 2023. "Association between COPD and Stage of Lung Cancer Diagnosis: A Population-Based Study" Current Oncology 30, no. 7: 6397-6410. https://doi.org/10.3390/curroncol30070471
APA StyleButler, S. J., Louie, A. V., Sutradhar, R., Paszat, L., Brooks, D., & Gershon, A. S. (2023). Association between COPD and Stage of Lung Cancer Diagnosis: A Population-Based Study. Current Oncology, 30(7), 6397-6410. https://doi.org/10.3390/curroncol30070471






